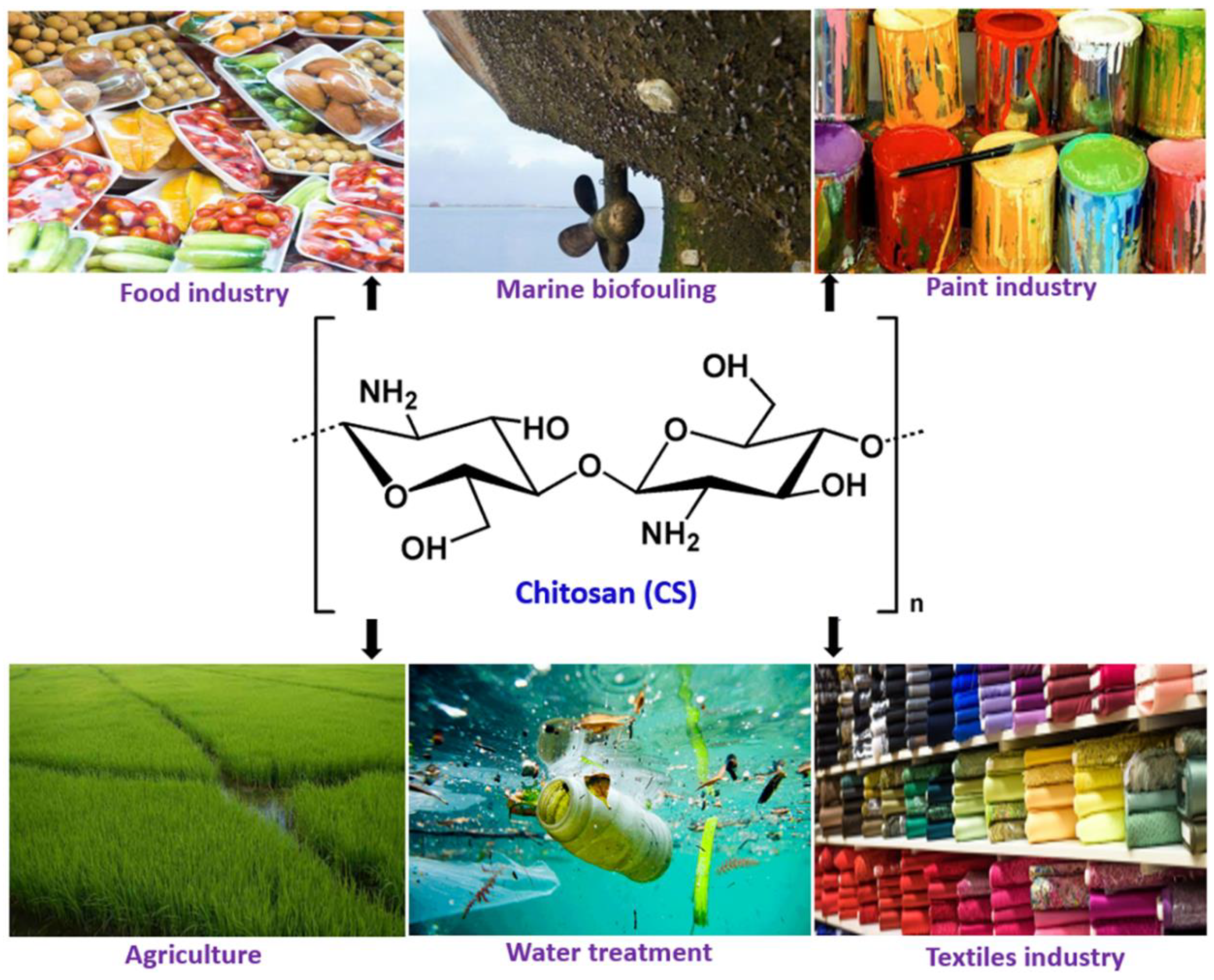
Millions of tons of crustaceans are produced every year and consumed as protein-rich seafood but the shells and other non-edible parts constituting about half the body mass are wasted. The crustacean shells are a prominent source of polysaccharide (chitin) and protein. Chitosan, a de-acetylated form of chitin obtained from the crustacean waste are used for a variety of medical applications. In recent times, it has also found use in food and paint industries including marine antifouling coatings, due to its characteristic properties, like solubility in weak acids, film-forming ability, pH-sensitivity, antifouling properties, biodegradability, and biocompatibility. Chitosan composite coatings in food, paint and water treatment solutions have been developed. In food industries, chitosan-based composite films and coatings are applied for prolonging the post-harvest life of fruits and vegetables, while anti-corrosion and self-healing properties are mainly explored for antifouling applications in paints and metal ion chelation and antifouling properties are useful for water treatment.
- Crustacean waste
- Chitosan
- Nanocomposite
- Films or Coatings
- Antimicrobial activity
- Anti-corrosion
- Antifouling
- Food preservation
- Fruits and vegetable
- Water Treatment
1. Introduction
2. Chitosan and its Properties
2.1. Source and Extraction
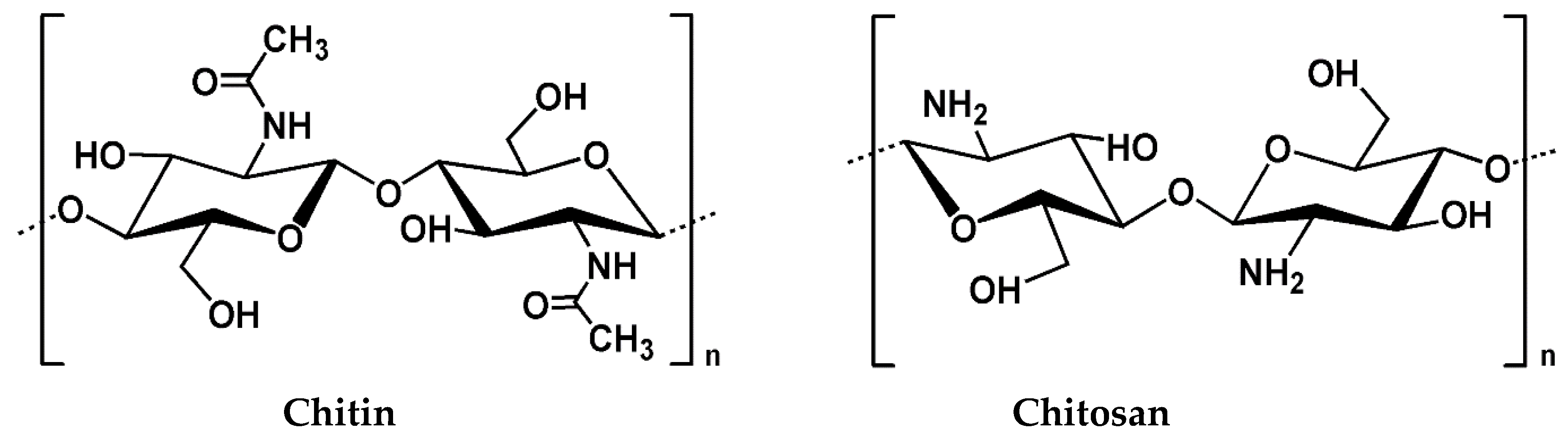
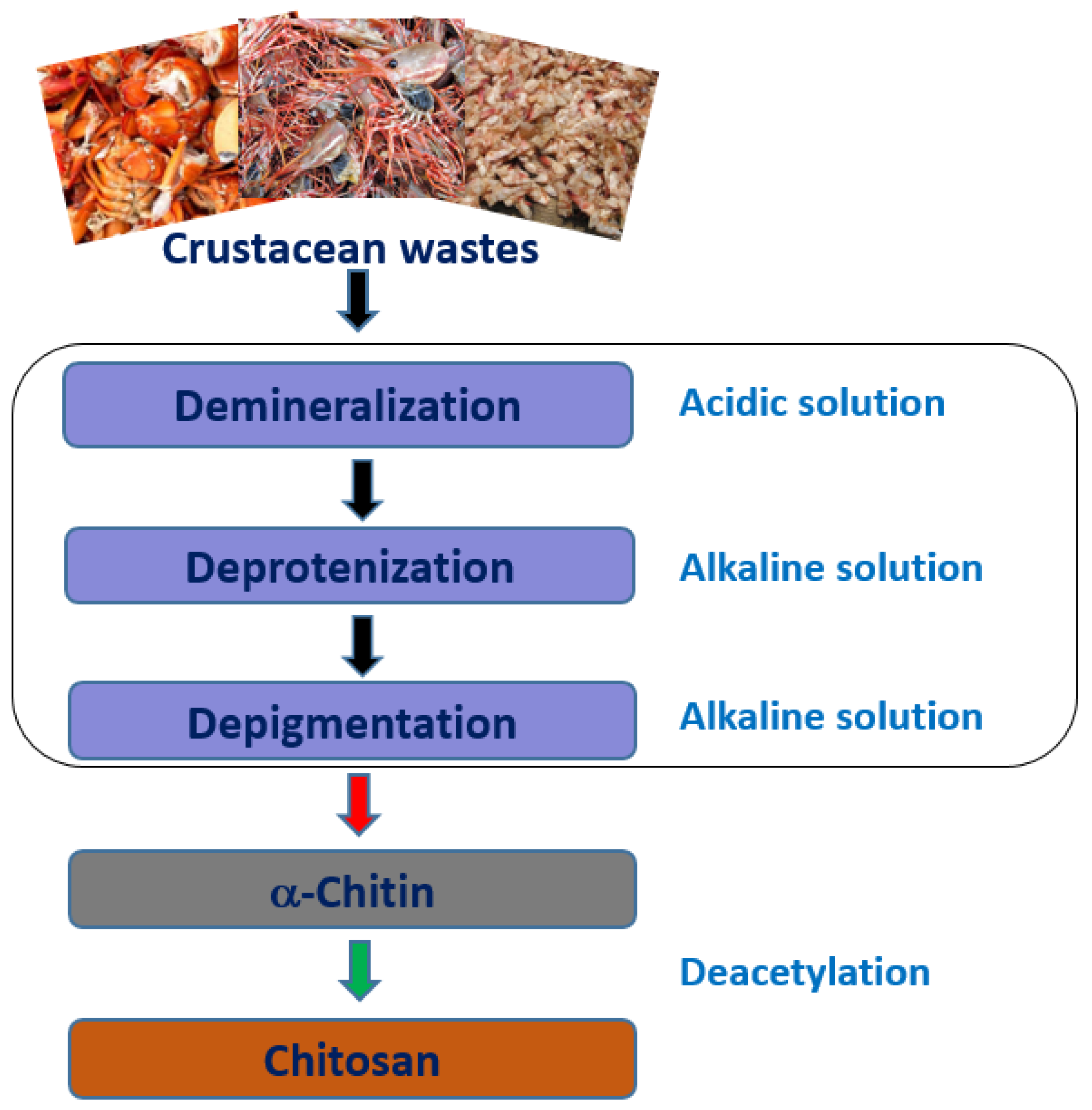
2.2. Physico-Chemical Properties of Chitosan
2.2.1. Degree of Deacetylation (DD)
2.2.2. Molecular Weight (MW)
2.2.3. Solubility
2.3. Antimicrobial Properties
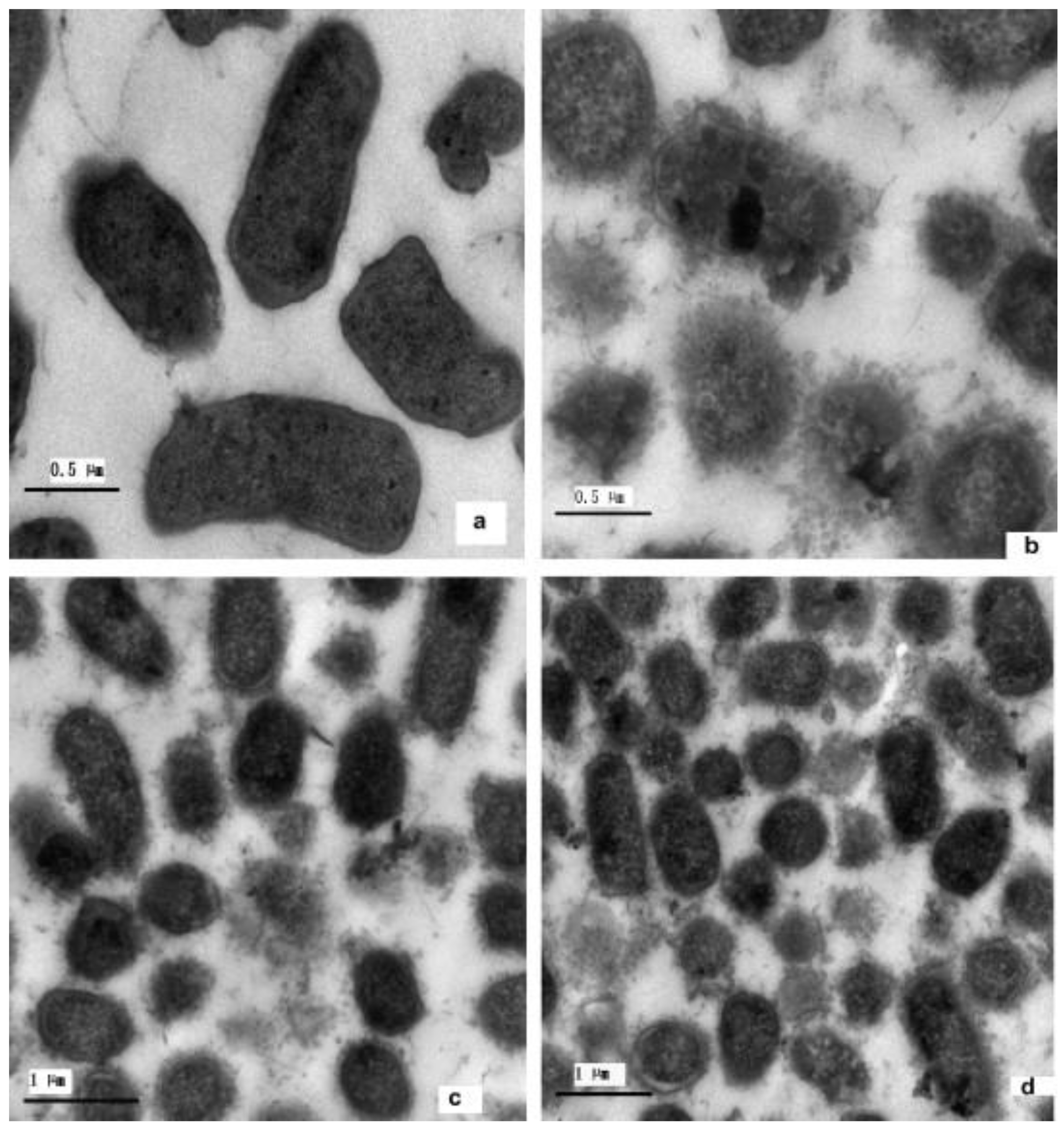
2.4. Self-Healing Properties
3. Chitosan-Based Nanocomposites
Previously, many studies have reported chemical modification of chitosan either by coupling with small molecules or grafting with polymers, for changing/improvement or better use of the intrinsic properties of chitosan. Chitosan has been grafted with poly-lactide to form polymeric amphiphilic micelles [75] or polyethyleneimine (PEI) to form a branched PEI-g-chitosan with lowered cytotoxicity but higher gene transfection efficiency compared to PEI [76]. More recently, chitosan-based nanocomposites have emerged, where both polymer and nanoparticles contribute to the improvement or enhancement of specific properties. Dispersed nanomaterials contained in chitosan polymer matrix not only improves the physical, mechanical, and thermal stability of chitosan but also endows the composite with its intrinsic properties, such as high surface area or extraordinary physicochemical properties. Chitosan nanocomposites formed between chitosan and metal/metal oxide, carbon, polymer, or clay materials via physical or chemical interaction and their applications in food, paints, and environmental fields are summarized in Table 1.3.1. Chitosan-Metal/Metal Oxide
3.2. Chitosan-Carbon Materials
3.3. Chitosan-Polymer Mixture or Copolymer
3.4. Chitosan-Clay Composites
- 2012
- ,
- 87
- , 110–116.
- Jeong, Y.-I.; Kim, D.-G.; Jang, M.-K.; Nah, J.-W. Preparation and spectroscopic characterization of methoxy poly(ethylene glycol)-grafted water-soluble chitosan.
- Res.
- 2008
- ,
- 343
- , 282–289.
- Mohamed, N.A.; Abd El-Ghany, N.A. Preparation and antimicrobial activity of some carboxymethyl chitosan acyl thiourea derivatives.
- J. Biol. Macromol.
- 2012
- ,
- 50
- , 1280–1285.
- Botelho da Silva, S.; Krolicka, M.; van den Broek, L.A.M.; Frissen, A.E.; Boeriu, C.G. Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO-laccase redox system.
- Polym.
- 2018
- ,
- 186
- , 299–309.
- Fu, Y.; Xiao, C.; Liu, J. Facile fabrication of quaternary water soluble chitosan-sodium alginate gel and its affinity characteristic toward multivalent metal ion.
- Technol. Innov.
- 2019
- ,
- 13
- , 340–345.
- Alfaro, L.; Chotiko, A.; Chouljenko, A.; Janes, M.; King, J.M.; Sathivel, S. Development of water-soluble chitosan powder and its antimicrobial effect against inoculated Listeria innocua NRRL B-33016 on shrimp.
- Food Control
- 2018
- ,
- 85
- , 453–458.
- Chouljenko, A.; Chotiko, A.; Reyes, V.; Alfaro, L.; Liu, C.; Dzandu, B.; Sathivel, S. Application of water-soluble chitosan to shrimp for quality retention.
- LWT
- 2016
- ,
- 74
- , 571–579.
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition.
- Mycol.
- 1979
- ,
- 3
- , 285–287.
- Sarwar, A.; Katas, H.; Zin, N.M. Antibacterial effects of chitosan–tripolyphosphate nanoparticles: Impact of particle size molecular weight.
- Nanoparticle Res. 2014, 16, 2517.
- Xing, K.; Chen, X.G.; Kong, M.; Liu, C.S.; Cha, D.S.; Park, H.J. Effect of oleoyl-chitosan nanoparticles as a novel antibacterial dispersion system on viability, membrane permeability and cell morphology of Escherichia coli and Staphylococcus aureus. Polym. 2009, 76, 17–22.
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Polym. 2017, 176, 257–265.
- Tamara, F.; Lin, C.; Mi, F.-L.; Ho, Y.-C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88.
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. J. Food Microbiol. 2004, 95, 147–155.
- Li, X.; Feng, X.; Yang, S.; Fu, G.; Wang, T.; Su, Z. Chitosan kills Escherichia coli through damage to be of cell membrane mechanism. Polym. 2010, 79, 493–499.
- Ataei, S.; Khorasani, S.N.; Neisiany, R.E. Biofriendly vegetable oil healing agents used for developing self-healing coatings: A review. Org. Coat. 2019, 129, 77–95.
- Scheiner, M.; Dickens, T.J.; Okoli, O. Progress towards self-healing polymers for composite structural applications. Polymer 2016, 83, 260–282.
- Garcia, S.J. Effect of polymer architecture on the intrinsic self-healing character of polymers. Polym. J. 2014, 53, 118–125.
- Mauldin, T.C.; Kessler, M.R. Self-healing polymers and composites. Mater. Rev. 2010, 55, 317–346.
- Szabó, T.; Molnár-Nagy, L.; Bognár, J.; Nyikos, L.; Telegdi, J. Self-healing microcapsules and slow release microspheres in paints. Org. Coat. 2011, 72, 52–57.
- Hefni, H.H.H.; Azzam, E.M.; Badr, E.A.; Hussein, M.; Tawfik, S.M. Synthesis, characterization and anticorrosion potentials of chitosan-g-PEG assembled on silver nanoparticles. J. Biol. Macromol. 2016, 83, 297–305.
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Chitosan-based self-healing protective coatings doped with cerium nitrate for corrosion protection of aluminum alloy 2024. Org. Coat. 2012, 75, 8–13.
- Bao, Q.; Zhang, D.; Wan, Y. 2-Mercaptobenzothiazole doped chitosan/11-alkanethiolate acid composite coating: Dual function for copper protection. Surface Sci. 2011, 257, 10529–10534.
- Ulaeto, S.B.; Pancrecious, J.K.; Rajan, T.P.D.; Pai, B.C. Chapter 17—Smart Coatings. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Woodhead Publishing, Duxford, United Kingdom: 2019; pp. 341–372.
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Functionalized chitosan-based coatings for active corrosion protection. Surface Coat. Technol. 2013, 226, 51–59.
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Mater. 2018, 3, 267–277.
- Ding, F.; Li, H.; Du, Y.; Shi, X. Recent advances in chitosan-based self-healing materials. Che. Intermed. 2018, 44, 4827–4840.
- Zheludkevich, M.L.; Tedim, J.; Freire, C.S.R.; Fernandes, S.C.M.; Kallip, S.; Lisenkov, A.; Gandini, A.; Ferreira, M.G.S. Self-healing protective coatings with “green” chitosan based pre-layer reservoir of corrosion inhibitor. Mater. Chem. 2011, 21, 4805.
- Wu, Y.; Zheng, Y.; Yang, W.; Wang, C.; Hu, J.Fu, S. Synthesis and characterization of a novel amphiphilic chitosan–polylactide graft copolymer. Carbohydrate Polymers 2005; 59, 165-171.
- Wong, K.; Sun, G.; Zhang, X.; Dai, H.; Liu, Y.; He, C.; Leong, K.W. PEI-g-chitosan, a Novel Gene Delivery System with Transfection Efficiency Comparable to Polyethylenimine in vitro and after Liver Administration in vivo. Bioconjugate Chem. 2006, 17, 152–158.
- Kumar, S.; Bhattacharya, W.; Singh, M.; Halder, D.; Mitra, A. Plant latex capped colloidal silver nanoparticles: A potent anti-biofilm and fungicidal formulation. Mol. Liq. 2017, 230, 705–713.
- Kumari, R.; Brahma, G.; Rajak, S.; Singh, M.; Kumar, S. Antimicrobial activity of green silver nanoparticles produced using aqueous leaf extract of Hydrocotyle rotundifolia. Pharm. Exp. Med. 2016, 16, 195–201.
- Swargiary, M.; Kumar, S. One pot phytosynthesis of gold nanoparticles using aqueous extract of elephant apple- an eco-friendly approach. Pharm. Exp. Med. 2017, 17, 285–289.
- Pang, X.; Zhitomirsky, I. Electrodeposition of hydroxyapatite–silver–chitosan nanocomposite coatings. Surface Coat. Technol. 2008, 202, 3815–3821.
- Mishra, S.K.; Ferreira, J.M.F.; Kannan, S. Mechanically stable antimicrobial chitosan–PVA–silver nanocomposite coatings deposited on titanium implants. Polym. 2015, 121, 37–48.
- Pounraj, S.; Somu, P.; Paul, S. Chitosan and graphene oxide hybrid nanocomposite film doped with silver nanoparticles efficiently prevents biofouling. Surface Sci. 2018, 452, 487–497.
- Lin, B.; Luo, Y.; Teng, Z.; Zhang, B.; Zhou, B.; Wang, Q. Development of silver/titanium dioxide/chitosan adipate nanocomposite as an antibacterial coating for fruit storage. LWT-Food Sci. Technol. 2015, 63, 1206–1213.
- Ortiz-Duarte, G.; Pérez-Cabrera, L.E.; Artés-Hernández, F.; Martínez-Hernández, G.B. Ag-chitosan nanocomposites in edible coatings affect the quality of fresh-cut melon. Postharvest Biol. Technol. 2019, 147, 174–184.
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Food Sci. Emerg. Technol. 2016, 38, 231–237.
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417.
- Roosen, J.; Spooren, J.; Binnemans, K. Adsorption performance of functionalized chitosan–silica hybrid materials toward rare earths. Mater. Chem. A 2014, 2, 19415–19426.
- Grande, C.D.; Mangadlao, J.; Fan, J.; De Leon, A.; Delgado-Ospina, J.; Rojas, J.G.; Rodrigues, D.F.; Advincula, R. Chitosan Cross-Linked Graphene Oxide Nanocomposite Films with Antimicrobial Activity for Application in Food Industry. Symp. 2017, 374, 1600114.
- Yan, H.; Yang, H.; Li, A.; Cheng, R. pH-tunable surface charge of chitosan/graphene oxide composite adsorbent for efficient removal of multiple pollutants from water. Eng. J. 2016, 284, 1397–1405.
- Dong, X.; Wei, C.; Liang, J.; Liu, T.; Kong, D.; Lv, F. Thermosensitive hydrogel loaded with chitosan-carbon nanotubes for near infrared light triggered drug delivery. Colloids Surfaces B Biointerfaces 2017, 154, 253–262.
- Papadimitriou, L.; Kaliva, M.; Vamvakaki, M.; Chatzinikolaidou, M. Immunomodulatory Potential of Chitosan-graft-poly(ε-caprolactone) Copolymers toward the Polarization of Bone-Marrow-Derived Macrophages. ACS Biomater. Sci. Eng. 2017, 3, 1341–1349.
- Pandiyaraj, K.N.; Ramkumar, M.C.; Arun Kumar, A.; Padmanabhan, P.V.A.; Pichumani, M.; Bendavid, A.; Cools, P.; De Geyter, N.; Morent, R.; Kumar, V.; et al. Evaluation of surface properties of low density polyethylene (LDPE) films tailored by atmospheric pressure non-thermal plasma (APNTP) assisted co-polymerization and immobilization of chitosan for improvement of antifouling properties. Sci. Eng. C 2019, 94, 150–160.
- Trivedi, P.; Saloranta-Simell, T.; Maver, U.; Gradišnik, L.; Prabhakar, N.; Smått, J.-H.; Mohan, T.; Gericke, M.; Heinze, T.; Fardim, P. Chitosan–Cellulose Multifunctional Hydrogel Beads: Design, Characterization and Evaluation of Cytocompatibility with Breast Adenocarcinoma and Osteoblast Cells. Bioengineering 2018, 5, 3.
- Wang, Z.; Shi, Y.; Yang, X.; Xiong, Y.; Li, Y.; Chen, B.; Lai, W.-F.; Rogach, A.L. Water-Soluble Biocompatible Copolymer Hypromellose Grafted Chitosan Able to Load Exogenous Agents and Copper Nanoclusters with Aggregation-Induced Emission. Funct. Mater. 2018, 28, 1802848.
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot study in vitro. Biotechnol. Equip. 2017, 31, 766–773.
- Njoku, D.I.; Cui, M.; Xiao, H.; Shang, B.; Li, Y. Understanding the anticorrosive protective mechanisms of modified epoxy coatings with improved barrier, active and self-healing functionalities: EIS and spectroscopic techniques. Rep. 2017, 7, 15597.
- Benucci, I.; Liburdi, K.; Cacciotti, I.; Lombardelli, C.; Zappino, M.; Nanni, F.; Esti, M. Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocoll. 2018, 74, 124–131.
- Azharul Islam, M.; Tan, Y.L.; Atikul Islam, M.; Romić, M.; Hameed, B.H. Chitosan–bleaching earth clay composite as an efficient adsorbent for carbon dioxide adsorption: Process optimization. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 554, 9–15.
- Li, X.; Li, Y.-C.; Chen, M.; Shi, Q.; Sun, R.; Wang, X. Chitosan/rectorite nanocomposite with injectable functionality for skin hemostasis. Mater. Chem. B 2018, 6, 6544–6549.
- Susilowati, E.; MaryaniAshadi, *. REPLACE .*. Preparation of silver-chitosan nanocomposites and coating
on bandage for antibacterial wound dressing application. AIP Conf. Proc. 2016, 1710, 030015 - El-Sherbiny, I.M.; Hefnawy, A.; Salih, E. New core–shell hyperbranched chitosan-based nanoparticles as
optical sensor for ammonia detection. Int. J. Biol. Macromol. 2016, 86, 782–788. - Ogugbue, C.J.; Sawidis, T. Bioremediation and Detoxification of Synthetic Wastewater Containing Triarylmethane Dyes by Aeromonas hydrophila Isolated from Industrial Effluent. Res. Int. 2011, 2011, 1–11.
- Brüschweiler, B.J. Toxicity of non-regulated aromatic amines from azo dyes in textiles: Knowns and unknowns. Lett. 2013, 221, S54.
- Wang, J.; Zhuang, S. Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386.
- Shen, C.; Shen, Y.; Wen, Y.; Wang, H.; Liu, W. Fast and highly efficient removal of dyes under alkaline conditions using magnetic chitosan-Fe(III) hydrogel. Water Res. 2011, 45, 5200–5210.
- Ali, F.; Khan, S.B.; Kamal, T.; Anwar, Y.; Alamry, K.A.; Asiri, A.M. Anti-bacterial chitosan/zinc phthalocyanine fibers supported metallic and bimetallic nanoparticles for the removal of organic pollutants. Polym. 2017, 173, 676–689.
- Zhou, J.; Lü, Q.-F.; Luo, J.-J. Efficient removal of organic dyes from aqueous solution by rapid adsorption onto polypyrrole–based composites. Clean. Prod. 2017, 167, 739–748.
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Ecol. Progr. Ser. 1989, 58, 175–189.
- Qian, P.Y.; Lau, S.C.K.; Dahms, H.U.; Dobretsov, S.; Harder, T. Marine Biofilms as Mediators of Colonization by Marine Macroorganisms: Implications for Antifouling and Aquaculture. Biotechnol. 2007, 9, 399–410.
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J.-F. Marine biofilms on artificial surfaces: Structure and dynamics. Microbiol. 2013, 15, 2879–2893.
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Org. Coat. 2004, 50, 75–104.
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2010, 27, 87–98.
- Pelletier, É.; Bonnet, C.; Lemarchand, K. Biofouling Growth in Cold Estuarine Waters and Evaluation of Some Chitosan and Copper Anti-Fouling Paints. J. Mol. Sci. 2009, 10, 3209–3223.
- Al-Naamani, L.S. Antifouling properties of chitosan coatings on plastic substrates. Agric. Mar. Sci. [JAMS] 2019, 23, 92.
- Dobretsov, S.; Abed, R.M.M.; Muthukrishnan, T.; Sathe, P.; Al-Naamani, L.; Queste, B.Y.; Piontkovski, S. Living on the edge: Biofilms developing in oscillating environmental conditions. Biofouling 2018, 34, 1064–1077.
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S.K. Fabrication, Characterization of Chitosan/Nanosilver Film and Its Potential Antibacterial Application. Biomater. Sci. Polym. Ed. 2009, 20, 2129–2144.
- Reighard, K.P.; Hill, D.B.; Dixon, G.A.; Worley, B.V.; Schoenfisch, M.H. Disruption and eradication of aeruginosabiofilms using nitric oxide-releasing chitosan oligosaccharides. Biofouling 2015, 31, 775–787.
- Elshaarawy, R.F.M.; Mustafa, F.H.A.; van Geelen, L.; Abou-Taleb, A.E.A.; Tadros, H.R.Z.; Kalscheuer, R.; Janiak, C. Mining marine shell wastes for polyelectrolyte chitosan anti-biofoulants: Fabrication of high-performance economic and ecofriendly anti-biofouling coatings. Polym. 2017, 172, 352–364.
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Eng. J. 2008, 40, 485–491.
- Nigmatullin, R.; Konovalova, V.; Pobigay, G. Development of antimicrobial membranes via the surface tethering of chitosan. Appl. Polym. Sci. 2009, 111, 1697–1705.
- Salehi, H.; Rastgar, M.; Shakeri, A. Anti-fouling and high water permeable forward osmosis membrane fabricated via layer by layer assembly of chitosan/graphene oxide. Surface Sci. 2017, 413, 99–108.
- Bulwan, M.; Wójcik, K.; Zapotoczny, S.; Nowakowska, M. Chitosan-Based Ultrathin Films as Antifouling, Anticoagulant and Antibacterial Protective Coatings. Biomater. Sci. Polym. Ed. 2012, 23, 1963–1980.
- Shanthana Lakshmi, D.; Jaiswar, S.; saxena, M.; Tasselli, F.; Raval, H.D. Preparation and performance of biofouling resistant PAN/chitosan hollow fiber membranes. 3 Biotech 2017, 7, 224.
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H.; Beygzadeh, M. Novel high flux antifouling nanofiltration membranes for dye removal containing carboxymethyl chitosan coated Fe3O4 nanoparticles. Desalination 2014, 349, 145–154.
- Wang, R.; Song, X.; Xiang, T.; Liu, Q.; Su, B.; Zhao, W.; Zhao, C. Mussel-inspired chitosan-polyurethane coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. Polym. 2017, 168, 310–319.
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT 2018,
| Chitosan Molecular Weight/Viscosity | Type of Nanomaterials in Composite | Name of Nanomaterial/Polymer/Clay | Preparation Method of Chitosan Nanocomposite | Form of Chitosan Nanocomposites | Specific Application | Key/Enhanced Properties | Application Field | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 kDa | Metal | Ag nanoparticles | In situ reduction on chitosan | Thin film coating on bandage | Antibacterial activity against | E. coli | and | S. aureus | Inactivation bacterial metabolism | Antimicrobial | [ | 100 | ] |
| Medium molecular weight | Metal | Ag nanoparticles | In situ reduction on chitosan | Ag nanoparticles anchored on chitosan particles | Sensing of ammonia in solution | Sensitive in optical absorption intensity and wavelength | Environment | [ | 101 | ] | |||
| Medium molecular weight | Metal oxide | ZnO nanoparticles | Blending | Thin film coating | Antifouling prevention | Anti-diatom activity and antibacterial activity against the marine bacterium | Anti-biofouling | [ | 85 | , | 86 | ] | |
| Low viscosity | Metal oxide | SiO | 2 | nanoparticles | In situ Stöber method grown on chitosan | Slurry packed in liquid chromatography (LC) column | Adsorption of rare-earth elements | High adsorption efficiency, selectivity, and reusability | Environmental | [ | 87 | ] | |
| 190–310 kDa | Carbon | Graphene oxide | Cross-linking | Thin film | Antimicrobial against | E. coli | and | B. subtilis | Improved mechanical and antimicrobial properties | Antimicrobial | [ | 88 | ] |
| 300 kDa | Carbon | Graphene oxide | Cross-linking | Hydrogel | Removal of dyes and metal ions from water | Tunable surface charge; efficient removal of pollutants | Environmental | [ | 89 | ] | |||
| N/A | Polymer | low density poly-ethylene (LDPE) film | Grafting | Coating | Significant changes in surface wettability | Improved anti-thrombogenic properties | Antifouling | [ | 92 | ] | |||
| N/A | Clay | Halloysite clay nanotubes | Electrostatical adsorption | Coating | Anticorrosive protective | Improved passive barrier protective and self-healing | Environmental | [ | 96 | ] | |||
| 50–190 kDa | Clay | Bentonite and sepiolite | Blend | Thin film | Winemaking application | Enhanced immobilization of protease but negatively affected catalytic properties | Antimicrobial | [ | 97 | ] | |||
| Medium molecular weight | Clay | Bentonite | Gelation and lyophilization | Bead | Carbon dioxide adsorption | High adsorption capacity under moderate condition | Environmental | [ | 98 | ] |
4. Applications of Chitosan-Based Nanocomposites
4.1. Water Purification
4.2. Antifouling Paints and Coatings
4.3. Shelf-Life Extension of Fruits and Vegetables
During postharvest transportation and storage, fresh produce (fruits and vegetables) undergo quality deteriorations due to the various physiology reactions and processes, such as postharvest respiration, ripening, ethylene production, and senescence. These physiological changes are directly influenced by the surrounding environment, i.e., temperature, oxygen, humidity, and light, that lead to loss of water, texture, color, and nutrients of the produce [126,127]. Microbiological spoilage leads to postharvest losses of about 15% to 50% of fruits and vegetables produced worldwide [128]. In developing countries, the percentages of product losses are quite high due to a lack of appropriate technologies for postharvest storage of fruits and vegetables. The physiological, biochemical, and environmental changes promote the growth of postharvest pathogens that are the major causes of loss/damages in the supply chain. However, controlling postharvest destruction of fresh produce involves the application of synthetic antimicrobials, fungicides, and insecticides. Consumer concerns about the adverse effect of synthetic chemical residues on human health and environment and the chances of the development of pathogen resistance have led global scientific research towards the development of alternative strategies for the preservation of fresh produce [129]. The application of a natural polymer (biopolymer)-based films and coatings on food surfaces have recently gained interest for the shelf-life extension of fresh produce due to their similar functions to those of conventional protection or synthetic packaging. Out of several natural polymers, the use of chitosan-based treatment at the postharvest stages has been considered to be a suitable alternative treatment to replace the use of synthetic chemicals [130]. Chitosan-based films and coatings have been reported to effectively extend quality and storability of food products in general, and of fresh agricultural produce in particular, due to its excellent natural antioxidant, antibacterial, and antifungal activities [131,132,133,134].4.3.1. Packaging Films
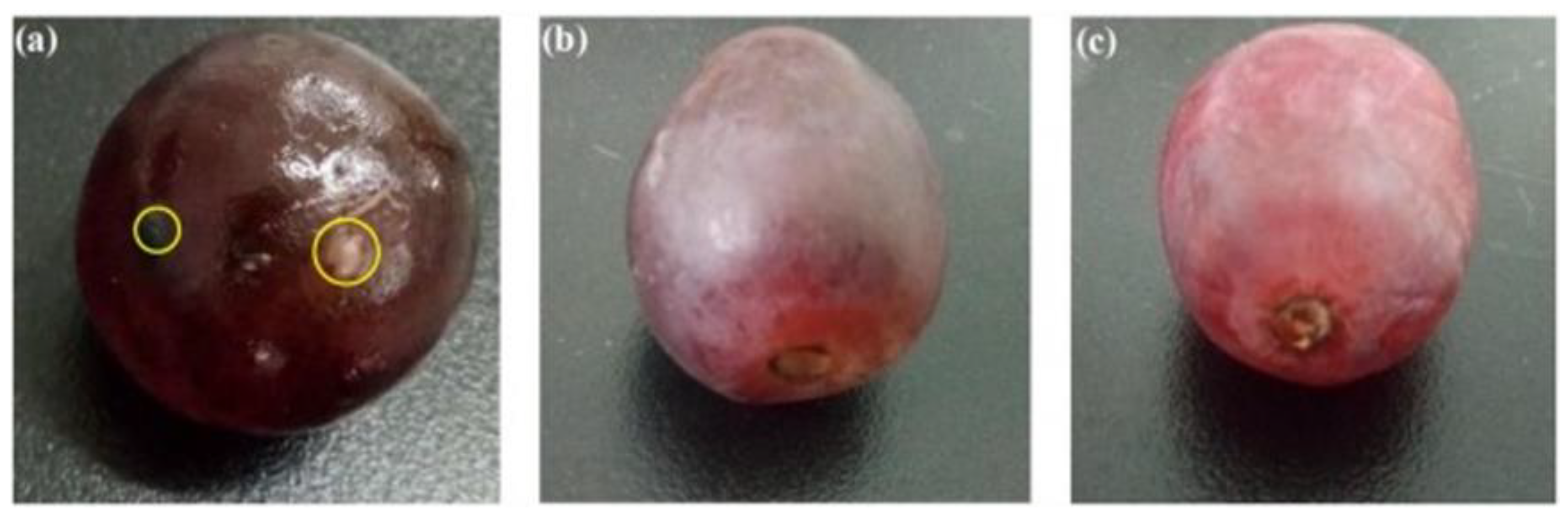
4.3.2. Coatings of Fruits and Vegetables
Coatings provide a thin layer of materials on food surfaces to maintain or control the ingress of gases, moisture, and solutes from the environment. Additionally, coatings can also act as a carrier for functional antimicrobial and antioxidant substances to additionally enhance its functionality for ensuring food quality and food safety of fresh produce (Figure 6). Coatings on the surface of fruits and vegetables could provide the following functions: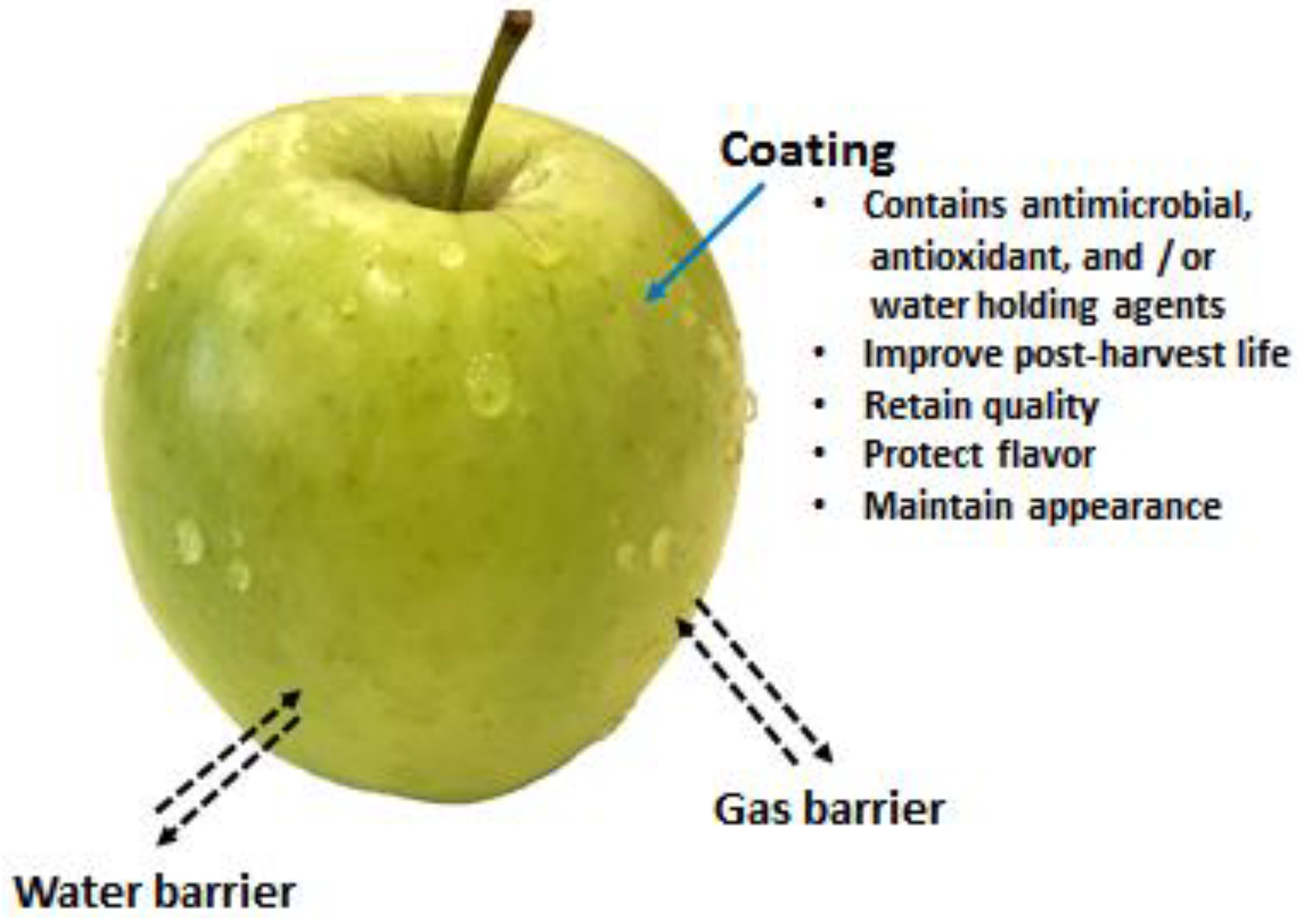
-
Offer barrier properties against moisture and oxygen
-
Help to deliver antimicrobial activity to inhibit or delay the microbial growth
-
Deliver antioxidant effects that help to reduce the oxidation process, loss of color, vitamins, etc.
-
Help to maintain the loss of volatile components and stop acquiring foreign odors
References
References
- Tsigos, I.; Martinou, A.; Kafetzopoulos, D.; Bouriotis, V. Chitin deacetylases: New, versatile tools in biotechnology. Trends Biotechnol. 2000, 18, 305–312.
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Technol. 2006, 97, 1061–1085.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Polym. Sci. 2006, 31, 603–632.
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Rev. Biotechnol. 2012, 33, 379–403.
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56.
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Drugs 2010, 8, 1567–1636.
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470.
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Polym. J. 2013, 49, 780–792.
- Kumari, S.; Rath, P.K. Extraction and Characterization of Chitin and Chitosan from (Labeo rohit) Fish Scales. Procedia Mater. Sci. 2014, 6, 482–489.
- Doiphode, N.; Joshi, C.; Ghormade, V.; Deshpande, M.V. Biotechnological Applications of Dimorphic Yeasts. In Yeast Biotechnology: Diversity and Applications; Springer: Dordrecht, The Netherlands, 2009; pp. 635–650.
- Amorim, R.V.S.; Ledingham, W.M.; Kennedy, J.F.; Campos-Takaki, G.M. Chitosan from Syncephalastrum racemosum Using Sugar Cane Substrates as Inexpensive Carbon Sources. Food Biotechnol. 2006, 20, 43–53.
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Rev. Food Sci. Nutr. 2003, 43, 61–87.
- Nouri, M.; Khodaiyan, F.; Razavi, S.H.; Mousavi, M. Improvement of chitosan production from Persian Gulf shrimp waste by response surface methodology. Food Hydrocoll. 2016, 59, 50–58.
- Teli, M.D.; Sheikh, J. Extraction of chitosan from shrimp shells waste and application in antibacterial finishing of bamboo rayon. J. Biol. Macromol. 2012, 50, 1195–1200.
- Sedaghat, F.; Yousefzadi, M.; Toiserkani, H.; Najafipour, S. Bioconversion of shrimp waste Penaeus merguiensis using lactic acid fermentation: An alternative procedure for chemical extraction of chitin and chitosan. J. Biol. Macromol. 2017, 104, 883–888.
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem. 2012, 47, 2032–2039.
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171.
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. J. Biol. Macromol. 2017, 104, 1697–1705.
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-Chitin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. J. Biol. Macromol. 2017, 104, 953–962.
- Marei, N.H.; El-Samie, E.A.; Salah, T.; Saad, G.R.; Elwahy, A.H.M. Isolation and characterization of chitosan from different local insects in Egypt. J. Biol. Macromol. 2016, 82, 871–877.
- Nemtsev, S.V.; Zueva, O.Y.; Khismatullin, M.R.; Albulov, A.I.; Varlamov, V.P. Isolation of Chitin and Chitosan from Honeybees. Biochem. Microbiol. 2004, 40, 39–43.
- Abdel-Gawad, K.M.; Hifney, A.F.; Fawzy, M.A.; Gomaa, M. Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 2017, 63, 593–601.
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Polym. 2006, 64, 98–103.
- Baron, R.D.; Pérez, L.L.; Salcedo, J.M.; Córdoba, L.P.; Sobral, P.J.d.A. Production and characterization of films based on blends of chitosan from blue crab (Callinectes sapidus) waste and pectin from Orange (Citrus sinensis Osbeck) peel. J. Biol. Macromol. 2017, 98, 676–683.
- Nguyen, T.T.; Barber, A.R.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioprocess. 2017, 4, 27.
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. J. Biol. Macromol. 2018, 107, 662–667.
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. J. Biol. Macromol. 2018, 120, 1181–1189.
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416.
- Jiang, X.; Chen, L.; Zhong, W. A new linear potentiometric titration method for the determination of deacetylation degree of chitosan. Polym. 2003, 54, 457–463.
- Dos Santos, Z.M.; Caroni, A.L.P.F.; Pereira, M.R.; da Silva, D.R.; Fonseca, J.L.C. Determination of deacetylation degree of chitosan: A comparison between conductometric titration and CHN elemental analysis. Res. 2009, 344, 2591–2595.
- Kasaai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Polym. 2010, 79, 801–810.
- Desbrières, J.; Martinez, C.; Rinaudo, M. Hydrophobic derivatives of chitosan: Characterization and rheological behaviour. J. Biol. Macromol. 1996, 19, 21–28.
- Kasaai, M. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Polym. 2008, 71, 497–508.
- Wu, T.; Zivanovic, S. Determination of the degree of acetylation (DA) of chitin and chitosan by an improved first derivative UV method. Polym. 2008, 73, 248–253.
- Wu, C.; Kao, C.Y.; Tseng, S.-Y.; Chen, K.C.; Chen, S.-F. Determination of the degree of deacetylation of chitosan by capillary zone electrophoresis. Polym. 2014, 111, 236–244.
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120.
- Li, X.; Xia, W. Effects of concentration, degree of deacetylation and molecular weight on emulsifying properties of chitosan. J. Biol. Macromol. 2011, 48, 768–772.
- Zhuang, C.; Zhong, Y.; Zhao, Y. Effect of deacetylation degree on properties of Chitosan films using electrostatic spraying technique. Food Control 2019, 97, 25–31.
- Paul, T.; Halder, S.K.; Das, A.; Ghosh, K.; Mandal, A.; Payra, P.; Barman, P.; Das Mohapatra, P.K.; Pati, B.R.; Mondal, K.C. Production of chitin and bioactive materials from Black tiger shrimp (Penaeus monodon) shell waste by the treatment of bacterial protease cocktail. 3 Biotech 2015, 5, 483–493.
- Peniche, C.; Peniche, H.; Pérez, J. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235.
- Jongsri, P.; Wangsomboondee, T.; Rojsitthisak, P.; Seraypheap, K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. LWT 2016, 73, 28–36.
- Zhong, Y.; Zhuang, C.; Gu, W.; Zhao, Y. Effect of molecular weight on the properties of chitosan films prepared using electrostatic spraying technique. Polym. 2019, 212, 197–205.
- Kim, K.W.; Min, B.J.; Kim, Y.-T.; Kimmel, R.M.; Cooksey, K.; Park, S.I. Antimicrobial activity against foodborne pathogens of chitosan biopolymer films of different molecular weights. LWT -Food Sci. Technol. 2011, 44, 565–569.
- Zheng, L.-Y.; Zhu, J.-F. Study on antimicrobial activity of chitosan with different molecular weights. Polym. 2003, 54, 527–530.
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. J. Food Microbiol. 2002, 74, 65–72.
- Shelma, R.; Sharma, C.P. Acyl modified chitosan derivatives for oral delivery of insulin and curcumin. Mater. Sci. Mater. Med. 2010, 21, 2133–2140.
- Wu, M.; Long, Z.; Xiao, H.; Dong, C. Preparation of N, N, N-trimethyl chitosan via a novel approach using dimethyl carbonate. Polym. 2017, 169, 83–91.
- Jagadish, R.S.; Divyashree, K.N.; Viswanath, P.; Srinivas, P.; Raj, B. Preparation of N-vanillyl chitosan and 4-hydroxybenzyl chitosan and their physico-mechanical, optical, barrier, and antimicrobial properties. Polym.
- 89
- , 198–209.
- Gong, T.; Li, C.; Bian, B.; Wu, Y.; Dawuda, M.M.; Liao, W. Advances in application of small molecule compounds for extending the shelf life of perishable horticultural products: A review.
- Hortic.
- 2018
- ,
- 230
- , 25–34.
- Swaminathan, M.S. Food Losses and Food Waste. In
- Combating Hunger and Achieving Food Security
- ; Cambridge University Press: Cambridge, UK, 2015, pp. 37–46.
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables.
- Trends Food Sci. Technol.
- 2017
- ,
- 64
- , 23–38.
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems.
- Food Res. Int.
- 2016
- ,
- 89
- , 117–128.
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO
- 2
- composite film with efficient antimicrobial activities under visible light for food packaging applications.
- Polym.
- 2017
- ,
- 169, 101–107.
- Shankar, S.; Rhim, J.-W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocoll. 2018, 82, 116–123.
- Xu, D.A.N.; Qin, H.-R.; Ren, D.A.N.; Yu, Y.-L. Influence of Coating Time on the Preservation Performance of Chitosan/Montmorillonite Composite Coating on Tangerine Fruits. In The 21st IAPRI World Conference on Packaging; DEStech Publications, Inc.: Zhuhai, China, 2018.
- Cosme Silva, G.M.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378.
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423.
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-biofilm activity and food packaging application of room temperature solution process based polyethylene glycol capped Ag-ZnO-graphene nanocomposite. Sci. Eng. C 2018, 91, 743–753.
- Kanmani, P.; Rhim, J.-W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652.
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Polym. 2018, 193, 19–27.
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184.
- Basumatary, K.; Daimary, P.; Das, S.K.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia speciosa fruit-mediated synthesis of silver nanoparticles and its application as filler in agar based nanocomposite films for antimicrobial food packaging. Food Packag. Shelf Life 2018, 17, 99–106.
- Kumar, S.; Mitra, A.; Halder, D. Centella asiatica leaf mediated synthesis of silver nanocolloid and its application as filler in gelatin based antimicrobial nanocomposite film. LWT 2017, 75, 293–300.
- Al-Naamani, L.; Dutta, J.; Dobretsov, S. Nanocomposite Zinc Oxide-Chitosan Coatings on Polyethylene Films for Extending Storage Life of Okra (Abelmoschus esculentus). Nanomaterials 2018, 8, 479.
