Among small organic molecules, perylene diimides (PDIs) are an important class of materials due to their outstanding thermal, chemical, electronic, and optical properties, all of which make them promising candidates for a wide range of organic electronic devices including sensors, organic solar cells, organic field-effect transistors, and organic light-emitting diodes. This is mainly due to their electron-withdrawing nature and significant charge transfer properties. Perylene-based sensors of this type show high sensing performance towards various analytes, particularly reducing gases like ammonia and hydrazine, but there are several issues that need to be addressed including the selectivity towards a specific gas, the effect of relative humidity, and operating temperature.
- n-type organic semiconductors
- perylene diimides
- gas sensors
- hydrazine
- ammonia
1. Introduction
The increase in globalization, digitization, and the rapid production techniques of industry come with the need to be able to measure our environment, either local or more global, to ensure safe working and living environments for our communities to prosper. In particular, the monitoring and detection of toxic and harmful gases, air pollutants, and explosives are an essential need in industries such as pharmaceuticals, propulsion systems, fertilizers, renewable energy, and manufacturing, as well as in everyday life including the need to safely travel by air. Sensors are the primary devices used for the detection and monitoring of the toxic, explosive, and flammable analytes present in our surroundings, and a great deal of attention has been paid to the development of various types of gas sensors, including electrical, optical, acoustic, and fluorescent [1][2][3][4][5][6][7][8][9][1–9]. Major analytes include hydrazine (N2H4), ammonia (NH3), hydrogen (H2), hydrogen peroxide (H2O2), nitrogen dioxide (NO2), methane (CH4), and volatile organic compounds (VOCs), all of which cause adverse effects on human and environmental health [10][11][12][10–12]. The commercially available sensors are primarily based on inorganic materials [13], and, although market leading, they suffer from a number of disadvantages, including complex fabrication techniques, a lack of selectivity, the need for a high working temperature, and above all, high-costs [13][14][14,15]. Therefore, it is still important to develop new and suitable alternatives to fabricate gas sensors that are reliable, low-cost, stable at room temperature, and have a high degree of selectivity towards the desired analyte.
Over the past few years, organic semiconductors have gained some attention in gas-sensing applications, primarily due to their unique properties, including solution processability, low-cost fabrication, room temperature (RT) operation, ease of integration, and scalable and straightforward synthetic strategies [15][16]. Along with the development of organic field-effect transistors (OFETs), the design and synthesis of novel organic materials has provided a vast material library for electrical gas-sensing applications, e.g., pigments and dyes such as phthalocyanines [16][17][18][19][17–20], perylene diimide derivatives [20][21][22][23][21–24], and conjugated polymers [24][25][26][27][28][29][30][25–31]. There are two main types of organic semiconductors: the so-called p- and n-types. The p-type organic semiconductors are more widely applied in gas-sensing applications [31][32] compared to their n-type counterparts due to their prominent instability in air. When p-type organic materials are exposed to oxidizing gases such as NO2, an increase in conductivity occurs, whereas the reverse happens for reducing gases, such as NH3 and H2; thus, a positive response result is attained over a reduction in response, which could be interpreted in other ways, such as device failure or not providing a measurable linear response past a certain level. A variety of p-type organic materials, including pentacene, phthalocyanines, and thiophene, have become of interest, and they have all been used for gas-sensing applications [31][32]. The n-type materials continue to have limited utility for gas-sensing applications, mainly due to their instability in the air [32]. However, there are several n-type organic materials that have been designed and developed for gas-sensing applications [32][33] by incorporating a variety of electron-withdrawing groups, e.g., cyano (CN), fluoro (F), and chloro (Cl) within their structure. Due to the inherent electron-deficient nature of n-type materials, an increase in conductivity occurs when they are exposed to reducing gases, and the opposite happens for oxidizing gases [21][22][33][34][22,23,34,35]. Hence, the development of a library of organic semiconductors that demonstrate specific properties for specific analytes and gases, with specific responses to reducing or oxidizing gases, could be an important advance to the field. In this review, we elaborate on the chemistry of perylene diimides as an example of the n-type class that led to its use in sensor development.
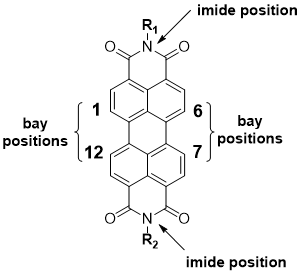
Perylene diimide (PDI), as shown in Figure 1, is an n-type organic semiconductor discovered by Kardos in 1913 [35][36]. Initially used as a textile dye, this high-performing pigment comes in red, violet, and near-black shades [36][37]. PDIs possess a high thermal stability and photostability under ambient conditions [37][38], making them viable for self-assembly processing and applications in various electronic devices [38][39]. Several PDI molecules have been prepared into well-shaped supramolecular nanostructures; these achievements have also encouraged the area of the self-assembly of many other n-type organic semiconductor molecules [39][40]. The existence of electron-withdrawing imide fragments in PDIs permits them to be simply reduced chemically or electrochemically, forming radical anions, yet remaining stable to oxidation [40][41]. Their resistance to oxidation is the main reason why PDIs generally act as stable n-type organic materials [41][42]. Together with their additional properties, including facile structural functionalization, excellent light-absorption in the visible region, and near-unity fluorescence quantum yields in the molecular state, PDIs have been known as appealing candidates for preparing organic optoelectronic materials and devices, such as organic photovoltaics (OPVs), organic field effect transistors (OFETs), dye lasers, organic light-emitting diodes (OLEDs), and sensors [41][42][43][22,42–44]. The well-shaped 1D nanostructures of PDIs can be prepared via supramolecular self-assembly procedures [43][44]. This 1D self-assembly is mostly governed by the intrinsic π–π stacking interaction between the aromatic perylene planes, which support the 1D growth of assembly in cooperation with other intermolecular noncovalent interactions. The 1D nanostructures of PDIs display excellent optical and electronic properties along the 1D π–π stacking direction, which offers great potential for electronic device application like OPVs and sensors [43][44][44,45].
Figure 1. Structure of perylene diimide (PDI) core.
PDI and its derivatives have been studied as active materials in numerous organic electronic devices such as OPVs, OLEDs, and OFETs [45][46][36,46,47]. As is the focus of this review, PDI derivatives have also been widely used in gas-sensing applications, mainly due to their electron-deficient nature, desired self-assembled and crystal structures, and high chemical and thermal stabilities [35][36]. To improve the sensing performance of PDI-based sensors, research has focused on the molecular modification of PDI [20][21][47][48][49][21,22,48–50]. The modifications highlighted in this review within the PDI structure can be achieved through either substitution on nitrogen atoms or via incorporating aryl and/or alkyl substituents on the core and, in particular, the bay positions [50][51][41,51,52]. PDIs generated through imide nitrogen substitution usually possess similar optoelectronic properties because the electron density nodes in the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) levels at imide positions reduce the coupling between the PDI unit and the imide substituents to a minimum [41]. In this respect, this is an excellent way of modification for processability or the introduction of connectors. PDIs obtained through substitution at the core are generally highly conjugated targets, and, depending upon the substitution, the optical band gap, crystal structure, absorption profile, and solubility of the target can be altered [52][53][53,54]. Moreover, such substitutions may help to minimize the π–π overlap between aggregating naphthalene subunits to improve the sensing performance of the resulting devices [43,55]. The structure modification through imide or core positions can affect the morphology and self-assembly of the PDIs, which can improve the sensing performance [21][54][22,56].
2. Electrical Gas Sensors and Sensing Mechanism
Electrical gas sensors have been extensively studied, reported, and utilized, mainly because of their ease of processing and fabrication, portability, low-cost, and compatibility with various standard electronics [55][57]. An electrical gas sensor usually consists of two major components: the active material and the transducer. Generally, the mechanism of electrical gas sensors using organic materials is based on gas adsorption/desorption at the active material surface. A charge-transfer complex between the active layer and an analyte is formed, thus leading to a change in the charge carrier mobility of the sensing material and. hence, in the electrical response through conductivity, resistivity, and current [27][56][57][28,58,59]. This mechanism of adsorption may contain either chemical or weak interactions, i.e., chemisorption or physisorption, depending upon the chemical properties of both the sensing materials and the analyte [58][60]. To bring the sensor to its initial state, the target gas is removed from the testing chamber and air or nitrogen as a reference gas is introduced. The sensitivity or response of active materials mainly depends on two factors: (1) a high surface area, which can provide effective diffusion of the gas molecules, and (2) the chemical nature of the active materials, such as molecular packing, redox potential, and energy level, which are associated with the formation of charge carriers and efficient transport into the active materials [56][58]. As an n-type organic semiconductor, PDI can accept electrons from the electron donor gas, such as ammonia and hydrazine, via donor–acceptor complexation. Thus, the electrons can be efficiently transported through π-electron delocalization along the long-axis of perylene dyes and ultimately lead to a measurable increase in resistance or current [20][21][56][21,22,58].
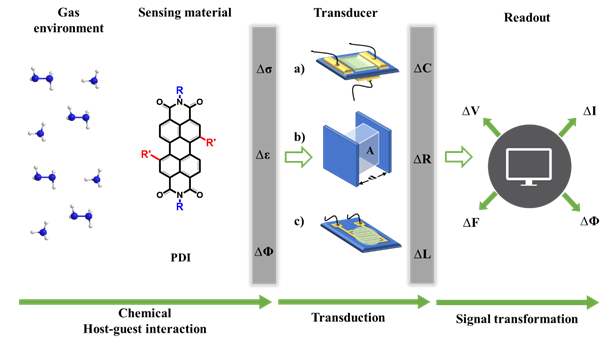
Applying standard electronic devices such as resistors, capacitors, and field-effect transistors (FETs) allows the variations in the sensors active layer’s physical properties to be recorded in the form of change in resistance or current (ΔR or ΔI), capacitance (ΔC), and voltage (ΔV), respectively, [59][60][61][61–63] as shown in Figure 2. Electrical gas sensors that record these gas–solid interactions mainly take the three device configurations of FET, capacitor, and chemiresistor (amperometric and resistive) [62][63][64][64–66].
Figure 2. Schematic diagram illustrating the major components required to evaluate an electrical mode gas-sensing mechanism. The transducers are a) FETs, b) capacitors and c) chemiresistors. Analytes interact with the sensing material changes some of its physical properties such as conductivity (s), work function (j), and permittivity (e). The transducer converts one of these physical quantities into the variation of its electric parameters such as capacitance, C, and resistance, R. Finally, the circuit to which the sensor is connected gives rise to the sensing electrical signal that can be in either current (I) or voltage (V), and each can be measured in frequency (F) and phase (Φ) [65][67].
In 1975, Lundstrom developed the core components of an FET-based gas sensor for the first time [66][68]. Generally, an FET contains two electrodes (the source and the drain), connected by a sensing layer as the channel, and a gate electrode usually located on the underside of the substrate (Figure 2a). When a source-drain voltage is applied, current flows through the channel, which is matched through the charge carriers by applying a gate voltage. This provides supplementary ways to control the current response in the sensing layer upon interacting with a target analyte. Outputs other than variations in channel current, such as the threshold voltage and sub-threshold swing, can also be used to reflect the sensing process [67][69]. Despite their complex structure and fabrication, these advantages make FETs more stable and reliable for gas sensing compared to chemiresistors. PDIs and other organic materials are being considered for FETs where flexibility, low processing temperatures, and print deposition are desired or additional chemical functionality to create the chemical selectivity needed for functioning gas sensors is required. As PDIs and other organic molecules have inferior mobilities than inorganic materials, they need higher drain and gate voltages to produce viable currents.
Capacitive-type sensors are mainly used for molecular detection including large entities such as DNA [68][70], biomolecules [69][71], smaller molecules related to humidity [70][72], and general gases [71][73]. In most cases, a capacitive-type sensor comprises a layer of active material sandwiched between two parallel electrodes (Figure 2b). The capacitance of the sensor is usually expressed as C = ε0εrA/d, where ε0 is the permittivity in vacuum, εr is the relative permittivity of the active material, A is the capacitor area, and d is the gap between the electrodes [72][74]. Gas or humidity adsorption on the active material alters εr and, as a result, changes the sensor capacitance. For the case of polymer-based active materials, gas-induced swelling and the subsequent alteration of d or A between the electrodes can also cause a change in the capacitance [73][75]. The capacitor set-up also permits the measurement of the change in material impedance, which is associated with both the resistive and capacitive variations in response to target analyte adsorption, typically in the range of zero to several megahertz [74][76]. PDIs and other organic molecules have been used in capacitive-type sensors for humidity and gas-sensing applications. PDI-based humidity sensors have only shown good sensitivity in high-humidity environments and are unable to show notable responses below 60% RH [70][72].
To date, the majority of PDI-based sensors have been developed as chemiresistive, i.e., resistive or amperometric, configurations, as shown in Figure 2c. A chemiresistor usually comprises interdigitated electrodes (IDEs) linked with a sensing material deposited onto an insulating substrate [75][77]. The resistance, i.e., current, of the sensing material changes upon exposure to the target analytes. Chemiresistive sensors comprise one of the most extensively used device structures for gas-sensing applications due to their simplicity, compatibility with the conventional direct current (DC) circuits, low-cost, predictable electrical properties, ease of high accuracy measurements [75][77], and suitability when speedy prototyping is essential. The simple design and operation of this type of device renders it appealing starting platforms for studying the chemical response of novel materials. Despite their simple setup, chemiresistors are restricted by their single type of output, which is easily influenced by environmental perturbations.
To analyze the general performance of a gas sensor against others, a number of parameters extracted from static and dynamic measurements are needed [76][77][78,79]. Critical parameters include sensitivity, selectivity, stability, hysteresis, response and recovery time, the limit of detection (LOD), and working temperature. Ideally, a gas sensor should possess a high sensitivity to the target gas with a linear response, quick response and recovery, high selectivity, low hysteresis, low LOD, and long-term stability. One of the disadvantages of organic materials is their perceived long-term instability when compared to inorganic materials.
Sensitivity is the minimum fractional variation in the output signal (ΔI/Io and ΔR/Ro) of a sensor upon exposure to an analyte, where Io and Ro are the current and resistance, before exposure to the target gas respectively, and ΔI and ΔR are the change in the current and resistance, respectively, when the sensor is exposed to a certain quantity of target analyte. Selectivity is one of the most significant sensing parameters because there are a variety of interfering gases that are detrimental to accurate target analyte detection. Selectivity is the capability of a sensor to recognize a specific target gas among a mixture of gases. Selectivity is generally examined by comparing the cross-sensitivity towards several analytes at a particular concentration. Stability is another critical factor that is defined as the capability of a sensor to repeat the same results for a target gas for a fixed period of time. Hysteresis is a variance of the sensor output at a specified point of the input signal when it is approached from the opposite direction. Low levels of hysteresis are desirable. Response and recovery times are measured to quantify the sensing speed of a sensor. Generally, the response time is defined as the time taken for the current/resistance to change from the baseline current/resistance (Io/Ro) to 90% of the maximum change in current/resistance at a specific level of the target analyte. On the other hand, the recovery time is defined as the time taken by a sensor to reach the baseline state after completely removing the target analyte. The LOD is the lowest analyte concentration that a sensor can sense or detect reliably. The LOD is generally defined based on signal to noise ratio (S/N) and is typically determined by an S/N of 3:1. The working temperature is the temperature at which a sensor can work effectively with high sensitivity and stability. Table 1 aims to summarize many of the reported PDI based gas sensors of merit and their overall performance.