Periodontal bacteria dissemination into the lower respiratory tract may create favorable conditions for severe COVID-19 lung infection. Once lung tissues are colonized, cells that survive persistent bacterial infection can undergo permanent damage and accelerated cellular senescence. Consequently, several morphological and functional features of senescent lung cells facilitate SARS-CoV-2 replication. The higher risk for severe SARS-CoV-2 infection, the virus that causes COVID-19, and death in older patients has generated the question whether basic aging mechanisms could be implicated in such susceptibility. Mounting evidence indicates that cellular senescence, a manifestation of aging at the cellular level, contributes to the development of age-related lung pathologies and facilitates respiratory infections. Apparently, a relationship between life-threatening COVID-19 lung infection and pre-existing periodontal disease seems improbable. However, periodontal pathogens can be inoculated during endotracheal intubation and/or aspirated into the lower respiratory tract.
- periodontitis
- periodontal disease
- COVID-19
- LUNG
- SARS-CoV-2
1. Introduction
Based on their genomic structure, coronaviruses can be divided into four subgroups: α, β, γ, and δ[1]. SARS-CoV-2 is a β-coronavirus, and similar to other respiratory pathogens, can be spread through droplets generated during talking, sneezing, and coughing. In addition, it can be transmitted by “infected” aerosols generated during medical or dental procedures[2][3][4]. The first step for SARS-CoV-2 infection is the binding to angiotensin-converting enzyme 2 (ACE2), a host cell receptor expressed in lung cells and multiple extrapulmonary tissues[4][5]. ACE2 is highly expressed in lung epithelial cells, myocardial cells, the gastrointestinal system, kidney proximal tubule cells, and arterial smooth muscle cells[6]]. Furthermore, the oral mucosa plays a crucial role as a portal for SARS-CoV-2 infection, as oral epithelial cells highly express ACE2 [7]. Interestingly, ACE2 expression in the nasal epithelium is age-dependent, with lower expression in children, which may explain the higher COVID-19 prevalence in older individuals[8][9]. Therefore, although different factors are needed for efficient viral infection, human cells with high ACE2 expression are potentially more susceptible to becoming infected with SARS-CoV-2.
After binding specific receptors, viruses manipulate host cells’ resources in order to replicate. Although SARS-CoV-2 is closely related to another coronavirus, it is considered a new β-coronavirus. To better understand a newly identified virus, many mechanisms can be learned from related strains, including invasion and replication properties[10]. For instance, SARS-CoV-2 is able to infect and replicate more efficiently in human lung tissues than SARS-CoV[11]. Some coronaviruses manipulate the mitotic cycle and apoptotic cell death as strategies to promote infection. They can induce cell growth arrest in different cell lines in order to enhance their replication, probably by increasing the availability of deoxynucleotides[12][13][14]. One of the mechanisms used by coronaviruses to induce mitotic arrest is the activation of DNA damage response (DDR) signaling. Xu et al. found that the suppression of ATR (ataxia telangiectasia and Rad3 related), a key modulator of DDR, inhibited viral replication[13]. Although DDR can act as an antiviral mechanism, some viruses can use DDR factors to stimulate replication[15]. Consistent with the concept that coronaviruses can induce cell growth arrest to promote their replication, Bouhaddou et al. recently demonstrated that SARS-CoV-2 causes both DNA damage during the early stage of infection and cell cycle arrest[16]. Therefore, an early cycle inhibitory effect in infected cells may delay their death, and at the same time allow coronaviruses to evade host immune surveillance and facilitate replication[14].
Following viral infection, cells react and secrete specific chemokines that modulate the recruitment of immune cells; this process represents the first line of defense to reduce virus replication and viral spread [17][18]. In contrast, low and dysregulated immune response and reduced levels of antiviral interferons are observed after SARS-CoV-2 infection; however, proinflammatory cytokine secretion remains increased [19][20][21][22]. High and uncontrolled secretion of proinflammatory cytokines, also known as a “cytokine storm”, can result in severe local tissue injury and also have systemic consequences [18][23]. In agreement with this, a cytokine storm caused as a reaction to SARS-CoV-2 infection is a major cause of direct lung injury, acute respiratory distress syndrome (ARDS), multiple organ failure, and unfavorable prognosis[18][24]. Therefore, lung tissue damage is the consequence not only of the direct damage from virus, but also from ROS-mediated oxidative stress associated with inflammation[25].
2. Predictors of Severe Lung COVID-19 Infection
Higher risk of developing severe COVID-19 infection is not the direct consequence of chronological aging itself; it is partially explained by the presence of pre-existing health problems. Indeed, the presence of one or more comorbidities can predict COVID-19 severity in older patients[26][27][28]. Given that severe COVID-19 infection can cause lung damage and dysfunction, it is reasonable to assume that chronic respiratory problems can increase the risk of mortality. Indeed, dyspnea, pneumonia, and COPD are considered the strongest predictive factors for disease severity[29][30]. In addition to the role of pre-existing comorbidities, a dysfunctional immune response has an important role in COVID-19 severity. It has been suggested that the combination of age-related decline of the host immune defense (immunosenescence), chronic low-grade systemic inflammation (inflammaging), and SARS-CoV-2 infection can predispose older patients to increased complications[31]. Along the same line, lymphopenia is also a reliable indicator for disease severity[32].
CT scans and chest radiographic findings, specific cytokines in serum, and the viral load also help to predict COVID-19 severity and potential complications. Given that bilateral lung involvement and pulmonary opacities increase in frequency during the late stage of infection, they are considered hallmarks of SARS-CoV-2 lung infection[33][34][35]. However, after the onset of symptoms (0–2 days), 56% of patients with SARS-CoV-2 infection had normal chest CT scans. In addition, only 28% had bilateral lung involvement, which suggests a limited predictive value during the early stages of infection[35]. On the other hand, during lung infection, immune and nonimmune cells display an excessive secretion of proinflammatory cytokines, including interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α)[36]. Consistent with this, Del Valle et al. identified that high IL-6 and TNF-α serum levels at the time of hospital admission are strong predictors for disease severity and patient survival[37]. In this inflammatory setting, the hyperactivation of NF-kB has a crucial role in modulating the secretion of proinflammatory mediators and the immune response[36]. Another important parameter relevant to predicting disease severity is the viral load, which in patients with severe infection is around 60 times higher than those with mild disease[38]. Altogether, different parameters can help with the stratification of patients with COVID-19, and the identification of additional clinical factors could increase the predictive capacity of physicians, improving the therapeutic management of patients.
3. Could Periodontal Bacteria Dissemination into the Lower Respiratory Tract Contribute to Severe COVID-19 Lung Infection?
The aspiration of oral fluids or food represents a plausible route for the translocation of oral pathogens into the lungs, especially in geriatric patients. In fact, aspiration pneumonia is one of the most common long-term complications of poor oral hygiene in elderly people, and adequate oral health care can decrease the risk of dying from that condition[39][40]. Considering that aspiration pneumonia is often caused by
P. gingivalis[41][42], and that oral bacteria have been identified in bronchoalveolar lavage samples from COVID-19 patients [43], there are at least three interconnected mechanisms that might facilitate SARS-CoV-2 replication in lung cells as a consequence of pre-existing pulmonary infection by periodontal bacteria: first,
P. gingivalis
3.1. LPS-Induced Senescence and SARS-CoV-2 Replication
Cells displaying senescence-like features may facilitate cell entry and promote efficient coronavirus replication. Prolonged exposure to LPS causes accelerated senescence in different cell types, including adipocyte precursors[44], microglial cells [45], and pulmonary epithelial cells [46]. Recent evidence indicates that cells can undergo DNA damage-driven cellular senescence as result of repeated exposure to
P. gingivalis LPS [47][48]. Although it is recognized that senescent cells contribute to deteriorating their local environment, these dysfunctional cells could also facilitate pathogen infection. Shivshankar et al. demonstrated that senescent cells promote bacterial adhesion to lung cells, eventually resulting in increased susceptibility to bacterial-induced pneumonia in older adults[49]. On the other hand, given that the entry of SARS-CoV-2 in host cells depends on the attachment to specific cell receptors, senescent cells may contribute to facilitating this process.
Vimentin is highly expressed in senescent cells. This filamentous cytoskeletal protein promotes the typical enlarged senescent morphology and plays an essential role in coronavirus cell binding and entry[50][51]. Yu et al. demonstrated that vimentin directly interacts with the spike protein of the coronavirus and that the susceptibility of coronavirus infection is notably reduced by eliminating vimentin activity [51]. Considering that ACE2 expression alone is not sufficient for viral infection, vimentin might act as a surface coreceptor for coronavirus, and likely for SARS-CoV-2 infection as well[51][52][53]. As consequence of this idea, it has been proposed that decreasing vimentin expression could be an attractive strategy for the treatment of COVID-19 infection[53]. Interestingly, Kara et al. recently reported that the strong relationship between severe periodontal disease and COVID-19 could be explained through increased viral attachment and immune response mediated by galectin-3 [54]. Galactin-3 has been identified in the cytoplasm of senescent human fibroblasts, indicating that cells that undergo proliferative arrest lack factors required for the nuclear import of this protein[55]. Furthermore, galectin-3 is highly secreted by senescent mesenchymal stem cells, which could promote the growth of colorectal cancer cells[56]. Consistent with the role of vimentin mentioned above, increased galectin-3 expression by senescent cells could facilitate SARS-CoV-2 attachment and cell entry. Of note, Diaz-Alvarez and Ortega reported that galectin-3 directly binds to invading pathogens and contributes to the immune system reaction against infection[57]. Indeed, galectin-3 is rapidly upregulated in gastric epithelial cells in response to
Helicobacter pylori, which through the expression of CagA, promotes cellular senescence in human gastric epithelial cells and extra-gastric cells[58][59].
Importantly, Bouhaddou et al. demonstrated that SARS-CoV-2 cell infection promotes the production of several cytokines, cell growth arrest, and p38 MAPK activation. These authors also identified that following SARS-CoV-2 infection, an upregulated IL-6 and TNF-α expression is observed, which was inhibited by SB203580 (a p38 inhibitor). Intriguingly, SARS-CoV-2 replication was also decreased by p38 inhibition[17]. P38 MAPK is a key regulatory pathway implicated in cell growth arrest and modulates the expression of most senescence-associated factors. Consequently, p38 inhibition delays the onset of senescence [60][61]. In agreement with the concept that viruses may use senescent cells to enhance replication, Kim et al. found that primary human bronchial epithelial cells that undergo replicative senescence were more susceptible to viral infection and enhanced replication than nonsenescent cells[62]. Therefore, we speculate that age-related accumulation of senescent cells in lung tissues is aggravated by
P. gingivalis
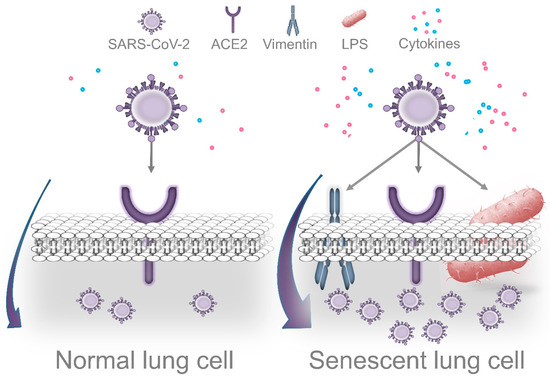
Figure 3.
P. gingivalis
3.2. Periodontal Bacteria and Exacerbation of Lung Inflammation in Response to SARS-CoV-2
ACE2 is crucial for SARS-CoV-2 cell binding and replication, but it also plays an essential role in protecting lung tissues from excessive inflammation. Whereas ACE2 mRNA is expressed in practically all human organs, the tissue distribution of ACE2 protein levels seems to be more restricted. Positive staining for ACE2 has been identified in specific sites of certain organs. For instance, in normal lungs, strong immunostaining for ACE2 is observed in alveolar epithelial cells [63]. In agreement with this, Zhao et al. demonstrated that 83% of cells expressing ACE2 in the lung are type II alveolar epithelial cells, suggesting that these cells can have an important role during SARS-CoV-2 infection[64]. In addition to mediating SARS-CoV-2 cell entry, ACE2 acts as an anti-inflammatory and antioxidant factor. Previous studies have demonstrated that ACE2 has a protective function against oxidative stress and severe inflammatory reactions, in part by inhibiting the NF-kB pathway[65][66][67]. Intriguingly, SARS-CoV—and probably SARS-CoV-2—spike protein binding results in ACE2 downregulation in the lungs of mice[68][69]. Along the same line, SARS-CoV-2 activates NF-kB, leading to cytokine secretion, including IL-6 and TNF-α [36]. Therefore, ACE2 expression is reduced after the attachment of the coronavirus spike in host cells, which results in the excessive secretion of key proinflammatory cytokines as a consequence of NF-kB upregulation.
Consistent with this protective role through the inhibition of NF-kB activity, ACE2 prevents both LPS-induced acute respiratory distress syndrome (ARDS) in a murine model and inflammatory injury in pulmonary microvascular endothelial cells [70][71]. Furthermore, ACE2 significantly decrease LPS-promoted IL-6 secretion by 75.6% and IL-1β by 86.7% in lung alveolar epithelial cells in vitro[72]. In addition, ACE2 protects mice from severe acute lung injury and failure induced by acid aspiration[73]. These studies suggest that ACE2 could defend lung tissues from LPS’s detrimental effects. A key finding was recently reported by Petruk et al.: they demonstrated the interaction between the SARS-CoV-2 spike and LPS from
Escherichia coli
Pseudomonas aeruginosa, a Gram-negative bacteria found in the intestines and an opportunistic pathogen implicated in the etiology of ventilator-associated pneumonia (VAP), respectively[74]. More specifically, the SARS-CoV-2 spike protein can interact with lipid A, a component of LPS, and result in significant upregulation of the inflammatory reaction compared with the individual effect of LPS. Moreover, these authors found that the combination of the spike protein and low levels of LPS potentiates NF-kB activation. In addition to decreasing inflammation, ACE2 also could protect lung tissues from LPS-induced oxidative stress. Kim et al. reported that sublethal concentrations of LPS promote the formation of hydrogen peroxide (H
2
2) in a dose-dependent manner in lung alveolar epithelial cells. Consistent with this, acute lung injury induced by LPS is characterized by inflammatory damage and decreased ACE2 expression[75]. On the other hand, Sahni et al. recently proposed that the connection between periodontitis and COVID-19 could be through a common proinflammatory cytokine expression profile; that is, patients with severe symptoms of COVID-19 have increased serum IL-1β, IL-7, IL-10, IL-17, IL-8, TNF-α, and MCP-1 levels, among others[76]. The secretion of many of these cytokines is increased in periodontal tissues of patients with periodontal disease compared to healthy controls. For instance, IL-17 seems to play an important role in exacerbating lung inflammation, and this proinflammatory cytokine can serve as a biomarker of COVID-19 severity [77]. Of note, IL-17 plays an important role in periodontal disease pathogenesis, and it is secreted by senescent alveolar bone osteocytes [78]. Altogether, pre-existing Gram-negative bacterial infection and the associated presence of LPS might exacerbate local lung inflammation as result of SARS-CoV-2 spike protein binding by enhancing NF-kB activation.
3.3. Impaired Immune Surveillance Caused by Periodontal Bacteria and SARS-CoV-2 Replication
Manipulation of the host immune defense by periodontal bacteria mediates the onset of inflammatory conditions locally, but also at distant organs colonized by those pathogens[79]. Although
P. gingivalis
P. gingivalis can delay the recruitment of neutrophils by inhibiting the expression of IL-8, a key chemokine that guides these immune cells into the site of infection. This absence of an IL-8 gradient severely affects local immune defense and promotes the overgrowth of these pathogens. This mechanism is called local chemokine paralysis[80]. However, periodontal pathogens are “inflammophilic” and they depend on inflammation for source nutrients. For that reason, once pathogenic bacteria increase in number and form a biofilm, they “proactively induce inflammation”[81] [82].
P. gingivalis is also able to invade and survive intracellularly in different cell types, including vascular and epithelial cells in vitro and in vivo[83][84]. These pathogens inside epithelial cells may be protected from antibiotics [85]. Interestingly, the ability to decrease immune recognition is not an exclusive feature of
P. gingivalis
T. forsythia, another pathogen implicated in periodontal disease etiology, can attenuate immune mechanisms of detection, leading to delayed bacterial elimination [86]. Another example is
A. actinomycetemcomitans, which is recognized by its immunosuppressive properties[87][88]. Considering that with advancing aging, the host immune surveillance is impaired [89][90], these studies suggest that bacterial mechanisms of immune evasion can not only promote their overgrowth, but also facilitate SARS-CoV-2 replication in lung cells.
4. Periodontal Status and Ventilator-Associated Pneumonia (VAP)
In patients infected with SARS-CoV-2, progressive respiratory failure is the most common cause for the admission to the ICU[33]. Intubation and invasive ventilation are required in approximately 3.2% of COVID-19 patients in order to provide respiratory support; however, it is also a high-risk procedure with potential complications[91][92][93]. The incidence of VAP ranges from 7% to 70% and represents a major cause of morbidity and mortality in the ICU [94]. VAP is a type of pneumonia caused by “infectious agents not present” at the time of mechanical ventilation, which triggers an inflammatory reaction after 48–72 h following intubation[95].
Whereas VAP is a polymicrobial infection, the major group of pathogens isolated from samples of patients with VAP is Gram-negative bacteria[96][97][98]. The inoculation of periodontal pathogens, bacteria from the dorsal surface of the tongue, and contaminated upper airway secretions have been directly implicated in the pathogenesis of VAP[99][100]. For example, Okuda et al. found that inoculation of
P. gingivalis
T. denticola into the trachea of mice resulted in increased secretion of cytokines and pneumonia[42]. Furthermore, the proteolytic enzyme gingipain, which is secreted by
P. gingivalis, mediates lung tissue damage produced during aspiration pneumonia[42]. Moreover, some periodontal pathogens can induce the secretion of proinflammatory cytokines in human lung epithelial cells, as well as in the lower respiratory system using a murine model[101]. These studies suggest that higher risk of oral bacteria inoculation exists when advanced periodontal lesions are present in those patients that require invasive mechanical ventilation. In other words, in older patients with neglected oral care, a greater number of oral bacteria can be attached to the endotracheal tube, translocated into the respiratory tract, and contribute to exacerbated SARS-CoV-2-mediated inflammation as highlighted above.
5. Conclusions
Elderly people are more susceptible to experiencing life-threatening SARS-CoV-2 lung infection. Why older patients experience increased COVID-19 severity remains poorly understood. It has been proposed that age-related processes, such as immune defense decline and low-grade systemic inflammation, may play an important role. The periodontal status of older people, on the other hand, has been overlooked. Considering that periodontal bacteria can disseminate into the lower respiratory tract of older people as a result of oral fluid aspiration, or inoculated during invasive mechanical ventilation, it is a plausible hypothesis that translocated Gram-negative periodontal bacteria may cause LPS-induced senescence in lung cells, aggravate age-related senescent cell accumulation, and facilitate SARS-CoV-2 replication. A relationship between established periodontitis and severe SARS-CoV-2 lung infection seems improbable, but P. gingivalis LPS-induced accelerated cellular senescence may be an overlooked event that links such conditions. Given that poor oral hygiene and the aspiration of periodontal bacteria could aggravate COVID-19 lung infection, appropriate oral care may be a crucial factor to prevent the exacerbation of lung inflammation. Furthermore, the decrease of the viral load in the oral cavity may reduce the risk of SARS-CoV-2 transmission, and the use of antiviral mouth rinses could prevent COVID-19 dissemination. Although multiple factors are implicated in the pathogenesis of severe COVID-19 lung infection, poor oral hygiene and periodontal infection (“gum disease”) are important parameters to be considered in older people
