Traumatic brachial plexus injuries are rare but serious consequences of major traumas. Pre-ganglionic lesions are considered irreparable, while post-ganglionic injuries can be potentially treated if an early diagnosis is available.
- brachial plexus
- MRI scan
- MRI diffusion weighted
- nervous system traumas
- peripheral nerves
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
The brachial plexus (BP) is the neural network that provides innervation to the upper chest, shoulders, and upper limbs. It is formed by the anterior branches of the last four cervical nerves (C5, C6, C7, and C8) and the first thoracic nerve (T1); the posterior and anterior nerve roots carry, respectively, sensory and motor fibers and exit from the spinal canal through the intervertebral foramen [1].
Before the union of the fibers there is an important structure, the posterior or dorsal root ganglion (DRG), which is considered an important landmark: lesions occurring proximally to DRG are defined pre-ganglionic, while lesions occurring distally to DRG are defined as post-ganglionic.
The second division of the BP is represented by three primary trunks: the superior trunk (formed by the union of C5 and C6 anterior roots), the middle trunk (which is the continuation of C7 anterior root), and the inferior trunk (C8 and T1 roots). The trunks are typically described as running into the interscalene triangle with the subclavian artery [2,3][2][3].
Near the lateral border of the first rib, each trunk splits into two branches: anterior and posterior. The six divisions form a triangular cluster that can be identified until the coracoid process occurs, where they form three cords.
The cords—lateral, posterior, and medial—run close to the axillary artery towards the pectoralis minor muscle, where they separate into five terminal branches: the axillary nerve, the median nerve, the musculocutaneous nerve, the radial nerve, and the ulnar nerve [1].
Traumatic BP injuries affect 1% of patients involved in major trauma (car accidents, occupational injuries, and falling), causing disability, pain, psychologic morbidity, and reduced quality of life [2,3,4][2][3][4].
According to the Seddon, Sunderland, and MacKinnon classifications, traumatic plexopathies can be divided into six degrees based on the number of layers damaged: neuropraxia (first degree), axonotmesis (from second to fourth degree), and neurotmesis (from fifth to sixth degree) [5,6][5][6].
Neuropraxia is a clinical condition characterized by temporary loss of function without denervation atrophy of the muscle. Axonotmesis is characterized by a Wallerian degeneration followed by nerve regeneration. While the latter can be managed conservatively, neurotmesis needs surgery for axon and myelin sheath disruption [7].
Another important classification of nerve injuries is based on their location: pre-ganglionic lesions are considered irreparable, while post-ganglionic injuries can be potentially surgically treated if an early diagnosis is available. Early surgical nerve repair leads to better functional recovery of the upper limb function [8,9][8][9].
As a consequence, diagnosis is important to distinguish low-grade lesions not requiring surgical treatment from high-grade lesions and to identify their location [10,11][10][11]. As magnetic resonance imaging (MRI) is a non-invasive, non-radiative imaging modality with multi-planar capability and great soft tissue characterization, it is a basic diagnostic imaging modality [12].
Many authors have examined the role of MRI in the diagnosis of traumatic BP injuries.
2. Strategy Search
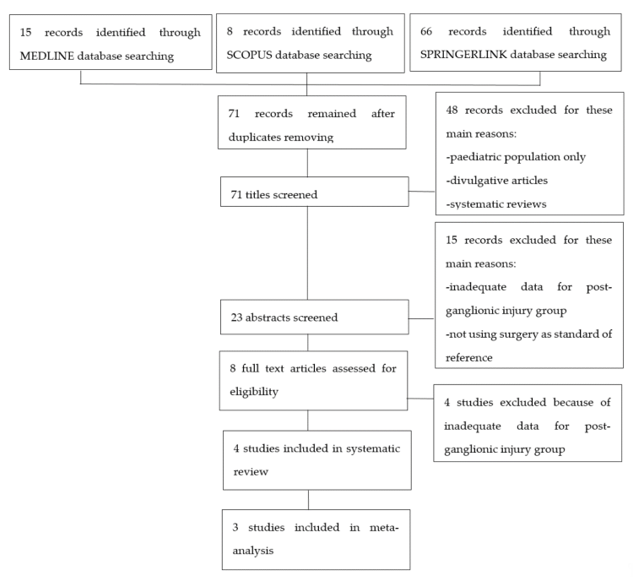
After searching in the aforementioned internet databases and removing duplicates, 71 articles were retrieved. These studies were then screened for eligibility as presented in the flow-chart (Figure 1). Eight articles underwent a full text screen and four of them were excluded because they were lacking adequate data regarding post-ganglionic BP injuries. Four studies were included in our systematic review, as summarized in Table 1. Of these, three were included in the meta-analysis [14–17][14][15][16][17], while Caporrino et al. [18][18] was excluded from the quantitative synthesis since TP, FP, TN, and FN were not reported in the text. All the included studies had prospective design and considered patients with traumatic BP injuries. All the studies but Caporrino et al. reported the number of patients included [15–17][15][16][17]. Two of the four studies provided information about the age range of the patients [16,17][16][17]. In Acharya, Caporrino, and Gad, a 1.5T MRI scanner was employed [15[15][16][18],16,18], while in Zhang, a 3T MRI scanner was used [17]. All the studies but Caporrino provided a precise description of the employed MRI protocol [15–17][15][16][17]. All the included studies used surgical findings as standard of reference [15–18][15][16][17][18].
Figure 1. Flowchart of study selection process.
3. Methodological Quality Assessment
Quality assessment of the included studies was conducted with the QUADAS-2 tool (Table 2) [11]. All the included studies but Zhang provided adequate information about patients’ inclusion and exclusion criteria [15,16,18][15][16][18]. MRI protocol was extensively described in all the studies but Caporrino [15–17][15][16][17]. It was mentioned in Gad only that surgeons were blinded to the MRI results, and therefore the reference standard was considered unlikely to have introduced biases [15]. Only Acharya’s article provided clear information about both the time intervals between injuries and MRI and between MRI and surgery; It was then considered at low risk of bias in terms of “flow and timing” [16]. Caporrino et al. only reported the time interval between injury and MRI [18].
4. Synthesis of Results
Table 1 shows characteristics and main conclusions of the selected studies.
MRI findings considered as significative for post-ganglionic injury of the BP were:
- nerve rupture: characterized by different degrees of nerve thickening caused by edema and inflammation with abnormal hyper intense signal in T2/short-tau inversion recovery (STIR) sequences;
- neuroma formation, characterized by a focal thickening of the injured segment of the nerve [17].
The selected studies did not clearly distinguish data among the different type of lesion.
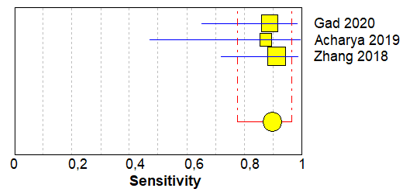
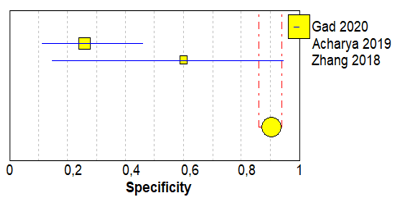
Table 3 shows sensitivity, which refers to the true positive rate (true positives)/(true positive + false negative), and specificity, which refers to the true negative rate (true negatives)/(true negative + false positive), values with 95% confidence intervals of MRI for traumatic post-ganglionic lesions for each study included in the meta-analysis and the relative forest plots [15–17][15][16][17].
The paper written by Caporrino et al. was also selected for the systematic review and reported a sensitivity of 60% (95% CI 32.3–83.7) and a specificity of 59.8% (95% CI 48.7–70.1%) [18].
The pooled sensitivity, pooled specificity, and 95% confidence interval of the three studies included in the meta-analysis are shown in Table 4. Pooled sensitivity turned out to be of 90% (95% CI 0.78–0.97) and the pooled specificity of 90% (0.86–0.94). The sensitivity value is, however, associated with a I2 rate >75%, due to the heterogeneous results of the selected literature.
Table 1. Summary of included studies.
|
|
Study Design |
Subject Features |
Postganglionic Lesions |
Age |
MRI Field Intensity |
MRI Sequences Employed |
MRI Timing |
Standard of Reference |
Level of Evidence |
Main Conclusion |
|
Acharya, 2019 [16] |
Prospective |
35 patients with traumatic brachial plexus injuries |
Eight surgically demonstrated postganglionic lesions |
Patients under the age of 60 |
1.5 T |
T1-T2-T2 weighted 3D neurography-T2 spin echo- short-tau inversion recovery (STIR) |
At least 3 weeks after injury |
Surgery |
2b |
Magnetic resonance imaging (MRI) is a useful tool in the diagnosis of brachial plexus injuries. |
|
Zhang, 2018 [17] |
Prospective |
28 patients with traumatic brachial plexus injuries |
23 surgically demonstrated postganglionic lesions, in 12 patients |
Mean age: 27.2 |
3 T |
T1-T2-STIR- balance FFE- diffusion-weighted imaging with background signal suppression (DWIBS) |
Not reported |
Surgery |
2b |
MRI is a valuable diagnostic tool for brachial plexus lesions, especially if balance-FFE, STIR, and DWIBS sequences are performed. |
|
Caporrino, 2014 [18] |
Prospective
|
34 patients with traumatic plexus injuries |
Not reported |
Mean age: 29.8 |
1.5 T |
Not reported |
2–3 months after injury |
Surgery |
2b |
MRI showed poor diagnostic performance in identifying brachial plexus lesions compared to physical examination. Notwithstanding, it is reasonable to think that the combination of physical examination and MRI could provide the best diagnostic accuracy. |
|
Gad, 2020 [15] |
Prospective |
22 patients with traumatic brachial plexus injuries |
18 surgically demonstrated postganglionic lesions |
Mean age: 26.3 |
1.5 T |
T1, STIR, T2, T2-STIR, and DWIBS
|
Not reported |
Surgery |
2b |
“MRI is the imaging modality of choice in the examination of traumatic and obstetric brachial plexus injuries; it is safe and non-invasive, having the multiplanar capability and better soft tissue characterization”. |
Table 2. Quality assessment of diagnostic accuracy studies (QUADAS)-2, quality assessment of the included studies.
|
|
Patient Selection |
Index Test |
Reference Standard |
Flow and Timing |
|
Acharya, 2019 [16] |
+ |
+ |
+ |
+ |
|
Zhang, 2018 [17] |
? |
+ |
? |
? |
|
Caporrino, 2014 [18] |
+ |
? |
+ |
? |
|
Gad, 2020 [15] |
+ |
+ |
+ |
? |
Table 3. Forest plot showing sensitivity and specificity for each included study.
|
|
|||||||
|
Study |
TPatient Selection | ||||||
|
Index Test |
Reference Standard |
||||||
Flow and | |||||||
FP |
FN |
||||||
T | iming | ||||||
N | Sensitivity |
(95% CI) |
Specificity (95% CI) |
Forrest Plots |
|||
|
Ach | |||||||
|
Garya,d 201920 [16] | |||||||
|
+ |
+ |
+ |
+ |
||||
[15] |
16 |
0 |
2 |
198 |
0.89 (0.65–0.99) |
1.00 (0.98–1.00) |
|
|
Z | |||||||
|
Achang,rya 20189 [17] | |||||||
|
? |
+ |
? |
? |
||||
[16] |
7 |
20 |
1 |
7 |
0.88 (0.47–1.00) |
0.26 (0.11–0.46) |
|
|
C | |||||||
|
Zhaporrino, 2014g 2018 [18] | |||||||
|
+ |
? |
+ |
? |
||||
[17] |
21 |
2 |
2 |
3 |
0.91 (0.72–0.99) |
0.60 (0.15–0.95) |
|
|
Gad, 2020 [15] |
+ |
+ |
+ |
? |
Table 3. Forest plot showing sensitivity and specificity for each included study.
|
Study |
TP |
FP |
FN |
TN |
Sensitivity (95% CI) |
Specificity (95% CI) |
Forrest Plots |
|
Gad 2020 [15] |
16 |
0 |
2 |
198 |
0.89 (0.65–0.99) |
1.00 (0.98–1.00) |
|
|
Acharya 2019 [16] |
7 |
20 |
1 |
7 |
0.88 (0.47–1.00) |
0.26 (0.11–0.46) |
|
|
Zhang 2018 [17] |
21 |
2 |
2 |
3 |
0.91 (0.72–0.99) |
0.60 (0.15–0.95) |
Abbreviations: TP, true positive; FP, false positive; FN, false negative; TN, true negative.
Table 4.
Results of pooled data.
|
TP |
FP |
FN |
TN |
Pooled Sensitivity |
Pooled Specificity |
Pooled LR+ |
Pooled LR− |
Pooled DOR |
|||||
|
Value (95% CI) |
I2 |
Value (95% CI) |
I2 |
Value (95% CI) |
I2 |
Value (95% CI) |
I2 |
Value (95%CI) |
I2 |
||||
|
44 |
22 |
5 |
208 |
0.90 (0.78–0.97) |
0.0% |
0.90 (0.86–0.94) |
98.1% |
7.70 (0.28–214.76) |
96.5% |
0.17 (0.07–0.39) |
0.0% |
40.71 (0.99–1666.3) |
84.6% |
Abbreviations: TP, true positive; FP, false positive; FN, false negative; TN, true negative; CI, confidence interval; LR+, positive likelihood ratio; LR−, negative likelihood ratio; DOR, diagnostic odd ratio.
References
- Gilcrease-Garcia, B.M.; Deshmukh, S.D.; Parsons, M.S. Anatomy, Imaging, and Pathologic Conditions of the Brachial Plexus. Radio Graph. 2020, 40, 1686–1714.
- Griffith, J. Ultrasound of the Brachial Plexus. Musculoskelet. Radiol. 2018, 22, 323–333, doi:10.1055/s-0038-1645862.
- Lutz, A.M.; Gold, G.; Beaulieu, C. MR Imaging of the Brachial Plexus. Neuroimaging Clin. N. Am. 2014, 24, 91–108, doi:10.1016/j.nic.2013.03.024.
- Wade, R.G.; Takwoingi, Y.; Wormald, J.C.; Ridgway, J.P.; Tanner, S.; Rankine, J.J.; Bourke, G. MRI for detecting root avulsions in traumatic adult brachial plexus injuries: A systematic review and meta-analysis of diagnostic accuracy. Radiology 249, 293, 125-133.
- Yoshikawa, T.; Hayashi, N.; Yamamoto, S.; Tajiri, Y.; Yoshioka, N.; Masumoto, T.; Mori, H.; Abe, O.; Aoki, S.; Ohtomo, K. Brachial plexus injury: Clinical manifestations, conventional imaging findings, and the latest imaging techniques. Radiographics 2006, 26 (Suppl. 1), S133–S143.
- Fox, I.K.; Mackinnon, S.E. Adult peripheral nerve disorders—Nerve entrapment, repair, transfer and brachial plexus disorders. Reconstr. Surg. 2011, 127, doi:10.1097/PRS.0b013e31820cf556.
- Silbermann-Hoffman, O.; Teboul, F. Post-traumatic brachial plexus MRI in practice. Interv. Imaging 2013, 94, 925–943.
- Franzblau, L.E.; Shauver, M.J.; Chung, K.C. Patient satisfaction and self-reported outcomes after complete brachial plexus avulsion injury. Hand Surg. 2004, 39.5, 948-955.
- Ochi, M.; Ikuta, Y.; Watanabe, M.; Kimori, K.; Itoh, K. The diagnostic value of MRI in traumatic brachial plexus injury. Hand Surg. 1994, 19, 55–59.
- Bischoff, C.; Kollmer, J.; Schulte-Mattler, W. State-of-the-Art Diagnosis of Peripheral Nerve Trauma: Clinical Examination, Electrodiagnostic, and Imaging. In Modern Concepts of Peripheral Nerve Repair; Springer: Cham, Germany, 2017; pp. 11–25.
- Balakrishna, S. A Pictorial essay of MRI findings–obstetric brachial plexopathy. Congr. Radiol. 2019, 2019, doi:10.26044/ecr2019/C-2491.
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097.
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Intern. Med. 2011, 155, 529–536.
- Zamora, J.; Abraira, V.; Muriel, A.; Khan, K.; Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med Res. Methodol. 2006, 6, 31.
- Gad, D.M.; Hussein, M.T.; Omar, N.N.M.; Kotb, M.M.; Abdel-Tawab, M.; Yousef, H.A.Z. Role of MRI in the diagnosis of adult traumatic and obstetric brachial plexus injury compared to intraoperative findings. J. Radiol. Nucl. Med. 2020, 51, 1–7.
- Acharya, A.M.; Cherian, B.S.; Bhat, A.K. Diagnostic accuracy of MRI for traumatic adult brachial plexus injury: A comparison study with surgical findings. Orthop. 2020, 17, 53–58.
- Zhang, L.; Xiao, T.; Yu, Q.; Li, Y.; Shen, F.; Li, W. Clinical value and diagnostic accuracy of 3.0 T multi-parameter magnetic resonance imaging in traumatic brachial plexus injury. Med Sci. Monit. Int. Med J. Exp. Clin. Res. 2018, 24, 7199.
- Caporrino, F.A.; Moreira, L.; Moraes, V.Y.; Belloti, J.C.; Gomes dos Santos, J.B.; Faloppa, F. Brachial plexus injuries: Diagnosis performance and reliability of everyday tools. Hand Surg. 2014, 19, 7–11.
References
- Gilcrease-Garcia, B.M.; Deshmukh, S.D.; Parsons, M.S. Anatomy, Imaging, and Pathologic Conditions of the Brachial Plexus. Radio Graph. 2020, 40, 1686–1714.
- Griffith, J. Ultrasound of the Brachial Plexus. Musculoskelet. Radiol. 2018, 22, 323–333, doi:10.1055/s-0038-1645862.
- Lutz, A.M.; Gold, G.; Beaulieu, C. MR Imaging of the Brachial Plexus. Neuroimaging Clin. N. Am. 2014, 24, 91–108, doi:10.1016/j.nic.2013.03.024.
- Wade, R.G.; Takwoingi, Y.; Wormald, J.C.; Ridgway, J.P.; Tanner, S.; Rankine, J.J.; Bourke, G. MRI for detecting root avulsions in traumatic adult brachial plexus injuries: A systematic review and meta-analysis of diagnostic accuracy. Radiology 249, 293, 125-133.
- Yoshikawa, T.; Hayashi, N.; Yamamoto, S.; Tajiri, Y.; Yoshioka, N.; Masumoto, T.; Mori, H.; Abe, O.; Aoki, S.; Ohtomo, K. Brachial plexus injury: Clinical manifestations, conventional imaging findings, and the latest imaging techniques. Radiographics 2006, 26 (Suppl. 1), S133–S143.
- Fox, I.K.; Mackinnon, S.E. Adult peripheral nerve disorders—Nerve entrapment, repair, transfer and brachial plexus disorders. Reconstr. Surg. 2011, 127, doi:10.1097/PRS.0b013e31820cf556.
- Silbermann-Hoffman, O.; Teboul, F. Post-traumatic brachial plexus MRI in practice. Interv. Imaging 2013, 94, 925–943.
- Franzblau, L.E.; Shauver, M.J.; Chung, K.C. Patient satisfaction and self-reported outcomes after complete brachial plexus avulsion injury. Hand Surg. 2004, 39.5, 948-955.
- Ochi, M.; Ikuta, Y.; Watanabe, M.; Kimori, K.; Itoh, K. The diagnostic value of MRI in traumatic brachial plexus injury. Hand Surg. 1994, 19, 55–59.
- Bischoff, C.; Kollmer, J.; Schulte-Mattler, W. State-of-the-Art Diagnosis of Peripheral Nerve Trauma: Clinical Examination, Electrodiagnostic, and Imaging. In Modern Concepts of Peripheral Nerve Repair; Springer: Cham, Germany, 2017; pp. 11–25.
- Balakrishna, S. A Pictorial essay of MRI findings–obstetric brachial plexopathy. Congr. Radiol. 2019, 2019, doi:10.26044/ecr2019/C-2491.
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097.
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Intern. Med. 2011, 155, 529–536.
- Zamora, J.; Abraira, V.; Muriel, A.; Khan, K.; Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med Res. Methodol. 2006, 6, 31.
- Gad, D.M.; Hussein, M.T.; Omar, N.N.M.; Kotb, M.M.; Abdel-Tawab, M.; Yousef, H.A.Z. Role of MRI in the diagnosis of adult traumatic and obstetric brachial plexus injury compared to intraoperative findings. J. Radiol. Nucl. Med. 2020, 51, 1–7.
- Acharya, A.M.; Cherian, B.S.; Bhat, A.K. Diagnostic accuracy of MRI for traumatic adult brachial plexus injury: A comparison study with surgical findings. Orthop. 2020, 17, 53–58.
- Zhang, L.; Xiao, T.; Yu, Q.; Li, Y.; Shen, F.; Li, W. Clinical value and diagnostic accuracy of 3.0 T multi-parameter magnetic resonance imaging in traumatic brachial plexus injury. Med Sci. Monit. Int. Med J. Exp. Clin. Res. 2018, 24, 7199.
- Caporrino, F.A.; Moreira, L.; Moraes, V.Y.; Belloti, J.C.; Gomes dos Santos, J.B.; Faloppa, F. Brachial plexus injuries: Diagnosis performance and reliability of everyday tools. Hand Surg. 2014, 19, 7–11.