The elevated concentrations of various trace metals beyond existing guideline recommendations in water bodies have promoted research on the development of various electrochemical nanosensors for the trace metals’ early detection. Inspired by the exciting physical and chemical properties of nanomaterials, advanced functional nanocomposites with improved sensitivity, sensitivity and stability, amongst other performance parameters, have been synthesized, characterized, and applied on the detection of various trace metals in water matrices.
- nanomaterials
- nanosensors
- sensitivity
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
The key factor to mitigating trace metals pollution and keeping the water quality up to standard is the early detection of trace metals within the environmental water bodies [1]. Undoubtedly, the global economic expansion and industrial developments necessary to meet demand and the continued sustainability of human lives have been appreciated worldwide. At the same time, the discharges from the increased chemical processes, agricultural processes, and energy conversions, amongst other applications, have elevated water pollution. These anthropogenic processes have been observed to be the major contributors to the elevated concentrations of trace metals in water bodies [2–6][2][3][4][5][6]. The statistics published by the World Health Organization (WHO), based on the 2017 figures, pronounce that 785 million people lack basic drinking-water services, including 144 million people who are dependent on surface water. Globally, at least 2 billion people use a drinking water source contaminated with faeces, leading to the transmission of diseases such as; diarrhea, cholera, dysentery, typhoid, and polio, amongst others. It is well known that a class of essential trace elements, such as; zinc (Zn), copper (Cu), molybdenum (Mo), selenium (Se), chromium III (Cr), cobalt (Co), manganese (Mn), amongst others, play significant roles in nutrition balance and other biological aspects [7,8][7][8]. However, their excessive ingestion above the maximum permitted guidelines provided by the World Health Organization (WHO) may lead to various adverse effects ranging from acute to chronic impacts, depending on the type, species and level of exposure [9,10][9][10]. Another class of toxic trace metals, comprised of mercury (Hg), arsenic (As), thallium (TI), tin (Sn), antimony (Sb) and lead (Pb), amongst others, are poisonous and provide minimal health benefits even at trace levels [7,11][7][11]. To avoid a trace metal-based catastrophe, the amount of trace metals, predominantly toxic trace metals, in environmental water bodies ought to be frequently monitored and regulated. Traditional methods for the detection and monitoring of trace metals in water bodies include, but are not limited to, spectrometric techniques such as; inductively coupled plasma-optical emission spectrometry (ICP-OES) [6], inductively coupled plasma-mass spectrometry (ICP-MS) [12], flame atomic absorption spectroscopy (FAAS) [13], graphite furnace atomic absorption spectroscopy (GFAAS) [14] and x-ray fluorescence (XRF) [15]. However, these techniques require sample collection, sample pre-treatment, high energy input, pure gas input, infrastructure and skilled engineers, scientists, or technicians to operate the instruments [6,12–16][6][12][13][14][15][16]. As an alternative to these techniques, electroanalytical devices, particularly electrochemical sensors, have been identified as potential candidates for the low cost, sensitive and selective detection of trace metals in environmental water bodies [17–19][17][18][19]. Nowadays, nanomaterial technology plays an important role in providing opportunities and possibilities for the development of a new generation of sensing tools [20,21][20][21]. Considering their low cost, high efficiency, multiple functionality, flexibility, selectivity, and sensitivity, nanosensor devices account for the shortcomings encountered by the traditional analytical detection techniques [22]. Due to the incorporation of nanomaterials into their working electrode systems, significant enhancements on the sensitivity and selectivity of electrochemical nanosensors, amongst other performance parameters, have been reported [23]. It is without any doubt that the properties of nanomaterials, such as; the shape, size, aggregation/agglomeration state, size distribution, crystallinity and defect structure, play a significant role on the electrode’s performance in electrochemical sensors [24–26][24][25][26]. Tuning/engineering the nanomaterials on the surface of the electrode enables the detection of various toxic trace metals simultaneously or independently [27,28][27][28]. In the past few decades, extensive research has been carried out on electrochemical sensors incorporating engineered carbon [29,30][29][30], transition metals [31[31][32],32], and transition metal oxide/hydroxide [33,34][33][34] nanostructured materials as recognition elements, with promising results reported. To this end, three major strategies, including the engineering of active sites, engineering the electronic conductivity, and constructing a porous structure in nanomaterials, have been identified to improve the electrode’s surface chemistry and therefore enhance the electrode’s performance [35–38][35][36][37][38].
2. Nanosensors
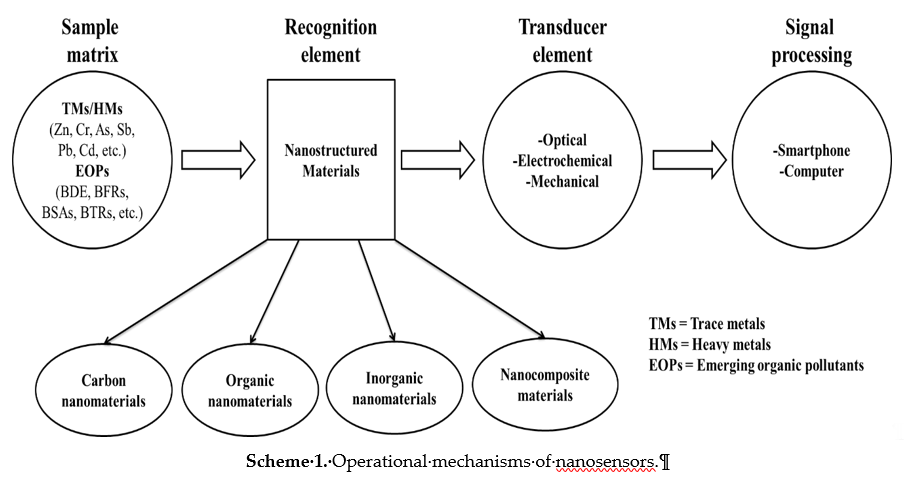
Nanosensors are sensing devices that are dependent on the unique properties of nanomaterials to recognize and detect the behavior of matter within a given environment at the nanoscale to macromolecular level [22]. These sensing devices can be large or small devices that utilize the nanomaterials incorporated as recognition elements to detect changes at a nanoscale [39]. The sensing mechanism follows either the optical, electrochemical or mechanical route of detection [22[22][40],40], as a result of the combination of the recognition element and the detector used as illustrated in Scheme 1. Evidently, as key technological and economic drivers, various nanosensors have been developed for the detection of chemical and toxic gases, food packaging, medical diagnostics, and water quality monitoring, amongst other applications [41–43][41][42][43]. Interestingly, the recognition element has been identified amongst these applications as the key element giving the identity of the nanosensor [44]. Owing to their unique physico-chemical properties enhancing the signal detection, nanomaterials have been incorporated as recognition elements in sensors [22]. Nanomaterials allow for the amplified binding of target molecules on their available active sites, which lead to detectable signals [44]. It is worth noting that if the recognition element is a nanostructured material, then a nanosensor is obtained [22]. To this end, various classes of nanostructured materials ranging from carbon-based nanomaterials to organic-based nanomaterials, inorganic-based nanomaterials, and nanocomposite materials (incorporated in Scheme 1) have been synthesized and deployed in the afore-mentioned applications [41–43][41][42][43]. Various synthetic methods for the synthesis of these classes of nanomaterials are outlined in the next section.
Scheme 1. Operational mechanisms of nanosensors.
3. Nanosensor Fabrication
Nanomaterials properties such as; high surface area-volume ratio, dimensionality (<100 nm), high adsorption surface area, high mobility of particles, reproducibility of the particles, uniformity, composition, rapid/delayed particle agglomeration and high electric/heat conductivity are crucial in nanosensors [45,46][45][46]. Miniaturization of the nanomaterials improves the surface-to-volume ratio of nanomaterials, resulting in variations in chemical, mechanical, optical, and magnetic properties, resulting in the increased sensitivity and specificity in nanosensors [47,48][47][48]. The nanomaterials synthesis method is therefore crucial for improving the performance of the nanosensors. Moreover, synthesis methods leading to the production of uniformly distributed particles and consistent shapes are necessary to improve the linearity and the detection limits of the nanosensor, amongst other performance parameters [47]. There are two common approaches for the synthesis of nanomaterials, namely top down and bottom up methods [49]. Common top down methods involve mechanical methods such as; mechanical grinding, high energy ball milling, mechanical alloying, reactive milling and lithography [16]. Common bottom up methods include chemical methods such as; hydrothermal, co-precipitation, microemulsion, sol-gel, chemical-vapor deposition, electrodeposition, epitaxial growth, and colloidal dispersion [50].
From the perspective of this review, the classification of the nanomaterials was done based on the structural composition, such as; carbon based nanomaterials, metallic nanomaterials, and metal oxides/hydroxide nanomaterials. After the identification and description of the key performance parameter, the three classes’ classifications are used in Section 5 to discuss various nanomaterials for sensing trace metals in environmental water samples for water quality assessment applications.
References
- Akanji, S.P.; Ama, O.M.; Ray, S.S.; Osifo, P.O. Metal Oxide Nanomaterials for Electrochemical Detection of Heavy Metals in Water. In Nanostructured Metal-Oxide Electrode Materials for Water Purification; Springer: Berlin, Germany, 2020; pp. 113–126.
- Sihlahla, M.; Mouri, H.; Nomngongo, P.N. Uptake of trace elements by vegetable plants grown on agricultural soils: Evaluation of trace metal accumulation and potential health risk. Afr. Earth Sci. 2019, 160, 103635, doi:10.1016/j.jafrearsci.2019.103635.
- Donner, M.W.; Arshad, M.; Ullah, A.; Siddique, T. Unravelled keratin-derived biopolymers as novel biosorbents for the simultaneous removal of multiple trace metals from industrial wastewater. Total Environ. 2019, 647, 1539–1546, doi:10.1016/j.scitotenv.2018.08.085.
- Embaby, A.; Redwan, M. Sources and behavior of trace elements in groundwater in the South Eastern Desert, Egypt. Monit. Assess. 2019, 191, 686, doi:10.1007/s10661-019-7868-3.
- Strzelec, M.; Proemse, B.C.; Barmuta, L.A.; Gault-Ringold, M.; Desservettaz, M.; Boyd, P.W.; Perron, M.M.G.; Schofield, R.; Bowie, A.R. Atmospheric Trace Metal Deposition from Natural and Anthropogenic Sources in Western Australia. Atmosphere (Basel) 2020, 11, 474, doi:10.3390/atmos11050474.
- Munonde, T.S.; Maxakato, N.W.; Nomngongo, P.N. Preconcentration and speciation of chromium species using ICP-OES after ultrasound-assisted magnetic solid phase extraction with an amino-modified magnetic nanocomposite prepared from Fe3O4, MnO2 and Al2O3. Acta 2017, 184, 1223–1232, doi:10.1007/s00604-017-2126-2.
- Jakavula, S.; Biata, N.R.; Dimpe, K.M.; Pakade, V.E.; Nomngongo, P.N. A Critical Review on the Synthesis and Application of Ion-Imprinted Polymers for Selective Preconcentration, Speciation, Removal and Determination of Trace and Essential Metals from Different Matrices. Rev. Anal. Chem. 2020, 1–13, doi:10.1080/10408347.2020.1798210.
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Martínez, M.Á.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part A. concentrations in maternal blood, urine and cord blood. Res. 2019, 177, 108599, doi:10.1016/j.envres.2019.108599.
- Nnorom, I.C.; Ewuzie, U.; Eze, S.O. Multivariate statistical approach and water quality assessment of natural springs and other drinking water sources in Southeastern Nigeria. Heliyon 2019, 5, e01123, doi:10.1016/j.heliyon.2019.e01123.
- Cotruvo, J.A. 2017 WHO Guidelines for Drinking Water Quality: First Addendum to the Fourth Edition. Am. Water Work. Assoc. 2017, 109, 44–51, doi:10.5942/jawwa.2017.109.0087.
- Khound, N.J.; Phukon, P.; Bhattacharyya, K.G. Toxic Trace Metals in the Surface Water Sources of Jia–Bharali river basin, North Brahmaputra Plain, India—A Hydrochemical Elucidation. Water Resour. 2019, 46, 117–127, doi:10.1134/s009780781901010x.
- Munonde, T.S.; Maxakato, N.W.; Nomngongo, P.N. Preparation of magnetic Fe3O4 nanocomposites modified with MnO2, Al2O3, Au and their application for preconcentration of arsenic in river water samples. Environ. Chem. Eng. 2018, 6, 1673–1681, doi:10.1016/j.jece.2018.02.017.
- Filik, H.; Avan, A.A. Ionic Liquid Based Dispersive Liquid-Liquid Microextraction Combined with Magnetic-Based Dispersive Micro-Solid-Phase Extraction for Determination of Trace Cobalt in Water Samples by FAAS. Anal. Chem. 2017, 13, 456–463, doi:10.2174/1573411013666170307093452.
- Han, Q.; Huo, Y.; Yang, L.; Yang, X.; He, Y.; Wu, J. Determination of Trace Nickel in Water Samples by Graphite Furnace Atomic Absorption Spectrometry after Mixed Micelle-Mediated Cloud Point Extraction. Molecules 2018, 23, 2597, doi:10.3390/molecules23102597.
- Zhou, S.; Yuan, Z.; Cheng, Q.; Zhang, Z.; Yang, J. Rapid in situ determination of heavy metal concentrations in polluted water via portable XRF: Using Cu and Pb as example. Pollut. 2018, 243, 1325–1333, doi:10.1016/j.envpol.2018.09.087.
- Wang, J.; Wu, S.; Suo, X.-K.; Liao, H. The Processes for Fabricating Nanopowders. In Advanced Nanomaterials and Coatings by Thermal Spray; Elsevier: Amsterdam, The Netherlands, 2019; pp. 13–25.
- Kokab, T.; Shah, A.; Nisar, J.; Khan, A.M.; Khan, S.B.; Shah, A.H. Tripeptide Derivative-Modified Glassy Carbon Electrode: A Novel Electrochemical Sensor for Sensitive and Selective Detection of Cd2+ Ions. ACS Omega 2020, 5, 10123–10132, doi:10.1021/acsomega.0c00760.
- Munir, A.; Shah, A.; Nisar, J.; Ashiq, M.N.; Akhter, M.S.; Shah, A.H. Selective and simultaneous detection of Zn2+, Cd2+, Pb2+, Cu2+, Hg2+ and Sr2+ using surfactant modified electrochemical sensors. Acta 2019, 323, 134592, doi:10.1016/j.electacta.2019.134592.
- Wang, Y.; Zhao, S.; Li, M.; Li, W.; Zhao, Y.; Qi, J.; Cui, X. Graphene quantum dots decorated graphene as an enhanced sensing platform for sensitive and selective detection of copper(II). Electroanal. Chem. 2017, 797, 113–120, doi:10.1016/j.jelechem.2017.05.031.
- Baig, N.; Sajid, M.; Saleh, T.A. Recent trends in nanomaterial-modified electrodes for electroanalytical applications. TrAC Trends Anal. Chem. 2019, 111, 47–61, doi:10.1016/j.trac.2018.11.044.
- Oliveira, T.M.B.F.; Morais, S. New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes. Sci. 2018, 8, 1925, doi:10.3390/app8101925.
- Munawar, A.; Ong, Y.; Schirhagl, R.; Tahir, M.A.; Khan, W.S.; Bajwa, S.Z. Nanosensors for diagnosis with optical, electric and mechanical transducers. RSC Adv. 2019, 9, 6793–6803, doi:10.1039/c8ra10144b.
- Li, Y.; Chen, Y.; Yu, H.; Tian, L.; Wang, Z. Portable and smart devices for monitoring heavy metal ions integrated with nanomaterials. TrAC Trends Anal. Chem. 2018, 98, 190–200, doi:10.1016/j.trac.2017.11.011.
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. J. Chem. 2019, 12, 908–931.
- Wu, Z.; Yang, S.; Wu, W. Shape control of inorganic nanoparticles from solution. Nanoscale 2016, 8, 1237–1259, doi:10.1039/c5nr07681a.
- Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23, doi:10.1016/j.teac.2017.02.001.
- Huang, H.; Chen, L.; Wang, S.; Kang, P.; Chen, X.; Guo, Z.; Huang, X.-J. Electrochemical monitoring of persistent toxic substances using metal oxide and its composite nanomaterials: Design, preparation, and application. TrAC Trends Anal. Chem. 2019, 119, 115636, doi:10.1016/j.trac.2019.115636.
- Lu, Z.; Zhang, J.; Dai, W.; Lin, X.; Ye, J.; Ye, J. A screen-printed carbon electrode modified with a bismuth film and gold nanoparticles for simultaneous stripping voltammetric determination of Zn(II), Pb(II) and Cu(II). Acta 2017, 184, 4731–4740, doi:10.1007/s00604-017-2521-8.
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. New approaches to antimony film screen-printed electrodes using carbon-based nanomaterials substrates. Chim. Acta 2016, 916, 17–23, doi:10.1016/j.aca.2016.03.003.
- Zuo, Y.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Yu, Y. Graphene-derived nanomaterials as recognition elements for electrochemical determination of heavy metal ions: A review. Acta 2019, 186, 1–17, doi:10.1007/s00604-019-3248-5.
- De Barros, A.; Constantino, C.J.L.; Da Cruz, N.C.; Bortoleto, J.R.R.; Ferreira, M. High performance of electrochemical sensors based on LbL films of gold nanoparticles, polyaniline and sodium montmorillonite clay mineral for simultaneous detection of metal ions. Acta 2017, 235, 700–708, doi:10.1016/j.electacta.2017.03.135.
- Eranjaneya, H.; Adarakatti, P.S.; Siddaramanna, A.; Malingappa, P.; Chandrappa, G.T. Citric acid assisted synthesis of manganese tungstate nanoparticles for simultaneous electrochemical sensing of heavy metal ions. Sci. Semicond. Process. 2018, 86, 85–92, doi:10.1016/j.mssp.2018.06.020.
- Jin, W.; Fu, Y.; Hu, M.; Wang, S.; Liu, Z. Highly efficient SnS-decorated Bi2O3 nanosheets for simultaneous electrochemical detection and removal of Cd(II) and Pb(II). Electroanal. Chem. 2020, 856, 113744.
- Mohamed, M.A.; El-Badawy, F.M.; El-Desoky, H.S.; Ghoneim, M.M. Magnetic cobalt ferrite nanoparticles CoFe2O4platform as an efficient sensor for trace determination of Cu(ii) in water samples and different food products. New J. Chem. 2017, 41, 11138–11147, doi:10.1039/c7nj01857f.
- Gan, X.; Zhao, H. Understanding signal amplification strategies of nanostructured electrochemical sensors for environmental pollutants. Opin. Electrochem. 2019, 17, 56–64, doi:10.1016/j.coelec.2019.04.016.
- Chen, X.; He, X.; Gao, J.; Jiang, J.; Jiang, X.; Wu, C. Three-dimensional porous Ni, N-codoped C networks for highly sensitive and selective non-enzymatic glucose sensing. Actuators B Chem. 2019, 299, 126945, doi:10.1016/j.snb.2019.126945.
- Chen, H.; Liang, X.; Liu, Y.; Ai, X.; Asefa, T.; Zou, X. Active Site Engineering in Porous Electrocatalysts. Mater. 2020, e2002435, doi:10.1002/adma.202002435.
- Liu, W.; Yin, R.; Xu, X.; Zhang, L.; Shi, W.; Cao, X. Structural Engineering of Low-Dimensional Metal–Organic Frameworks: Synthesis, Properties, and Applications. Sci. 2019, 6, 1802373, doi:10.1002/advs.201802373.
- Tuantranont, A. Applications of nanomaterials in sensors and diagnostics. In Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2013.
- Kranz, C. Carbon-Based Nanosensor Technology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 17, ISBN 3030118649.
- Wang, Z.L. Self-Powered Nanosensors and Nanosystems. Mater. 2012, 24, 280–285, doi:10.1002/adma.201102958.
- Vikesland, P.J. Nanosensors for water quality monitoring. Nanotechnol. 2018, 13, 651–660, doi:10.1038/s41565-018-0209-9.
- Fuertes, G.; Soto, I.; Carrasco, R.; Vargas, M.; Sabattin, J.; Lagos, C. Intelligent Packaging Systems: Sensors and Nanosensors to Monitor Food Quality and Safety. Sens. 2016, 2016, 1–8, doi:10.1155/2016/4046061.
- Justino, C.I.L.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Santos, T.A.P.R. Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends Anal. Chem. 2015, 68, 2–17, doi:10.1016/j.trac.2015.03.006.
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Chen, B.; Zhang, H. Two-Dimensional Metal Nanomaterials: Synthesis, Properties, and Applications. Rev. 2018, 118, 6409–6455, doi:10.1021/acs.chemrev.7b00727.
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934, doi:10.1039/c8nr02278j.
- Graboski, A.M.; Martinazzo, J.; Ballen, S.C.; Steffens, J.; Steffens, C. Nanosensors for water quality control. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 115–128.
- Mahbub, T.; Hoque, M.E. Introduction to nanomaterials and nanomanufacturing for nanosensors. In Nanofabrication for Smart Nanosensor Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–20.
- Gregorczyk, K.; Knez, M. Hybrid nanomaterials through molecular and atomic layer deposition: Top down, bottom up, and in-between approaches to new materials. Mater. Sci. 2016, 75, 1–37, doi:10.1016/j.pmatsci.2015.06.004.
- Ganachari, S.V.; Banapurmath, N.R.; Salimath, B.; Yaradoddi, J.S.; Shettar, A.S.; Hunashyal, A.M.; Venkataraman, A.; Patil, P.; Shoba, H.; Hiremath, G.B. Synthesis techniques for preparation of nanomaterials. In Handbook of Ecomaterials; Springer: Berlin/Heidelberg, Germany, 2017.
References
- Akanji, S.P.; Ama, O.M.; Ray, S.S.; Osifo, P.O. Metal Oxide Nanomaterials for Electrochemical Detection of Heavy Metals in Water. In Nanostructured Metal-Oxide Electrode Materials for Water Purification; Springer: Berlin, Germany, 2020; pp. 113–126.
- Sihlahla, M.; Mouri, H.; Nomngongo, P.N. Uptake of trace elements by vegetable plants grown on agricultural soils: Evaluation of trace metal accumulation and potential health risk. Afr. Earth Sci. 2019, 160, 103635, doi:10.1016/j.jafrearsci.2019.103635.
- Donner, M.W.; Arshad, M.; Ullah, A.; Siddique, T. Unravelled keratin-derived biopolymers as novel biosorbents for the simultaneous removal of multiple trace metals from industrial wastewater. Total Environ. 2019, 647, 1539–1546, doi:10.1016/j.scitotenv.2018.08.085.
- Embaby, A.; Redwan, M. Sources and behavior of trace elements in groundwater in the South Eastern Desert, Egypt. Monit. Assess. 2019, 191, 686, doi:10.1007/s10661-019-7868-3.
- Strzelec, M.; Proemse, B.C.; Barmuta, L.A.; Gault-Ringold, M.; Desservettaz, M.; Boyd, P.W.; Perron, M.M.G.; Schofield, R.; Bowie, A.R. Atmospheric Trace Metal Deposition from Natural and Anthropogenic Sources in Western Australia. Atmosphere (Basel) 2020, 11, 474, doi:10.3390/atmos11050474.
- Munonde, T.S.; Maxakato, N.W.; Nomngongo, P.N. Preconcentration and speciation of chromium species using ICP-OES after ultrasound-assisted magnetic solid phase extraction with an amino-modified magnetic nanocomposite prepared from Fe3O4, MnO2 and Al2O3. Acta 2017, 184, 1223–1232, doi:10.1007/s00604-017-2126-2.
- Jakavula, S.; Biata, N.R.; Dimpe, K.M.; Pakade, V.E.; Nomngongo, P.N. A Critical Review on the Synthesis and Application of Ion-Imprinted Polymers for Selective Preconcentration, Speciation, Removal and Determination of Trace and Essential Metals from Different Matrices. Rev. Anal. Chem. 2020, 1–13, doi:10.1080/10408347.2020.1798210.
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Martínez, M.Á.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part A. concentrations in maternal blood, urine and cord blood. Res. 2019, 177, 108599, doi:10.1016/j.envres.2019.108599.
- Nnorom, I.C.; Ewuzie, U.; Eze, S.O. Multivariate statistical approach and water quality assessment of natural springs and other drinking water sources in Southeastern Nigeria. Heliyon 2019, 5, e01123, doi:10.1016/j.heliyon.2019.e01123.
- Cotruvo, J.A. 2017 WHO Guidelines for Drinking Water Quality: First Addendum to the Fourth Edition. Am. Water Work. Assoc. 2017, 109, 44–51, doi:10.5942/jawwa.2017.109.0087.
- Khound, N.J.; Phukon, P.; Bhattacharyya, K.G. Toxic Trace Metals in the Surface Water Sources of Jia–Bharali river basin, North Brahmaputra Plain, India—A Hydrochemical Elucidation. Water Resour. 2019, 46, 117–127, doi:10.1134/s009780781901010x.
- Munonde, T.S.; Maxakato, N.W.; Nomngongo, P.N. Preparation of magnetic Fe3O4 nanocomposites modified with MnO2, Al2O3, Au and their application for preconcentration of arsenic in river water samples. Environ. Chem. Eng. 2018, 6, 1673–1681, doi:10.1016/j.jece.2018.02.017.
- Filik, H.; Avan, A.A. Ionic Liquid Based Dispersive Liquid-Liquid Microextraction Combined with Magnetic-Based Dispersive Micro-Solid-Phase Extraction for Determination of Trace Cobalt in Water Samples by FAAS. Anal. Chem. 2017, 13, 456–463, doi:10.2174/1573411013666170307093452.
- Han, Q.; Huo, Y.; Yang, L.; Yang, X.; He, Y.; Wu, J. Determination of Trace Nickel in Water Samples by Graphite Furnace Atomic Absorption Spectrometry after Mixed Micelle-Mediated Cloud Point Extraction. Molecules 2018, 23, 2597, doi:10.3390/molecules23102597.
- Zhou, S.; Yuan, Z.; Cheng, Q.; Zhang, Z.; Yang, J. Rapid in situ determination of heavy metal concentrations in polluted water via portable XRF: Using Cu and Pb as example. Pollut. 2018, 243, 1325–1333, doi:10.1016/j.envpol.2018.09.087.
- Wang, J.; Wu, S.; Suo, X.-K.; Liao, H. The Processes for Fabricating Nanopowders. In Advanced Nanomaterials and Coatings by Thermal Spray; Elsevier: Amsterdam, The Netherlands, 2019; pp. 13–25.
- Kokab, T.; Shah, A.; Nisar, J.; Khan, A.M.; Khan, S.B.; Shah, A.H. Tripeptide Derivative-Modified Glassy Carbon Electrode: A Novel Electrochemical Sensor for Sensitive and Selective Detection of Cd2+ Ions. ACS Omega 2020, 5, 10123–10132, doi:10.1021/acsomega.0c00760.
- Munir, A.; Shah, A.; Nisar, J.; Ashiq, M.N.; Akhter, M.S.; Shah, A.H. Selective and simultaneous detection of Zn2+, Cd2+, Pb2+, Cu2+, Hg2+ and Sr2+ using surfactant modified electrochemical sensors. Acta 2019, 323, 134592, doi:10.1016/j.electacta.2019.134592.
- Wang, Y.; Zhao, S.; Li, M.; Li, W.; Zhao, Y.; Qi, J.; Cui, X. Graphene quantum dots decorated graphene as an enhanced sensing platform for sensitive and selective detection of copper(II). Electroanal. Chem. 2017, 797, 113–120, doi:10.1016/j.jelechem.2017.05.031.
- Baig, N.; Sajid, M.; Saleh, T.A. Recent trends in nanomaterial-modified electrodes for electroanalytical applications. TrAC Trends Anal. Chem. 2019, 111, 47–61, doi:10.1016/j.trac.2018.11.044.
- Oliveira, T.M.B.F.; Morais, S. New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes. Sci. 2018, 8, 1925, doi:10.3390/app8101925.
- Munawar, A.; Ong, Y.; Schirhagl, R.; Tahir, M.A.; Khan, W.S.; Bajwa, S.Z. Nanosensors for diagnosis with optical, electric and mechanical transducers. RSC Adv. 2019, 9, 6793–6803, doi:10.1039/c8ra10144b.
- Li, Y.; Chen, Y.; Yu, H.; Tian, L.; Wang, Z. Portable and smart devices for monitoring heavy metal ions integrated with nanomaterials. TrAC Trends Anal. Chem. 2018, 98, 190–200, doi:10.1016/j.trac.2017.11.011.
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. J. Chem. 2019, 12, 908–931.
- Wu, Z.; Yang, S.; Wu, W. Shape control of inorganic nanoparticles from solution. Nanoscale 2016, 8, 1237–1259, doi:10.1039/c5nr07681a.
- Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23, doi:10.1016/j.teac.2017.02.001.
- Huang, H.; Chen, L.; Wang, S.; Kang, P.; Chen, X.; Guo, Z.; Huang, X.-J. Electrochemical monitoring of persistent toxic substances using metal oxide and its composite nanomaterials: Design, preparation, and application. TrAC Trends Anal. Chem. 2019, 119, 115636, doi:10.1016/j.trac.2019.115636.
- Lu, Z.; Zhang, J.; Dai, W.; Lin, X.; Ye, J.; Ye, J. A screen-printed carbon electrode modified with a bismuth film and gold nanoparticles for simultaneous stripping voltammetric determination of Zn(II), Pb(II) and Cu(II). Acta 2017, 184, 4731–4740, doi:10.1007/s00604-017-2521-8.
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. New approaches to antimony film screen-printed electrodes using carbon-based nanomaterials substrates. Chim. Acta 2016, 916, 17–23, doi:10.1016/j.aca.2016.03.003.
- Zuo, Y.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Yu, Y. Graphene-derived nanomaterials as recognition elements for electrochemical determination of heavy metal ions: A review. Acta 2019, 186, 1–17, doi:10.1007/s00604-019-3248-5.
- De Barros, A.; Constantino, C.J.L.; Da Cruz, N.C.; Bortoleto, J.R.R.; Ferreira, M. High performance of electrochemical sensors based on LbL films of gold nanoparticles, polyaniline and sodium montmorillonite clay mineral for simultaneous detection of metal ions. Acta 2017, 235, 700–708, doi:10.1016/j.electacta.2017.03.135.
- Eranjaneya, H.; Adarakatti, P.S.; Siddaramanna, A.; Malingappa, P.; Chandrappa, G.T. Citric acid assisted synthesis of manganese tungstate nanoparticles for simultaneous electrochemical sensing of heavy metal ions. Sci. Semicond. Process. 2018, 86, 85–92, doi:10.1016/j.mssp.2018.06.020.
- Jin, W.; Fu, Y.; Hu, M.; Wang, S.; Liu, Z. Highly efficient SnS-decorated Bi2O3 nanosheets for simultaneous electrochemical detection and removal of Cd(II) and Pb(II). Electroanal. Chem. 2020, 856, 113744.
- Mohamed, M.A.; El-Badawy, F.M.; El-Desoky, H.S.; Ghoneim, M.M. Magnetic cobalt ferrite nanoparticles CoFe2O4platform as an efficient sensor for trace determination of Cu(ii) in water samples and different food products. New J. Chem. 2017, 41, 11138–11147, doi:10.1039/c7nj01857f.
- Gan, X.; Zhao, H. Understanding signal amplification strategies of nanostructured electrochemical sensors for environmental pollutants. Opin. Electrochem. 2019, 17, 56–64, doi:10.1016/j.coelec.2019.04.016.
- Chen, X.; He, X.; Gao, J.; Jiang, J.; Jiang, X.; Wu, C. Three-dimensional porous Ni, N-codoped C networks for highly sensitive and selective non-enzymatic glucose sensing. Actuators B Chem. 2019, 299, 126945, doi:10.1016/j.snb.2019.126945.
- Chen, H.; Liang, X.; Liu, Y.; Ai, X.; Asefa, T.; Zou, X. Active Site Engineering in Porous Electrocatalysts. Mater. 2020, e2002435, doi:10.1002/adma.202002435.
- Liu, W.; Yin, R.; Xu, X.; Zhang, L.; Shi, W.; Cao, X. Structural Engineering of Low-Dimensional Metal–Organic Frameworks: Synthesis, Properties, and Applications. Sci. 2019, 6, 1802373, doi:10.1002/advs.201802373.
- Tuantranont, A. Applications of nanomaterials in sensors and diagnostics. In Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2013.
- Kranz, C. Carbon-Based Nanosensor Technology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 17, ISBN 3030118649.
- Wang, Z.L. Self-Powered Nanosensors and Nanosystems. Mater. 2012, 24, 280–285, doi:10.1002/adma.201102958.
- Vikesland, P.J. Nanosensors for water quality monitoring. Nanotechnol. 2018, 13, 651–660, doi:10.1038/s41565-018-0209-9.
- Fuertes, G.; Soto, I.; Carrasco, R.; Vargas, M.; Sabattin, J.; Lagos, C. Intelligent Packaging Systems: Sensors and Nanosensors to Monitor Food Quality and Safety. Sens. 2016, 2016, 1–8, doi:10.1155/2016/4046061.
- Justino, C.I.L.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Santos, T.A.P.R. Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends Anal. Chem. 2015, 68, 2–17, doi:10.1016/j.trac.2015.03.006.
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Chen, B.; Zhang, H. Two-Dimensional Metal Nanomaterials: Synthesis, Properties, and Applications. Rev. 2018, 118, 6409–6455, doi:10.1021/acs.chemrev.7b00727.
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934, doi:10.1039/c8nr02278j.
- Graboski, A.M.; Martinazzo, J.; Ballen, S.C.; Steffens, J.; Steffens, C. Nanosensors for water quality control. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 115–128.
- Mahbub, T.; Hoque, M.E. Introduction to nanomaterials and nanomanufacturing for nanosensors. In Nanofabrication for Smart Nanosensor Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–20.
- Gregorczyk, K.; Knez, M. Hybrid nanomaterials through molecular and atomic layer deposition: Top down, bottom up, and in-between approaches to new materials. Mater. Sci. 2016, 75, 1–37, doi:10.1016/j.pmatsci.2015.06.004.
- Ganachari, S.V.; Banapurmath, N.R.; Salimath, B.; Yaradoddi, J.S.; Shettar, A.S.; Hunashyal, A.M.; Venkataraman, A.; Patil, P.; Shoba, H.; Hiremath, G.B. Synthesis techniques for preparation of nanomaterials. In Handbook of Ecomaterials; Springer: Berlin/Heidelberg, Germany, 2017.