Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Arũnas Ramanavičius and Version 1 by Arũnas Ramanavičius.
Amperometric biosensors and biofuel cells are mostly based on immobilized enzymes or living cells. Among the many oxidoreductases, glucose oxidase (GOx) is used mostly in biosensor design. The same GOx can be well applied for the development of biofuel cells and self-charging capacitors based on the operation of biofuel cells.
- Enzymatic Biosensors
- Biofuel Cells
1. Introduction
CPs-based layers play a number of different roles in the design of amperometric sensors because they can serve: (i) as an immobilization matrix [1]; (ii) as a diffusional barrier for enzymatic reaction substrate, which increases the so called apparent Michaelis constant (KM(app.)) for immobilized enzymes and, therefore, in this way can extend the linear-range of amperometric biosensors [2]; and (iii) and in some cases, they act as charge transfer mediators [3,4]. Therefore, the entrapment of enzymes within conducting polymer-based structures enables some bioanalytical characteristics (such as limits of detection and linear ranges) of biosensing systems to be changed. Biosensors based on GOx, which is modified by conducting polymers (e.g., polyaniline, polypyrrole, or polythiophene) have been reported and in such systems, soluble redox mediators (ferrocene, benzoquinone, 2,6-dichlorophenol indophenol, phenazine methosulfate, and some others) were applied in order to facilitate charge transfer between the enzyme and electrode. Facilitation of indirect CT by the application of some nanomaterials (such as metal and semiconductor nanoparticles) is rather simple, therefore it is applied in most electrochemical enzymatic biosensors [5,6,7,8] and biofuel cells [9].
2. Development
Several ‘generations’ of amperometric biosensors are determined according to the applied charge principle. In ‘first-generation’ amperometric biosensors, charge is transferred via enzymatic reaction products [10] (e.g., if oxidases are applied, then electrons from one substrate are transferred to dissolved oxygen and hydrogen peroxide is formed [11,12]); in the case of such amperometric sensors, the analytical signal can be based on electrochemical registration of decreasing oxygen concentration or increasing hydrogen peroxide concentration (Figure 1A).
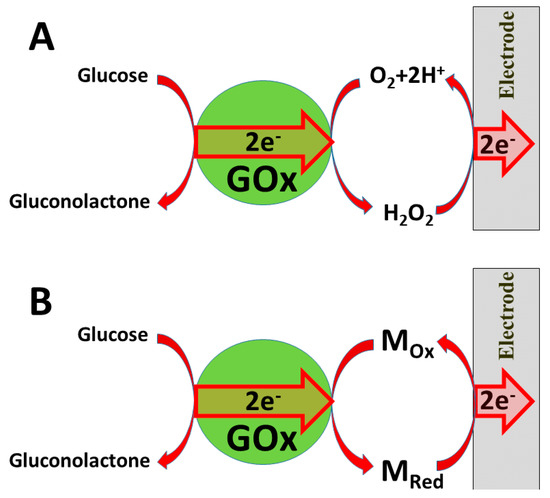
Figure 1. Charge transfer from glucose oxidase (GOx): (A) via formed hydrogen peroxide; (B) via redox mediator Mox/MRed.
In the ‘second generation’ of amperometric biosensors, dissolved charge transfer mediators are applied that transfer the charge while oxidized/reduced forms of these redox mediators diffuse between the redox-able active site of the enzyme to the electrode (Figure 2B). In the ‘third generation’ of amperometric biosensors, charge transfer is based on the direct exchange of charge carriers between the enzyme’s active site and electrode [13,14], and the same effect can be exploited in direct electron transfer-based biofuel cells [15] (Figure 2); to improve/facilitate this process, conducting polymers can be applied [3,14,15]. Sometimes, additional sophisticated ‘wiring’ routes are applied in order to establish the charge transfer between the redox sites of enzymes and electrodes [3,14,15,16].
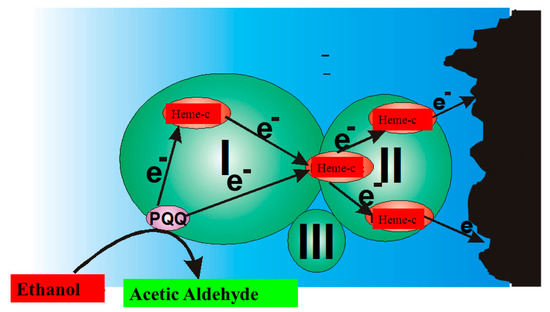
Figure 2. Charge transfer within PQQ-Heme dependent alcohol dehydrogenase, and direct electron transfer from PQQ-Heme dependent alcohol dehydrogenase, which can be applied in the design of biofuel cells [15] and amperommetric biosensors of the third generation.
It should be noted that fast and efficient charge transfer is especially critical for the action of biofuel cells. It should be noted that in addition to the charge transfer between the enzyme and electrode, very critical is the understanding of the charge carrier pathways and their dynamics within oxidoreductases applied in the design of bioelectronics-based devices [4,14,15]. From the scientific point of view, understanding of the charge transfer pathways and mechanisms is extremely important in order to exploit enzymes efficiently [4,14], which is important during the design of biofuel cells and biosensors. In some redox enzymes, charge transfer pathways are rather complex because some radicals of amino acids can be involved in intrinsic charge transfer pathways [4,14,17]. Such amino acids (e.g., tryptophan and tyrosine [18]) are mostly based on aromatic radicals and they can attend not only in intrinsic, but also in extrinsic charge transfer pathways that are typical for various biological systems [19-21]. Both electron- and hole-based charge transfer pathways can be observed between some redox enzymes (e.g., in GOx) and some conducting polymers that act as p-type organic semiconductors (e.g., carbazole-derivatives) [4,14].
Some other p-type semiconducting polymers (including poly(3,4-ethylenedioxythiophene (PEDOT) due to suitable ionization potential, which is below 5.0 eV) show sufficient ability to transfer holes [22]. It was predicted that charge transfer via hole hopping to some extent protects enzymes from oxidative damages [23]. Such polymers are able to not only transfer charge via holes, but can even inject them into the intrinsic charge transfer pathway of some redox enzymes including glucose oxidase as reported for some carbazole derivatives [4,14] or PEDOT [24-26]. Application of such p-type semiconducting polymers is very promising for biosensors and biofuel cells because it enables the stability of the enzymes to be retained for a longer period of time, therefore, in some of our works, we applied several p-type semiconducting carbazole-based derivates for the development of rather stable glucose oxidase-based biosensors [4,14]. The action of such glucose biosensors is well supported by DFT-based computations [14], which enabled the charge transfer mechanism to be elaborated not only in polymer, but also inside the enzyme, and to calculate charge transfer characteristics [14] that were in agreement with those determined by experimental approaches [4]. All these properties of conducting polymers can be applied during the development of advanced biosensors, which will have analytical characteristics better suitable for particular analytical purposes (e.g., the entrapment of redox enzymes), which initially possess rather low KM(app.), within CPs enables the increase in the ‘upper limits of analyte determination’ due to the formed CP-based ‘diffusion layer’ [1,5,26]. In this way, glucose biosensors can be based on glucose oxidases that mostly have rather low KM(app.), which are mostly much lower than the glucose concentration in the blood serum [27].
Some CPs can facilitate electron transfer between the active-site of the enzyme and electrode [3], which is important during the development of biofuel cells and amperometric biosensors [1,8]. However, active-sites in some redox enzymes are located within the protein backbone. Therefore, charge transfer to/from these active-sites is not possible even through conducting polymer-based structures.
In some of our previously published studies, we determined that charge transfer could be established by structures based on polyphenontraline [3] and carbazole-based derivatives [4,14]. During the modeling of amperometric biosensors, glucose oxidase is applied as a model enzyme. Therefore, glucose oxidase was entrapped within some CPs [1,3,8]. However, electron transfer from the active-site of enzymes and the electrode still remains a challenging problem in these structures, the most frequent charge transfer is established by dissolved redox mediators or by electrodeposited conducting polymers [3]. Therefore, in some biosensors, conducting polymers can serve as electron transfer mediators and as a matrix within which redox enzymes are immobilized [28,29]. The applicability of conducting polymers can be improved by the formation of various copolymers based on monomers that form conducting polymers (e.g., in this way, specific functional groups, which are required for covalent immobilization of enzymes (namely, carboxylic groups, amino groups, etc.), can be introduced) [30]. In this way, the pyrrole-2-carboxylic acid was polymerized into particles of poly-(pyrrole-2-carboxylic acid) (PCPy) by chemical polymerization initiated by H2O2, and then covalently modified by glucose oxidase via formed amide bonds, which were formed after the activation of carboxylic groups by N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS). During this activation step, EDC reacts with carboxyl groups and forms active O-acylisourea intermediates, which couple NHS and form amine-reactive N-sulfosuccinimidyl esters on the surface of the PCPy layer that during the next step react with the amino groups of glucose oxidase. Then, this GOx/PCPy nanocomposite was applied for the modification of graphite electrodes and applied in the design of a glucose sensor [30] (Figure 3).
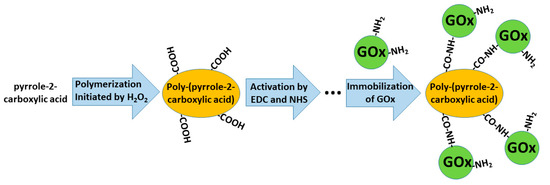
Figure 3. Formation of poly-(pyrrole-2-carboxylic acid), followed by the activation of carboxylic groups and covalent immobilization of glucose oxidase (GOx).
The immobilization of enzymes enables biosensors to be applied for continuous and/or repeating measurements of analytes, however, despite numerous efforts to retain the stable activity of enzymes, they gradually lose their activity, which negatively influences the accuracy of the analytical signal [31]. Hence, limited stability of amperometric biosensors is a drawback that requires special attention and, therefore, these biosensors require additional calibration procedures that are performed periodically and/or periodical exchange/replacement of enzyme-based structures.
It should be noted that various charge transfer reactions play a very important role in photosynthesis, metabolic pathways, and many other biological and artificial redox systems [32-34]. Both electron and hole transfer mechanisms are important for charge transfer in biosensors and biofuel cells. However, recent developments in electrochemistry and bioelectronics mostly take into consideration only the electron transfer-based reactions. For this reason, advanced understanding of the charge transfer mechanisms and pathways is required for the development of advanced bioelectronics-based devices. In addition, charge hopping and/or tunneling mechanisms [35,36] can be involved for the charge transfer between electrodes and redox enzymes or other redox proteins [37,38]. These mechanisms provide the ability to transfer charge through rather long distances, however, the efficiency of these charge transfer mechanisms is not very high and is always determined by the electrical potential of electrodes and redox potentials of used materials. Conducting polymers, which are used for the modification of electrodes applied in the design of amperometric biosensors and/or biofuel cells, can provide hole- or electron-based conductivity. Therefore, charge transfer between these conducting polymers and redox enzymes could also be evaluated in such a way that takes into account the many different mechanisms of charge transfer between redox enzymes and conducting polymers [4,14].
3. Conclusions
Efficient charge transfer (CT) plays a crucial role in the design of biosensors and especially in the development of reliable biofuel cells. In order to improve CT in these bioelectronics-based devices, some nanomaterials are applied. Indirect CT is the most frequently exploited during the design of biosensors and even during the development of biofuel cells, here, nanomaterials, especially metal and semiconductor nanostructures, play an important role. Nanocomposites based on conducting polymers and some nanomaterials can provide various technological advantages including increased surface area, which is required for the establishment of higher current densities that are of special interest during the development of biofuel cells. In addition, CPs offer very attractive ways for the immobilization of enzymes. Among the many different methods used for the formation of conducting polymer-based structures and nanostructures, electrochemical deposition is one of the most efficient due to the possibility of precise control of the polymerization process by the adjustment of the most suitable electrochemical parameters. In addition to electrochemical synthesis, oxidizing agents, redox enzyme, and microbes can be applied as initiators of CP synthesis, which all confirmed that through these ways, formed CP-based enzyme/CP composites could be applied in the design of biosensors and biofuel cells. In enzymatic-electrochemical biosensors and biofuel cells, some other characteristics of the enzyme/CP-based layer are also very important, namely, density, permeability, and thickness of the structure, which is formed over the electrode.
Direct charge transfer (DCT), which is very often simply called direct electron transfer (DET), from enzymes, except DCT/DET for a few types of enzymes (hemoproteins, Cu ion-based proteins, and some other enzymes), is still very rarely realized. Despite of this evaluation of DCT/DET is a very interesting and promissing research direction, which in the future will provide many interesting solutions. Recently, some rather sophisticated DCT/DET pathways from enzymes toward electrodes have been established by several research groups. In some of these DCT/DET routes, conducting polymers (CPs) such as polypyrrole and carbazole-derivatives were applied. In some of our research and theoretical calculations, we have demonstrated that not only electron transfer, but also hole transfer, can play a role and can be well exploited in the design of bioelectronics-based devices.
The good biocompatibility of some conducting polymers provides new possibilities for their application as ‘stealth coatings’ in the design of implantable biosensors and biofuel cells.
References
1. Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Self-encapsulation of oxidases as a basic approach to tune upper detection limit of amperometric bosensors. Analyst 2008, 133, 1083–1089.
2. Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49.
3. Oztekin, Y.; Ramanaviciene, A.; Yazıcıgil, Z.; Solak, A.O.; Ramanavicius, A. Direct electron transfer from glucose oxidase immobilized on polyphenanthroline-modified glassy carbon electrode. Biosens. Bioelectron. 2011, 26, 2541–2546, doi:10.1016/j.bios.2010.11.001.
4. Bagdžiūnas, G.; Žukauskas, Š.; Ramanavicius, A. Insights into a hole transfer mechanism between glucose oxidase and a p-type organic semiconductor. Biosens. Bioelectron. 2018, 102, 449–455, doi:10.1016/j.bios.2017.11.053.
5. German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of enzymatic formation of polyaniline nanoparticles. Polymer 2017, 115, 211–216, doi:10.1016/j.polymer.2017.03.028.
6. German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Formation and Electrochemical Characterisation of Enzyme-Assisted Formation of Polypyrrole and Polyaniline Nanocomposites with Embedded Glucose Oxidase and Gold Nanoparticles. J. Electrochem. Soc. 2020, 167, 165501, doi:10.1149/1945-7111/abc9dc.
7. German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation of Polyaniline and Polypyrrole Nanocomposites with Embedded Glucose Oxidase and Gold Nanoparticles. Polymer 2019, 11, 377, doi:10.3390/polym11020377.
8. German, N.; Ramanavicius, A.; Voronovic, J.; Ramanaviciene, A. Glucose biosensor based on glucose oxidase and gold nanoparticles of different sizes covered by polypyrrole layer. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 413, 224–230, doi:10.1016/j.colsurfa.2012.02.012.
9. Kizling, M.; Draminska, S.; Stolarczyk, K.; Tammela, P.; Wang, Z.; Nyholm, L.; Bilewicz, R. Biosupercapacitors for powering oxygen sensing devices. Bioelectrochemistry 2015, 106, 34–40, doi:10.1016/j.bioelechem.2015.04.012.
10. Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825, doi:10.1021/cr068123a.
11. Romero, E.; Castellanos, J.R.G.; Gadda, G.; Fraaije, M.W.; Mattevi, A. Same Substrate, Many Reactions: Oxygen Activation in Flavoenzymes. Chem. Rev. 2018, 118, 1742–1769, doi:10.1021/acs.chemrev.7b00650.
12. German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Enzymatic Formation of Polyaniline, Polypyrrole, and Polythiophene Nanoparticles with Embedded Glucose Oxidase. Nanomaterials 2019, 9, 806, doi:10.3390/nano9050806.
13. Scheiblbrandner, S.; Breslmayr, E.; Csarman, F.; Paulner, R.; Führer, J.; Herzog, P.L.; Shleev, S.V.; Osipov, E.M.; Tikhonova, T.V.; Popov, V.O.; et al. Evolving stability and pH-dependent activity of the high redox potential Botrytis aclada laccase for enzymatic fuel cells. Sci. Rep. 2017, 7, 1–3.
14. Bagdžiūnas, G.; Ramanavičius, A. Towards Direct Enzyme Wiring: A Theoretical Investigation of Charge Carriers Transfer Mechanisms between Glucose Oxidase and Organic Semiconductors. Phys. Chem. Chem. Phys. 2019, 21, 2968–2976.
15. Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Biofuel cell based on direct bioelectrocatalysis. Biosens. Bioelectron. 2005, 20, 1962–1967, doi:10.1016/j.bios.2004.08.032.
16. Liu, Y.; Dolidze, T.D.; Singhal, S.; Khoshtariya, D.E.; Wei, J. New Evidence for a Quasi-Simultaneous Proton-Coupled Two-Electron Transfer and Direct Wiring for Glucose Oxidase Captured by the Carbon Nanotube–Polymer Matrix. J. Phys. Chem. C 2015, 119, 14900–14910, doi:10.1021/acs.jpcc.5b02796.
17. Page, C.C.; Moser, C.C.; Dutton, P.L. Mechanism for electron transfer within and between proteins. Curr. Opin. Chem. Biol. 2003, 7, 551–556, doi:10.1016/j.cbpa.2003.08.005.
18. DeFelippis, M.R.; Murthy, C.P.; Broitman, F.; Weinraub, D.; Faraggi, M.; Klapper, M.H. Electrochemical properties of tyrosine phenoxy and tryptophan indolyl radicals in peptides and amino acid analogs. J. Phys. Chem. 1991, 95, 3416–3419, doi:10.1021/j100161a081.
19. Müller, P.; Ignatz, E.; Kiontke, S.; Brettel, K.; Essen, L.-O. Sub-nanosecond tryptophan radical deprotonation mediated by a protein-bound water cluster in class II DNA photolyases. Sci. 2018, 9, 1200–1212, doi:10.1039/c7sc03969g.
20. Warren, J.J.; Ener, M.E.; Vlček, A.; Winkler, J.R.; Gray, H.B. Electron hopping through proteins. Chem. Rev. 2012, 256, 2478–2487, doi:10.1016/j.ccr.2012.03.032.
21. Martin, R.; Lacombat, F.; Espagne, A.; Dozova, N.; Plaza, P.; Yamamoto, J.; Müller, P.; Brettel, K.; De La Lande, A. Ultrafast flavin photoreduction in an oxidized animal (6-4) photolyase through an unconventional tryptophan tetrad. Phys. Chem. Chem. Phys. 2017, 19, 24493–24504, doi:10.1039/C7CP04555G.
22. Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater. 2000, 12, 481–494.
23. Winkler, J.R.; Gray, H.B. Electron flow through biological molecules: Does hole hopping protect proteins from oxidative damage? Q. Rev. Biophys. 2015, 48, 411–420.
24. Kros, A.; van Hövell, S.W.F.M.; Sommerdijk, N.a.J.M.; Nolte, R.J.M. Poly(3,4-ethylenedioxythiophene)-based Glucose Biosensors. Adv. Mater. 2001, 13, 1555–1557.
25. Park, J.; Kim, H.K.; Son, Y. Glucose biosensor constructed from capped conducting microtubules of PEDOT. Sens. Actuators B Chem. 2008, 133, 244–250, doi:10.1016/j.snb.2008.02.029.
26. Krikstolaityte, V.; Kuliesius, J.; Ramanaviciene, A.; Mikoliunaite, L.; Kausaite-Minkstimiene, A.; Oztekin, Y.; Ramanavicius, A. Enzymatic polymerization of polythiophene by immobilized glucose oxidase. Polymer 2014, 55, 1613–1620, doi:10.1016/j.polymer.2014.02.003.
27. German, N.; Kausaite-Minkstimiene, A.; Ramanavicius, A.; Semashko, T.; Mikhailova, R.; Ramanaviciene, A. The use of different glucose oxidases for the development of an amperometric reagentless glucose biosensor based on gold nanoparticles covered by polypyrrole. Electrochim. Acta 2015, 169, 326–333, doi:10.1016/j.electacta.2015.04.072.
28. Cabaj, J.; Sołoducho, J.; Chyla, A.; Jędrychowska, A. Hybrid phenol biosensor based on modified phenoloxidase electrode. Sens. Actuators B Chem. 2011, 157, 225–231, doi:10.1016/j.snb.2011.03.054.
29. Nazari, M.; Kashanian, S.; Rafipour, R. Laccase immobilization on the electrode surface to design a biosensor for the detection of phenolic compound such as catechol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 130–138, doi:10.1016/j.saa.2015.01.126.
30. Kausaite-Minkstimiene, A.; Glumbokaite, L.; Ramanaviciene, A.; Dauksaite’, E.; Ramanavicius, A. An Amperometric Glucose Biosensor Based on Poly (Pyrrole-2-Carboxylic Acid)/Glucose Oxidase Biocomposite. Electroanalysis 2018, 30, 1642–1652, doi:10.1002/elan.201800044.
31. German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation and Electrochemical Evaluation of Polyaniline and Polypyrrole Nanocomposites Based on Glucose Oxidase and Gold Nanostructures. Polymer 2020, 12, 3026, doi:10.3390/polym12123026.
32. Page, C.C.; Moser, C.C.; Chen, X.; Dutton, P.L. Natural engineering principles of electron tunnelling in biological oxidation–reduction. Nat. Cell Biol. 1999, 402, 47–52, doi:10.1038/46972.
33. Prytkova, T.R.; Kurnikov, I.V.; Beratan, D.N. Coupling Coherence Distinguishes Structure Sensitivity in Protein Electron Transfer. Science 2007, 315, 622–625, doi:10.1126/science.1134862.
34. Cordes, M.; Giese, B. Electron transfer in peptides and proteins. Chem. Soc. Rev. 2009, 38, 892–901.
35. Ponce, A.; Gray, H.B.; Winkler, J.R. Electron Tunneling through Water: Oxidative Quenching of Electronically Excited Ru(tpy)22+(tpy = 2,2‘:6,2‘ ‘-terpyridine) by Ferric Ions in Aqueous Glasses at 77 K. J. Am. Chem. Soc. 2000, 122, 8187–8191, doi:10.1021/ja000017h.
36. Gray, H.B.; Winkler, J.R. Electron tunneling through proteins. Q. Rev. Biophys. 2003, 36, 341–372, doi:10.1017/s0033583503003913.
37. Saen-Oon, S.; Lucas, M.F.; Guallar, V. Electron transfer in proteins: Theory, applications and future perspectives. Phys. Chem. Chem. Phys. 2013, 15, 15271–15285, doi:10.1039/c3cp50484k.
38. Bostick, C.D.; Mukhopadhyay, S.; Pecht, I.; Sheves, M.; Cahen, D.; Lederman, D. Protein bioelectronics: A review of what we do and do not know. Rep. Prog. Phys. 2018, 81, 026601, doi:10.1088/1361-6633/aa85f2.
