Infectious diseases caused by bacteria, viruses, or parasites are the major causes of mortality and economic losses in commercial aquaculture. Some pathologies, especially those of bacterial origin, can be treated with commercially available drugs, while others are poorly managed. In fact, despite having been recognized as a useful preventive measure, no effective vaccination against many economically relevant diseases exist yet, such as for viral and parasitic infections.
- adjuvants
- aquaculture
- experimental challenge
- fish immunology
- fish welfare
- vaccines
- infectious diseases
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Aquaculture has experienced an enormous growth in productive terms, accounting to >527% in the 1990–2018 time frame. In 2018, aquaculture contributed to approximately 46% of the global total production of aquatic organisms (179 M tons) and 52% of seafood for human consumption (fish, crustaceans, mollusks, and other aquatic animals, excluding aquatic mammals, reptiles, seaweeds, and other aquatic plants) [1]. Capture-wise, any further increment in global productions will have to strictly ensure the preservation of natural resources, the 59.6% of which is currently being maximally sustainably fished, and avoid overfishing practices, also because of the severe ecological problems they are linked to (e.g., damages to coastal and marine ecosystems, alteration of multiple trophic levels, and algal blooms) [2][3][2,3]. Because of the increasing world population and per capita consumption [4][1,4], aquaculture is expected to continue growing, with conservative projections estimating 186 M tons production by year 2030 [5].
Commercial aquaculture is impacted by infectious diseases caused primarily by bacteria, viruses, parasites, and, to a lesser extent, fungi. Bacterial diseases can inflict significant biological, thus economic losses [6][7][8] [6–8]. While these are usually controllable with antibiotics, the indiscriminate use of these pharmaceuticals is ultimately a threat to human health because of the development and transfer of resistance mechanisms among bacterial species, some of which are also human pathogens. Their employment is therefore strongly regulated in many countries [9].
Various prevention strategies are currently used such as (i) biocontainment measures (e.g., quarantine and disease screening of newly introduced fishes) [10], (ii) water treatment systems (e.g., magnetic, ultraviolet, and ozone sterilization, all practically applicable only in recirculating systems) [11][12][11,12], and (iii) probiotics/prebiotics supplementation for immune system stimulation and growth promotion [13].
Fish vaccination can prevent or mitigate disease spreading with proven effectiveness against many relevant pathogens. The vaccine against enteric redmouth disease (caused by Yersinia ruckeri) developed in 1970s was the first to become commercially available [14], later followed by vaccines against cold water vibriosis (caused by Aliivibrio salmonicida) [15]. Since then, various vaccines have been developed, commercialized, successfully employed and reviewed [16][17][16,17]. Still, because of their high development and production costs and general lower efficacy than bacterins, few vaccines exist against viral diseases, and no commercial vaccines at all are available to date against parasitic diseases [18][15,18].
2. The factors affecting the efficacy of vaccines
This entry discusses the most promising and updated state-of-the-art vaccine research on three economically relevant aquaculture commodities chosen because of their distinct biological traits and geographical distribution as well as for being representative of different culture systems: European sea bass (Dicentrarchus labrax), Nile tilapia (Oreochromis niloticus), and Atlantic salmon (Salmo salar). From here on, the term “vaccine” is used to describe any substance used to stimulate the immune response or protect fish from pathogens, regardless of their classification (i.e., bacterial, viral, and parasitic). A compilation of mainly experimental formulations against bacterial, viral and parasitic infections is presented for each species (Tables 1–3; Figures 1–3). Commercial vaccines were considered only in particular cases (e.g., when a commercial product was adjuvanted with a recombinant molecule, when the study was of particular interest because of its large scale or analytical methods, or when commercial and experimental vaccines were compared). Because it is quite difficult to determine the exact variables affecting vaccine efficacy [19] [19], multiple factors such as (i) antigen dose, exposure and uptake, (ii) boost immunization strategy, (iii) adjuvant inclusion, type and performance, (iv) water temperature, (v) fish size, (vi) type, virulence, and route of experimental challenge need to be considered prior to being able to extrapolate fundamental scientific observations. For this reason, we herein provide readers with the essential procedural elements and findings from the available literature with the aim of delivering the most comprehensive understanding on the features and performances of protective vaccines and immunostimulants/adjuvants and, ultimately, on the fish immune response, a crucial end-point for further science-based vaccine developments.
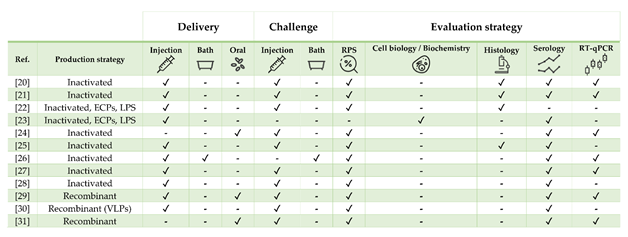
Table 1. Literature regarding experimental and commercial vaccines presented and discussed for European sea bass Dicentrarchus labrax. Approximate size refers to the fish body weight (BW) at the time of challenge or relative percentage of survival (RPS) calculation, as stated in or inferred from references. In case of commercial vaccines, the product description was linked. Challenges must be intended as homologous except when stated otherwise. List of abbreviations: ECPs—extracellular products; LPS—lipopolysaccharide; rTNFα—recombinant tumor necrosis factor alpha.
|
Pathogen |
Vaccine Status |
Adjuvant |
Approx. Size (g) |
Challenge |
Ref. |
|
Mycobacterium marinum |
Experimental |
50 |
Yes |
[20] |
|
|
M. marinum |
Experimental |
No |
20 |
Yes |
[21] |
|
Tenacibaculum maritimum |
Experimental |
No |
30 |
Yes |
[22] |
|
T. maritimum |
Experimental |
No |
5 |
No |
[23] |
|
Vibrio anguillarum + Vibrio ordalii |
Commercial (AquaVac Vibrio Oral) |
rTNFα |
30 |
Yes |
[24] |
|
V. anguillarum + Photobacterium damselae |
Commercial (AlphaJect 2000™ and AquaVac™ Vibrio-Pasteurella) |
Non-mineral |
35 |
Yes |
[25] |
|
Betanodavirus |
Experimental |
No |
2 and 6 |
Yes (only one exp. group) |
[26] |
|
Betanodavirus |
Experimental |
No |
11 |
Yes |
[27] |
|
Betanodavirus |
Experimental |
No |
6 |
Yes |
[28] |
|
Betanodavirus |
Experimental |
No |
11 |
Yes |
[29] |
|
Betanodavirus |
Experimental |
No |
30 |
Yes |
[30] |
|
Betanodavirus |
Experimental |
No |
6 |
Yes |
[31] |
Figure 1. Strategies for vaccine development, administration, and evaluation applied by referenced studies on European sea bass Dicentrarchus labrax.
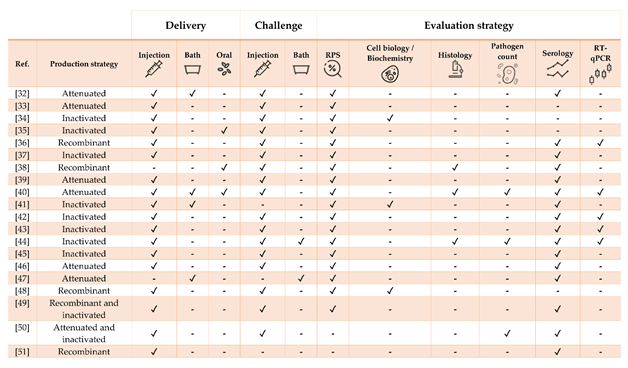
Table 2. Literature regarding experimental and commercial vaccines herein presented and discussed for Nile tilapia Oreochromis niloticus. Approximate size refers to the fish BW at the time of challenge or RPS calculation, as stated in or inferred from references. In case of commercial vaccines, the product description was linked. Challenges must be intended as homologous except when stated otherwise.
|
Pathogen |
Vaccine Status |
Adjuvant |
Approx. Size (g) |
Challenge |
Ref. |
|
Streptococcus iniae |
Experimental |
No |
10 |
Yes (homologous and heterologous) |
[32] |
|
S. iniae |
Experimental |
No |
40 |
Yes |
[33] |
|
S. iniae |
Experimental |
No |
5 |
Yes (heterologous) |
[34] |
|
S. iniae |
Experimental |
Oralject™ |
13 |
Yes |
[35] |
|
S. iniae |
Experimental |
No |
25 |
Yes |
[36] |
|
S. iniae |
Experimental |
No |
3 and 16 |
Yes |
[37] |
|
Streptococcus agalactiae |
Experimental |
No |
100 |
Yes |
[38] |
|
S. agalactiae |
Experimental |
No |
30 |
Yes (heterologous) |
[39] |
|
S. agalactiae |
Experimental |
No |
30 |
Yes |
[40] |
|
Polyvalent (S. agalactiae, S. iniae, Lactococcus garvieae and Enterococcus faecalis) |
Commercial (Mevac Aquastrept) |
500 and 1-month-old fry |
Yes |
[41] |
|
|
Francisella orientalis |
Experimental |
10 |
Yes |
[42] |
|
|
F. orientalis |
Experimental |
15 |
Yes (heterologous) |
[43] |
|
|
F. orientalis |
Experimental |
35 |
Yes |
[44] |
|
|
Aeromonas hydrophila |
Experimental |
No |
55 |
Yes |
[45] |
|
A. Hydrophila |
Experimental |
No |
10 |
Yes |
[46] |
|
Flavobacterium columnare |
Experimental |
No |
9 |
Yes (heterologous) |
[47] |
|
Vibrio anguillarum |
Experimental |
No |
3.5 |
Yes |
[48] |
|
Edwardsiella tarda |
Experimental |
102 |
Yes |
[49] |
|
|
E. tarda |
Experimental |
No |
42 |
Yes |
[50] |
|
Caligus rogercresseyi |
Experimental |
80 |
No |
[51] |
Figure 2. Strategies for vaccine development, administration, and evaluation applied by referenced studies on Nile tilapia Oreochromis niloticus.
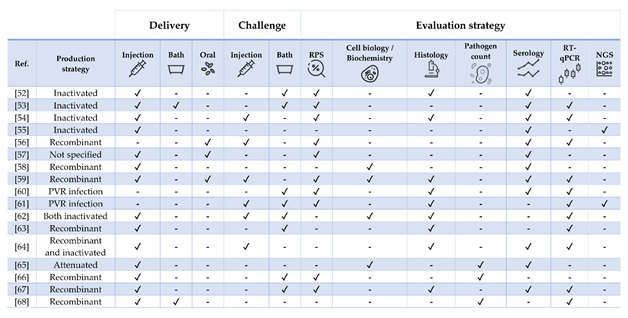
Table 3. Literature regarding experimental and commercial vaccines presented and discussed for Atlantic salmon Salmo salar. Approximate size refers to the fish BW at the time of challenge or RPS calculation, as stated in or inferred from references. In case of commercial vaccines, the product description was linked. Challenges must be intended as homologous except when stated otherwise. List of abbreviations: IFN—interferon; ISAV—infectious salmon anemia virus; IPNV—Infectious pancreatic necrosis virus; IHNV—infectious hematopoietic necrosis virus; SAV—salmonid alphavirus; PRV—piscine orthoreovirus; FCA—Freund’s complete adjuvant; FIA—Freund’s incomplete adjuvant.
|
Pathogen |
Vaccine Status |
Adjuvant |
Approx. Size (g) |
Challenge |
Ref. |
|
Tenacibaculum finnmarkense |
Experimental |
Mineral oil |
26 |
Yes (homologous and heterologous) |
[52] |
|
Yersinia ruckeri |
Experimental |
No |
9 |
Yes |
[53] |
|
Flavobacterium psychrophilum |
Experimental |
Squalene/alum or MontanideTM ISA 760 VG |
23 |
Yes |
[54] |
|
Polyvalent |
Commercial (Aquavac® PD7) |
Paraffin |
40 |
No |
[55] |
|
ISAV |
Experimental |
No |
40 |
Yes |
[56] |
|
ISAV and Piscirickettsia salmonis |
Commercial (Virbac-Centrovet) |
Oil |
40 |
No |
[57] |
|
ISAV |
Experimental |
IFNa- or IFNc |
40 |
No |
[58] |
|
IPNV |
Experimental |
No |
0.5 and 20 |
Yes |
[59] |
|
IHNV |
NA |
No |
5 g |
Yes (heterologous) |
[60] |
|
SAV |
NA |
No |
Post-smolt |
Yes (heterologous) |
[61] |
|
PRV |
Experimental and commercial (ALPHA JECT micro® 6) |
Paraffin |
55 |
Yes |
[62] |
|
PRV |
Experimental |
No |
35 |
Yes |
[63] |
|
SAV |
Experimental and commercial (Norvax® Compact PD) |
Montanide ISA 763A VG (only in the latter) |
30 |
Yes |
[64] |
|
Cryptobia salmositica |
Experimental |
No |
300 |
No |
[65] |
|
Caligus rogercressey |
Experimental |
80 |
Yes |
[66] |
|
|
Neoparamoeba perurans |
Experimental |
FCA (first immunization) and FIA (booster) |
100 |
Yes (two, 5-week apart) |
[67] |
|
Lepeophtheirus salmonis |
Experimental |
90 |
Yes |
[68] |
Figure 3. Strategies for vaccine development, administration, and evaluation applied by referenced studies on Atlantic salmon Salmo salar. For readability purposes, the bath and NGS columns also include cohabitation challenges and microarray experiments, respectively.