Non-alcoholic fatty liver disease (NAFLD) is the most frequent chronic liver disease in adults in developed countries, with a global prevalence as high as one billion. The pathogenesis of NAFLD is a multifactorial and multi-step process. Nowadays, a growing body of research suggests the considerable role of the endocannabinoid system (ECS) as a complex cell-signaling system in NAFLD development.
- endocannabinoid system
- endocannabinoid receptors
1. Introduction
The term “non-alcoholic fatty liver disease” (NAFLD) involves simple fat accumulation in the liver and may progress to steatohepatitis, fibrosis, cirrhosis, and, in some cases, hepatocellular carcinoma (HCC) [1]. Over the past decade, the prevalence of NAFLD (nearly 30% in the general adult population) is increasing worldwide with each passing year due to sedentary lifestyles and the unlimited availability of fat- and calorie-rich diets in modern western society [2]. The incidence of NAFLD increases with age, with a tendency to occur in men before 50 years of age and women after 50 years of age [3]. NAFLD is considered the most frequent liver disease in the world, the second most common cause of liver transplantation, and a primary cause of the development of hepatocellular carcinoma. Given these facts and the lack of effective treatment, NAFLD is a relevant problem for all health systems. Unsurprisingly, the pathogenesis of NAFLD is associated with fat deposition in the liver. Particularly, increased accumulation of triacylglycerols (TAG) is characteristic of NAFLD development. Steatosis occurs as a result of the imbalance between lipid storage (from accelerated free fatty acids (FFA) influx and de novo synthesis) and hepatic lipid clearance (decreased oxidation of FFA in the liver and decreased synthesis of low-density lipoproteins (VLDLs)). The complex and multifactorial process of NAFLD development was explained initially by the “two hits” model. The “first hit” included hepatic steatosis as a consequence of metabolic syndrome and excessive TAG deposition in hepatocytes. The “second hit” seemed to be necessary to develop non-alcoholic steatohepatitis (NASH) from NAFLD. However, this model was too simple to fully describe the complexity of NAFLD. In 2010, Tilg and Moschen proposed the “multiple hit” model, suggesting that different risk factors such as insulin resistance, adipocytes dysfunction, nutritional factors, gut microbiota, and genetic and epigenetic factors act simultaneously on both intrahepatic and extrahepatic pathways, which finally leads to steatosis or inflammation [4]. The previous model assumed that NAFLD always precedes inflammation. According to the “multiple hit” model, depending on which signaling pathways are activated by risk factors, hepatic lipid overload or NASH development may occur [5]. Currently, there are only a few specific pharmaceutical strategies available to treat NAFLD. However, none of them is ideal. Many of the promising results from rodent studies on phytocannabinoids and the endocannabinoid system (ECS) have fueled hopes of implementing novel therapeutic approaches and targets in humans. In our review, we aim to discuss the latest reports describing the changes in the ECS and its components on the development and progression of NAFLD. Furthermore, we will summarize the clinical studies analyzing the effects of natural cannabinoids in NAFLD treatment.
2. Phytocannabinoids
Cannabis sativa
Cannabis
Cannabis inflorescence [6].
Cannabis, the most studied agents are Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), and tetrahydrocannabivarin (THCV) [7].
Cannabis sativa
Cannabis extracts [8].
Cannabis also contains a large number of acidic precursors of the aforementioned molecules, respectively: Δ9-tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), and tetrahydrocannabivarinic acid (THCVA). These compounds may reveal interesting therapeutic properties, such as attenuation of body weight gain and amelioration of glucose-insulin homeostasis in a mouse model of HFD-induced obesity after administration of THCA [9]. However, the current knowledge in the field of phytocannabinoid acids is limited and requires further examination [10]. Therefore, the effect of medicinal
Cannabis
cannabis as a whole [6]. When analyzing the effects of the individual phytocannabinoids presented in our review, their complex pharmacology should be considered. The different effects on the response of several phytocannabinoids studied
in vivo are possibly related to the competition and displacement of endogenous cannabinoids, with the different centers (orthosteric and allosteric) and with the biased signalling of cannabinoid target receptors [8]. Additionally, phytocannabinoids interact with each other. For instance, CBD has the ability to antagonize THC effects by CBR1 and non-CB1 receptor mechanisms of action. However, CBD may also potentiate some THC effects in an additive or synergistic fashion [11].
Cannabis [12]. This was accompanied by a growing awareness of the role of the endocannabinoid system in our body. Currently, THC and CBD are the two major active compounds of Cannabis.
| Feature | THC | CBD | Reference |
|---|
| Interaction with receptors: a) CB1R b) CB2R |
+ (partial agonist) - (weak antagonist) |
- (negative allosteric modulator) - (inverse agonist) |
[13][14][15] | [77,78,79] | ||||
| Psychoactive effect | Yes | No | [16] | [80] | ||||
| Appetite stimulation | Yes | No | [16] | [80] | ||||
| Cardiovascular stimulation (inducing tachycardia and hypertension) | Yes | No | [16] | [80] | ||||
| Anticonvulsant effect | Yes | No | [16] | [80] | ||||
| Therapeutic indications approved by the FDA | 1 | Anorexia associated with weight loss in AIDS | 3 | patients Nausea and vomiting associated with anticancer chemotherapy Multiple sclerosis spasticity |
Lennox-Gastaut syndrome Dravet syndrome |
[17][18][19][20] | [81,82,83,84] | |
| Formulations available on US pharmaceutical market | Nabilone (trade name Cesamet) synthetic THC analog aviable as oral capsule Dronabinol (trade name Marinol)—synthetic formulation of the main THC constituent enantiomer found in | Cannabis | : [(−)-trans-Δ9-tetrahydrocannabinol] as an oily resin in capsules | Epidiolex | ® | —pharmaceutical formulation of CBD as an oral solution | [18][19][20] | [82,83,84] |
| Combination drugs available on US | 2 | pharmaceutical market | Nabiximol (trade name Sativex)—oral spray standardized in composition, formulation, and dose, delivering of 2.7 mg THC and 2.5 mg CBD per dose. | [21] | [85] | |||
1 FDA, Food and Drug Administration; 2 US, United States; 3 AIDS, Acquired Immunodeficiency Syndrome.
3. Effects of Prolonged
Cannabis
Use in the Context of NAFLD and its Comorbidities
Cannabis
Cannabis
cannabis use (CCU) has been associated with metabolic disturbances ascertained as detrimental factors leading to NAFLD development. Firstly, CCU is undoubtedly linked with increased appetite and calorie overconsumption [22][23]. What is more, this appetite dysregulation is augmented by a higher intake of highly-palpable, unhealthy foods that are rich in refined sugar and fat [24][25][26]. On the other hand, a multitude of studies analyzing the metabolic effects of CCU observed plenty of beneficial effects that could counteract the development of NAFLD. It has been shown that prolonged
Cannabis use is linked with decreased prevalence of insulin resistance [27][28] and hyperlipidemia and metabolic syndrome [29][30], as well as decreased frequency of diabetes mellitus occurrence [25]. In the aspect of CCU and obesity, an overwhelming majority of studies have shown a decreased prevalence of obesity among marijuana users. However, one study showed increased visceral adiposity in
Cannabis
cannabis smokers [31][32][33]. Mounting evidence indicates that current or past marijuana use is associated with a lower risk of NAFLD development, regardless of the presence of metabolic risk factors [31][34][35][36]. At first glance, this association is contradictory to the role of ECS in NAFLD development. Although it is clearly proven that increased endocannabinoid tone in liver and brain relates to obesity and metabolic syndrome and contributes to the development of NAFLD, the complex effects and impact of phytocannabinoids in relation to NAFLD pathophysiology are not yet fully clear (
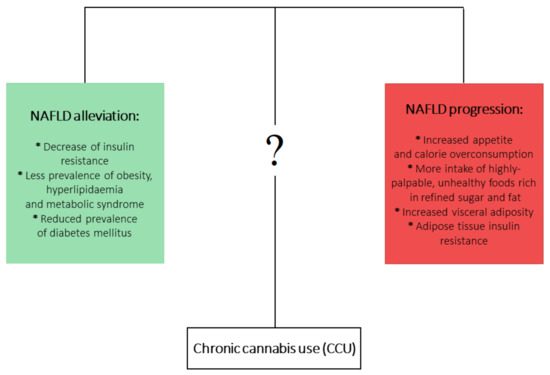
One of the possible mechanisms responsible for the positive influence of
Cannabis use on the prevalence of NAFLD and other metabolic diseases may include the antagonistic action of CBD and THCV on CB1R [37][38]. Additionally, CBD has been described as acting as a negative allosteric modulator of CB1R in HEK 293A and in STHdhQ7/Q7 cells, two model systems that highly express CB1R [26]. Antagonism of CB1R improves the insulin sensitivity of hepatocytes [39], decreases intrahepatic triglyceride synthesis [40], and decreases secretion of very-low-density lipoprotein (VLDL) [41]. Diminished IR may contribute to improved hepatic steatosis, hepatomegaly, and metabolic syndrome by restoring the optimal hepatic glucose metabolism and decreasing liver fat accumulation [42].
Moreover, the anti-inflammatory effects of phytocannabinoids should be emphasized, as they can inhibit secretion of pro-inflammatory cytokines (TNF-a, IL-6) and adipokines (leptin) and lead to the upregulation of inflammatory mediators such as cell transcription factor (NF-kB) [43]. Excessive inflammatory response plays a robust role in NAFLD development and progression to NASH [44]. CBD has been shown to alleviate liver inflammation induced by a high fat-cholesterol (HFC) diet in mice by inhibition of NF-kB and can likewise restrain NLRP3 inflammasome activation, which both lead to a reduction in inflammatory response [45][46][47]. Another possible mechanism that is involved in the beneficial properties of
Cannabis is development of tolerance and down-regulation of CB1R from repetitive THC use. THC should theoretically induce or worsen NAFLD by its agonistic role regarding CB1R. However, it has been proven that repetitive use of THC may result in decreased CB1R density, which may contribute to a dose-dependent inverse relationship between marijuana use and NAFLD occurrence [14][29][48]. Finally, the most important factor is that
Cannabis is a source of not only THC, CBD, and THCV but also various other phytocannabinoids such as cannabidivarin (CBDV), cannabigerol (CBG), cannabigerovarian (CBGV), cannabigerolic acid (CBGA), and cannabinol (CBN). The therapeutic potential of these compounds remains largely unexplored. Thus, there is a need for further research directed at establishing whether phytocannabinoids are indeed ‘a neglected pharmacological treasure trove’ [14][49].
