Lignocellulosic sources are the world’s largest renewable sources for bioethanol production and can be divided into three main types: (1) marine algae, (2) agricultural residues and municipal solid wastes, (3) and forest woody feedstocks.
- enzymatic hydrolysis
- bioethanol production
- biofuels
- lignocellulosic biomass
- agricultural waste
- wood feedstock
- marine algae
1. Introduction
Different groups of raw materials are available for bioethanol production, dependent on their structure and composition. Many research reports describe different lignocellulosic waste for the production of bioethanol, such as corn stover, rice straw, bagasse, grass, and others [1][2][3][4][5][8,24,25,26,27]. Marine algae are the new and promising alternative for the production of bioethanol, since they can grow fast, but they still face some challenges, such as their high pretreatment costs. The primary crop for bioethanol production is switch grass that grows in the northern hemisphere and is of great interest because of its low cost, as well as its abundance and high content of sugar substrates. Different grasses also require almost no or very low maintenance and no fertilization.
1.1. Marine Algae
Marine algae present a renewable biomass source, whose main advantage is in fast and sustainable growth [6][7][28,29]. Moreover, they are gaining interest as third-generation feedstock because of the rapid development of biorefineries designed for bioethanol production. Since marine algae have a high content of carbohydrates in their composition, they are able to yield almost 60 times more alcohol than other agricultural or forestry feedstocks [8][30]. However, there are still some challenges present in using algae as a source for bioethanol production, since the presence of hydrocolloid polymers present in the cell walls of algae makes the walls stronger and therefore requires a pretreatment of such algal feedstock to break down those complex cell wall structures (Figure 3). Such process is expensive and presents around 20% of production cost [9][10][31,32].
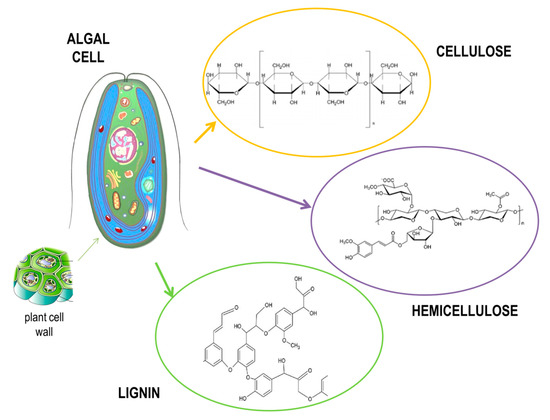
Figure 3.
Structure of an algal cell.
Macroalgae or seaweed are divided into three main types: brown algae (Phaeophyta), red algae (Rhodophyta), and green algae (Chlorophyta). In general, macroalgae contain around 25%–60% carbohydrates, 5%–20% proteins, 0.5%–5% lipids, and around 15%–40% ash. Regarding sugar composition (Table 1), brown algae generally consist of alginate, cellulose, mannitol, fucoidin, and laminarin, while red algae consist of agar, carrageenan, cellulose, and lignin; green alga consist of starch, cellulose, mannan, and ulvan [11][12][33,34]. In order to hydrolyze seaweed into fermentable sugars, pretreatment and saccharification processes are required. A typical pretreatment method for seaweed into hydrolysate conversion for bioethanol production is based on using acid pretreatment with fairly high temperatures (100–150 °C) [13][35]. However, other methods for the pretreatment of lignocellulosic bioethanol production, such as microwave [14][36] and alkali [15][37] pretreatments, were also used in hydrolysis processes. Enzymatic saccharification is often required following pretreatment using cellulosic enzyme solutions, such as alginate lyase or laminarinase, which are successful in the effective hydrolysis of brown algae [16][17][38,39]. On the other hand, microalgae have the ability to grow fast with high lipid content in some species, such as Chlorella sp. In addition, some species, such as Synechococcus sp., contain around 60% carbohydrates [18][40]. Factors influencing the lipid and carbohydrate content of microalgae are temperature, light, salinity, nutrient content, O2, CO2 level, and pH [19][41]. The microalgal cell wall is easily broken down with pretreatment with lysozymes or diluted acids when compared to macroalgae or other biomass. Cellulose, hemi-cellulose, lignin, pectin, and other carbohydrates converted to monomers by enzymatic or acid hydrolysis are the most common components in the microalgal cell wall [20][42].
| Macroalgae | Sugar Composition |
|---|---|
| Phaeophyta (brown) | alginate cellulose mannitol fucoidin laminarin |
| Rhodophyta (red) | agar carrageenan cellulose lignin |
| Chlorophyta (green) | starch cellulose mannan ulvan |
1.2. Agricultural Residues and Municipal Wastes
There are four major agricultural wastes that are the most favorable biomass feedstocks for the production of bioethanol. Those are rice straw, wheat straw, corn straw, and sugarcane baggase, which are also used as animal fodder and domestic fuel [22][4]. Most potential feedstocks for bioethanol production are wheat and rice straws and corn stalks, since they contain approximately 35% hemicellulosic material [23][44]. As agricultural residues present an environmentally friendly step in the process, they also help prevent deforestation. Different crops go through short-term harvest rotations and are therefore more available for bioethanol production [22][24][4,45]. Rice straw disposal is limited by its slow degradation, big bulk material, and high mineral content. Only a small part of rice straw is used as animal feed, while the rest of rice straw (more than 90%) is removed by field burning. Among the four mentioned major agricultural wastes, rice straw can produce more than 200 billion liters biofuel per year, being the most abundant waste biomass feedstock in the world [25][46].
As an alternative to agricultural cellulosic residues, a good candidate for raw materials that has potential for bioethanol production is municipal solid wastes, which can solve the household garbage disposal and therefore limit the environmental problems that may occur due to such problems [26][47]. All kinds of high yielding crops are gaining great interest as an alternative to woody and agricultural residues, since they present almost 70% of total available feedstocks for bioethanol production.
1.3. Forest Feedstocks
Two types of forest feedstocks are available for bioethanol production: those are hardwoods and softwoods. Softwoods, such as pine, spruce, cypress, fir, and others have lower density and can grow on a higher rate, while hardwoods, such as oak, willow, poplar, cottonwood, and others are angiosperm and mostly deciduous [27][48]. Cottonwood is believed to be the most suitable woody feedstock for bioethanol production, since it is the most productive tree with several important advantages, such as a large amount of clones, restoration possibility by multiple cuttings, and uniformity in planting material quality [28][49]. Different forest feedstocks possess more lignin and less ash content, which makes such woody feedstocks a very attractive raw material to improve and increase bioethanol conversions in its production processes [29][50].
2. Lignocellulosic Biomass Composition
Lignocellulosic materials are divided into three main components: cellulose, hemicellulose, and lignin (Figure 4), where cellulose and hemicellulose together present around 70% of all biomass. Both cellulose and hemicellulose are closely connected to component lignin through covalent and hydrogen bonds, which make its structure more robust and treatment resistant [30][51].
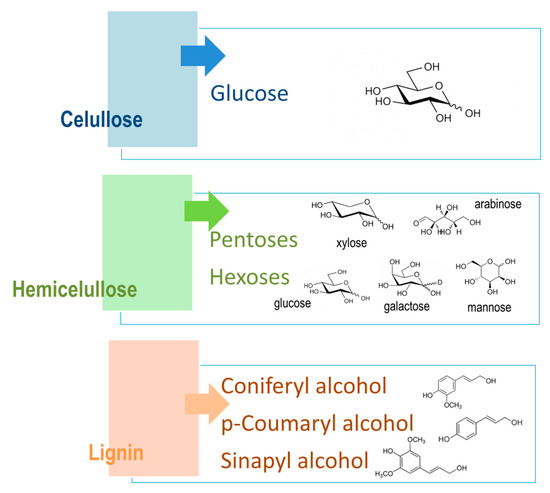
2.1. Cellulose
Cellulose is a linear component that consists of long-chain glucose monomer β-D-glucopyranose linked with β-(1,4)-glycosidic bonds, which can also reach more than one thousand units of glucose in length, with cellobiose being its repeating unit. Cellulosic chains are composed of around 1000 D-glucose units, which are arranged in microfibrils. Those fibrils form a lignocellulosic matrix with hydrogen linkages, which makes it very resistant, strong, and compact in its structure [31][52]. Cellulose is organized by intermolecular and intramolecular hydrogen bonds, as well as van der Waals forces, which make the cellulose crystalline. Where weaker bonds occur in the structure, the cellulose structure is amorphous (Figure 5). Cellulose, being the most widespread organic polymer in nature, requires a temperature of around 300 °C to be converted to an amorphous structure from crystalline. There are two ending groups in each chain of cellulose: a non-reducing and a reducing group. The non-reducing group is at the closed ring structure, while the reducing group is present at the opposite end of the chain, consisting of an aliphatic structure and a carbonyl group [32][33][53,54]. Cellulose being highly abundant in plants can be synthesized by animals, algae, bacteria, and fungi as well [34][55]. A study by Sacui et al. describes the production of cellulose nanocrystals and cellulose nanofibrils by, among others, enzymatic hydrolysis from three types of raw materials: wood, tunicate, and bacteria [35][56]. A study by Uzyol et al. reports on the production of bacterial cellulose from algae Chlorella vulgaris, which was used as a source of glucose for the production of bacterial cellulose [36][57].
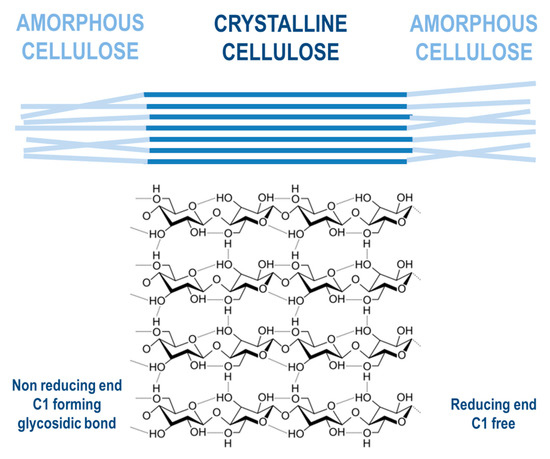
Figure 5. Cellulose structure.
2.2. Hemicellulose
Hemicellulose is a heterogenous and amorphous structure of polymers that contains different monosaccharide subunits, which include d-glucose, d-mannose, d-galactose, d-xylose, and L-arabinose, as well as other sugar acids, such as d-galacturonic and D-glucuronic acids [37][58]. The structure of hemicellulose is amorphous and is not physically strong, being easily hydrolyzed by hemicellulose enzymes [38][59]. It was reported that hemicellulose removal in the pretreatment process can increase the cellulose conversion due to the accessibility of enzymes to cellulose [39][60]. Through aromatic esters, hemicelluloses can also be linked with lignin, as well as to celluloses with hydrogen bonds, therefore providing a bond between cellulose and lignin. Polymers of hemicelluloses are constituted of different sugars, which the hemicelluloses are classified after. For example, xylan consists of xylose units linked with β-1,4 bonds to the L-arabinose substituted unit of the xylopyranose unit [34][40][55,61].
2.3. Lignin
Lignin is an aromatic polymer that is linked with covalent bonds to different xylans. It is a very complex heteropolymer of phenylpropanoied units that is composed of phenolic monomers, such as coniferyl, coumaryl, and sinapyl alcohol. Lignin contributes to the rigidity of the structure and its hydrophobicity [41][62]. Lignin, the linking part between cellulose and hemicellulose in the cell walls, obstructs cellulose conversion because of several factors, such as total lignin content and lignin structure. Lignin acts as a physical barrier and can limit the accessibility to polysaccharides [42][63]. The highest levels of lignin are present in softwood, around 30–60%, while grasses and other agricultural wastes contain only around 10–30% of lignin. Components of lignin have a dilution effect when added together with solid components to the pretreatment process, and that affects the hydrolysis process. For that reason, lignin is gaining more and more interest in the hydrolysis process itself [43][64].
Reference (Editors will rearrange the references after the entry is submitted)
- Menon, V.; Rao, M. Trends in Bioconversion of Lignocellulose: Biofuels, Platform Chemicals & Biorefinery Concept. Prog. Energy Combust. Sci. 2012, 38, 522–550.
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The Path Forward for Lignocellulose Biorefineries: Bottlenecks, Solutions, and Perspective on Commercialization. Bioresour. Technol. 2018, 264, 370–381.
- Alzagameem, A.; Khaldi-Hansen, B.E.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic Biomass as Source for Lignin-Based Environmentally Benign Antioxidants. Molecules 2018, 23, 2664.
- Limayem, A.; Ricke, S.C. Lignocellulosic Biomass for Bioethanol Production: Current Perspectives, Potential Issues and Future Prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467.
- Prasad, R.K.; Chatterjee, S.; Mazumder, P.B.; Gupta, S.K.; Sharma, S.; Vairale, M.G.; Datta, S.; Dwivedi, S.K.; Gupta, D.K. Bioethanol Production from Waste Lignocelluloses: A Review on Microbial Degradation Potential. Chemosphere 2019, 231, 588–606.
- Soares, I.; Ferreira, P.; Hens, L. Energy and Environmental Challenges: Bringing Together Economics and Engineering (ICEE’17). Environ. Dev. Sustain. 2018, 20, 1–5.
- Kim, J.; Sunagawa, M.; Kobayashi, S.; Shin, T.; Takayama, C. Developmental Localization of Calcitonin Gene-Related Peptide in Dorsal Sensory Axons and Ventral Motor Neurons of Mouse Cervical Spinal Cord. Neurosci. Res. 2016, 105, 42–48.
- Yao, S.; Nie, S.; Yuan, Y.; Wang, S.; Qin, C. Efficient Extraction of Bagasse Hemicelluloses and Characterization of Solid Remainder. Bioresour. Technol. 2015, 185, 21–27.
- Zhang, Z.; Liu, B.; Zhao, Z. Efficient Acid-Catalyzed Hydrolysis of Cellulose in Organic Electrolyte Solutions. Polym. Degrad. Stab. 2012, 97, 573–577.
- Maitan-Alfenas, G.P.; Visser, E.M.; Guimarães, V.M. Enzymatic Hydrolysis of Lignocellulosic Biomass: Converting Food Waste in Valuable Products. Curr. Opin. Food Sci. 2015, 1, 44–49.
- Saha, K.; Mashewari, U.; Sikder, J.; Chakraborty, S.; da Silva, S.S.; dos Santos, J.C. Membranes as a Tool to Support Biorefineries: Applications in Enzymatic Hydrolysis, Fermentation and Dehydration for Bioethanol Production. Renew. Sustain. Energy Rev. 2017, 74, 873–890.
- Banerjee, S.; Mudliar, S.; Sen, R.; Giri, B.; Satpute, D.; Chakrabarti, T.; Pandey, R.A. Commercializing Lignocellulosic Bioethanol: Technology Bottlenecks and Possible Remedies. Biofuels Bioprod. Biorefining 2010, 4, 77–93.
- Börjesson, J.; Peterson, R.; Tjerneld, F. Enhanced Enzymatic Conversion of Softwood Lignocellulose by Poly(Ethylene Glycol) Addition. Enzym. Microb. Technol. 2007, 40, 754–762.
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q. Pretreatment of Lignocellulosic Biomass with Ionic Liquids and Ionic Liquid-Based Solvent Systems. Molecules 2017, 22.
- Balat, M. Production of Bioethanol from Lignocellulosic Materials via the Biochemical Pathway: A Review. Energy Convers. Manag. 2011, 52, 858–875.
- Tabatabaei, M.; Aghbashlo, M. The Critical Role of Advanced Sustainability Assessment Tools in Enhancing the Real-World Application of Biofuels. Acta Innov. 2020, 67–73.
- Holzleitner, M.; Moser, S.; Puschnigg, S. Evaluation of the Impact of the New Renewable Energy Directive 2018/2001 on Third-Party Access to District Heating Networks to Enforce the Feed-in of Industrial Waste Heat. Util. Policy 2020, 66, 101088.
- Chiaramonti, D.; Goumas, T. Impacts on Industrial-Scale Market Deployment of Advanced Biofuels and Recycled Carbon Fuels from the EU Renewable Energy Directive II. Appl. Energy 2019, 251, 113351.
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of First and Second Generation Biofuels: A Comprehensive Review. Renew. Sustain. Energy Rev. 2010, 14, 578–597.
- Ullah, K.; Sharma, V.K.; Ahmad, M.; Lv, P.; Krahl, J.; Wang, Z. The Insight Views of Advanced Technologies and Its Application in Bio-Origin Fuel Synthesis from Lignocellulose Biomasses Waste, a Review. Renew. Sustain. Energy Rev. 2018, 82, 3992–4008.
- Tahir, A.; Arshad, M.; Anum, F.; Abbas, M.; Javed, S.; Shahzad, M.I.; Ansari, A.R.; Bano, I.; Shah, F.A. Ecofuel future prospect and community impact. In Advances in Eco-Fuels for a Sustainable Environment; Azad, K., Ed.; Woodhead Publishing: Sawston, UK, 2019; Chapter 17; pp. 459–479. ISBN 978-0-08-102728-8.
- Saha, S.; Sharma, A.; Purkayastha, S.; Pandey, K.; Dhingra, S. Bio-plastics and Biofuel: Is it the Way in Future Development for End Users? In Plastics to Energy; Al-Salem, S.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; Chapter 14; pp. 365–376. ISBN 978-0-12-813140-4.
- Aro, E.-M. From First Generation Biofuels to Advanced Solar Biofuels. Ambio 2016, 45, 24–31.
- Anu, A.; Kumar, A.; Jain, K.K.; Singh, B. Process Optimization for Chemical Pretreatment of Rice Straw for Bioethanol Production. Renew. Energy 2020, 156, 1233–1243.
- Zhao, Y.; Damgaard, A.; Liu, S.; Chang, H.; Christensen, T.H. Bioethanol from Corn Stover—Integrated Environmental Impacts of Alternative Biotechnologies. Resour. Conserv. Recycl. 2020, 155, 104652.
- Jugwanth, Y.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Valorization of Sugarcane Bagasse for Bioethanol Production through Simultaneous Saccharification and Fermentation: Optimization and Kinetic Studies. Fuel 2020, 262, 116552.
- Keshwani, D.R.; Cheng, J.J. Switchgrass for Bioethanol and Other Value-Added Applications: A Review. Bioresour. Technol. 2009, 100, 1515–1523.
- Nanda, S.K.; Lin, W.-Y.; Lee, M.-Y.; Chen, R.-S. A Quantitative Classification of Essential and Parkinson’s Tremor Using Wavelet Transform and Artificial Neural Network on SEMG and Accelerometer Signals. In Proceedings of the IEEE International Conference on Networking, Sensing and Control, Taipei, Taiwan, 9–11 April 2015.
- Kamyab, H.; Din, M.F.M.; Ponraj, M.; Keyvanfar, A.; Rezania, S.; Taib, S.M.; Majid, M.Z.A. Isolation and Screening of Microalgae from Agro-Industrial Wastewater (POME) for Biomass and Biodiesel Sources. Desalination Water Treat. 2016, 57, 29118–29125.
- Phwan, C.K.; Ong, H.C.; Chen, W.-H.; Ling, T.C.; Ng, E.P.; Show, P.L. Overview: Comparison of Pretreatment Technologies and Fermentation Processes of Bioethanol from Microalgae. Energy Convers. Manag. 2018, 173, 81–94.
- Keris-Sen, U.D.; Gurol, M.D. Using Ozone for Microalgal Cell Disruption to Improve Enzymatic Saccharification of Cellular Carbohydrates. Biomass Bioenergy 2017, 105, 59–65.
- Guedes, A.C.; Amaro, H.M.; Sousa-Pinto, I.; Malcata, F.X. Algal spent biomass—A pool of applications. In Biofuels from Algae, 2nd ed.; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 16; pp. 397–433. ISBN 978-0-444-64192-2.
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and Microalgae as a Potential Source for Commercial Applications along with Biofuels Production: A Biorefinery Approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941.
- Sudhakar, M.P.; Kumar, B.R.; Mathimani, T.; Arunkumar, K. A Review on Bioenergy and Bioactive Compounds from Microalgae and Macroalgae-Sustainable Energy Perspective. J. Clean. Prod. 2019, 228, 1320–1333.
- Jiang, R.; Ingle, K.N.; Golberg, A. Macroalgae (Seaweed) for Liquid Transportation Biofuel Production: What Is Next? Algal Res. 2016, 14, 48–57.
- Yuan, Y.; Macquarrie, D.J. Microwave Assisted Acid Hydrolysis of Brown Seaweed Ascophyllum Nodosum for Bioethanol Production and Characterization of Alga Residue. ACS Sustain. Chem. Eng. 2015, 3, 1359–1365.
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol Production from Gracilaria Verrucosa, a Red Alga, in a Biorefinery Approach. Bioresour. Technol. 2013, 135, 150–156.
- Ryu, M.; Lee, E.Y. Saccharification of Alginate by Using Exolytic Oligoalginate Lyase from Marine Bacterium Sphingomonas Sp. MJ-3. J. Ind. Eng. Chem. 2011, 17, 853–858.
- Adams, J.M.; Gallagher, J.A.; Donnison, I.S. Fermentation Study on Saccharina Latissima for Bioethanol Production Considering Variable Pre-Treatments. J. Appl. Phycol. 2008, 21, 569.
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232.
- Chen, J.-J.; Li, Y.-R.; Lai, W.-L. Application of Experimental Design Methodology for Optimization of Biofuel Production from Microalgae. Biomass Bioenergy 2014, 64, 11–19.
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.S.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B.; et al. An Engineered Microbial Platform for Direct Biofuel Production from Brown Macroalgae. Science 2012, 335, 308–313.
- Smachetti, M.E.S.; Rizza, L.S.; Coronel, C.D.; Nascimento, M.D.; Curatti, L. Microalgal Biomass as an Alternative Source of Sugars for the Production of Bioethanol. In Principles and Applications of Fermentation Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 351–386. ISBN 978-1-119-46038-1.
- Demirbaş, A. Bioethanol from Cellulosic Materials: A Renewable Motor Fuel from Biomass. Energy Sources 2005, 27, 327–337.
- Kim, S.; Dale, B.E. Global Potential Bioethanol Production from Wasted Crops and Crops Residues. Biomass Bioenergy 2005, 29, 361–375.
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol Production from Agricultural Wastes: An Overview. Renew. Energy 2012, 37, 19–27.
- Nikku, M.; Deb, A.; Sermyagina, E.; Puro, L. Reactivity Characterization of Municipal Solid Waste and Biomass. Fuel 2019, 254, 115690.
- Carević, I.; Baričević, A.; Štirmer, N.; Šantek Bajto, J. Correlation between Physical and Chemical Properties of Wood Biomass Ash and Cement Composites Performances. Constr. Build. Mater. 2020, 256, 119450.
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol Production from Renewable Sources: Current Perspectives and Technological Progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501.
- Stolarski, M.J.; Krzyżaniak, M.; Łuczyński, M.; Załuski, D.; Szczukowski, S.; Tworkowski, J.; Gołaszewski, J. Lignocellulosic Biomass from Short Rotation Woody Crops as a Feedstock for Second-Generation Bioethanol Production. Ind. Crop. Prod. 2015, 75, 66–75.
- Ufodike, C.O.; Eze, V.O.; Ahmed, M.F.; Oluwalowo, A.; Park, J.G.; Okoli, O.I.; Wang, H. Evaluation of the Inter-Particle Interference of Cellulose and Lignin in Lignocellulosic Materials. Int. J. Biol. Macromol. 2020, 147, 762–767.
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7.
- Deguchi, S.; Mukai, S.; Tsudome, M.; Horikoshi, K. Facile Generation of Fullerene Nanoparticles by Hand-Grinding. Adv. Mater. 2006, 18, 729–732.
- Quiroz-Castañeda, R.E.; Folch-Mallol, J.L. Hydrolysis of Biomass Mediated by Cellulases for the Production of Sugars. Sustain. Degrad. Lignocellul. Biomass Tech. Appl. Commer. 2013.
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from Enzymatic Degradation of Cellulose and Hemicellulose to Fermentable Sugars—A Review. Biomass Bioenergy 2020, 134, 105481.
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the Properties of Cellulose Nanocrystals and Cellulose Nanofibrils Isolated from Bacteria, Tunicate, and Wood Processed Using Acid, Enzymatic, Mechanical, and Oxidative Methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138.
- Uzyol, H.K.; Saçan, M.T. Bacterial Cellulose Production by Komagataeibacter Hansenii Using Algae-Based Glucose. Environ. Sci. Pollut. Res. Int. 2017, 24, 11154–11162.
- Saha, B.C. Hemicellulose Bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291.
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559.
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the Structural and Chemical Changes of Plant Biomass Following Steam Explosion Pretreatment. Biotechnol. Biofuels 2017, 10, 36.
- Bhalla, A.; Bischoff, K.M.; Sani, R.K. Highly Thermostable Xylanase Production from a Thermophilic Geobacillus Sp. Strain WSUCF1 Utilizing Lignocellulosic Biomass. Front. Bioeng. Biotechnol. 2015, 3, 1–8.
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass Pretreatment: Fundamentals toward Application. Biotechnol. Adv. 2011, 29, 675–685.
- Santos, R.B.; Lee, J.M.; Jameel, H.; Chang, H.-M.; Lucia, L.A. Effects of Hardwood Structural and Chemical Characteristics on Enzymatic Hydrolysis for Biofuel Production. Bioresour. Technol. 2012, 110, 232–238.
- Cheng, F.; Bayat, H.; Jena, U.; Brewer, C.E. Impact of Feedstock Composition on Pyrolysis of Low-Cost, Protein- and Lignin-Rich Biomass: A Review. J. Anal. Appl. Pyrolysis 2020, 147, 104780.
