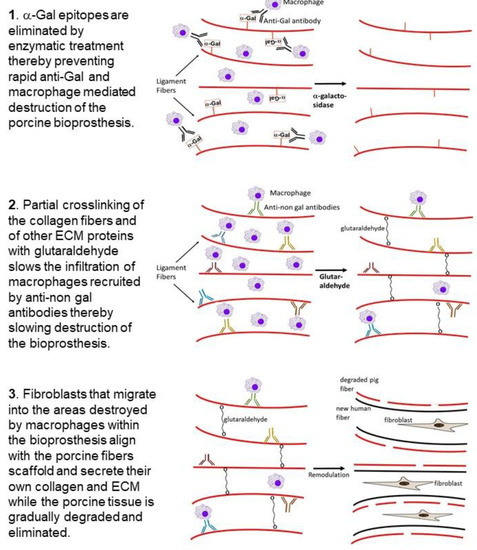
4. Processing of Porcine Patellar-Tendon into Bioprostheses, and Pre-Clinical Studies in Monkeys
Porcine patellar-tendons and the attached patellar and tibial bone-plugs () were processed to remove α-gal epitopes by incubation of the tendon for 12 h in recombinant (r)α-galactosidase solution [
53,
71]. Tendons may be of various sizes according to the age of the pig. The complete removal of α-gal epitopes was confirmed by an ELISA Inhibition Assay [
54], displaying no binding of the monoclonal anti-Gal antibody M86 [
78] to the homogenate of the treated tendon [
71]. The patellar-tendons were washed and partially crosslinked by incubation in 0.1% glutaraldehyde for 12 h. Subsequently, these processed tendons (referred to as BTB bioprostheses) were washed, and residual active aldehyde groups of glutaraldehyde were blocked with 0.1 M glycine.
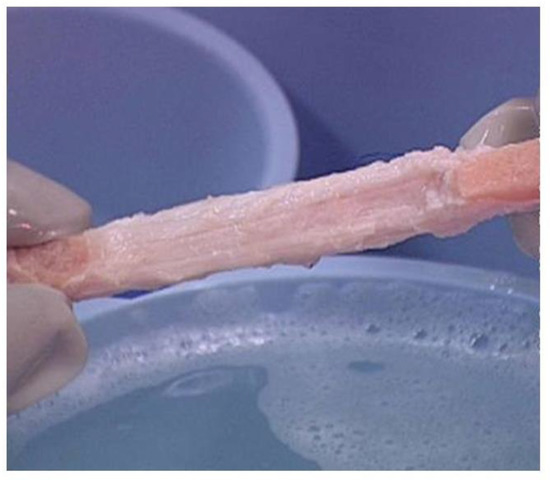
Figure 4. Bioprosthesis prepared from porcine bone-patellar-tendon-bone (length of ~10 cm and width of ~1 cm) for reconstructing torn ACL in humans. Note the two bone-plugs of the patella and tibia bones.
The optimal concentration of 0.1% glutaraldehyde used for partial crosslinking was determined empirically by incubation for 12 h of α-galactosidase treated patellar-tendon specimens in solutions containing glutaraldehyde at various concentrations, then washing and blocking of free aldehyde groups with glycine. These tendon specimens were implanted in the suprapatellar pouch of rhesus monkeys. The implants were explanted after 2 months, and their histopathology evaluated. The optimal glutaraldehyde concentration for partial crosslinking was determined as the concentration that subsequently enabled infiltration of macrophages to the extent that they occupied 20–30% of the implant. The crosslinked tendons were further preserved in 0.1 M glycine, and not in glutaraldehyde, to prevent additional crosslinking. The BTB bioprostheses were stored frozen after low level (17.8 kGy) e-Beam irradiation for final sterilization. In vitro stress tests indicated that this processing of the porcine tendons did not affect their biomechanical characteristics [
53]. It should be noted that the optimal concentration of glutaraldehyde has to be determined empirically for each type of tissue, to ensure the appropriate macrophage infiltration rate in soft tissues that may contain cellular and ECM components at concentrations that differ from those in tendons.
A pre-clinical study of BTB bioprostheses implantation was performed in 20 rhesus monkeys, in which the safety and efficacy of the method were evaluated [
53]. The ACL in the monkeys was removed and reconstructed by using treated porcine BTB implants, or allograft controls. Animals were stratified into 2-, 6-, and 12-month post implantation cohorts. Porcine BTB bioprostheses and rhesus patellar-tendon allografts were found to be incorporated by the host as functional ACL. There was no indication of toxicity with any of the bioprostheses. Both porcine BTB and rhesus tendon allografts revealed gradual host cellular infiltration and collagen remodeling similar to the ligamentization process observed in humans grafted with autologous or allogeneic (cadaveric) patellar tendon. Tensile tests of the strength of explanted transplants at the various time-points, demonstrated similar functional integration of allografts and treated porcine BTB reconstructions, and a similarity in gait between the two groups [
53].
Despite the elimination of α-gal epitopes by recombinant (r)α-galactosidase, titers of anti-Gal were elevated by ~30 fold, 2–4 weeks post implantation, as measured in ELISA assays with synthetic α-gal epitopes linked to albumin as solid-phase antigen () [
53]. This elicited anti-Gal response occurred because the of stimulation of the immune system to increase production of anti-Gal by α-gal epitopes on porcine RBC and bone marrow cells encased in the small cavities of the bone-plugs. The rα-galactosidase cannot reach these cells. However, α-gal epitopes on cells released from the bone-plugs in the course of their remodeling activate quiescent anti-Gal B cells to produce increased amounts of anti-Gal for a period of 2–10 weeks post implantation (). The subsequent decrease in anti-Gal production, to a level close to that observed in the pre-implantation serum, suggests that the porcine bone-plugs underwent near complete remodeling into autologous monkey bone, devoid of porcine cells, within ~3 months post implantation (). Accordingly, analysis of changes in anti-Gal titers post implantation of this bioprosthesis in humans can provide information on the extent of humanization of the bone-plugs in implanted patients at various time points [
71]. The elevation in anti-Gal activity in monkeys implanted with processed porcine BTB further supports the assumption that if α-gal epitopes are not removed from the BTB, the increased anti-Gal activity elicited by α-gal epitopes on soft tissue, and on RBC released from the bone cavities, will reach very high levels, and thus destroy the bioprosthesis prior to achieving appropriate humanization of the implant.
ELISA assays for anti-non gal antibody activity could provide information on the extent of replacement of the porcine bioprostheses with autologous ACL monkey tissue at various time points. In these assays the solid-phase antigen was porcine tendon homogenate, and the sera assayed were depleted of anti-Gal (by adsorption on glutaraldehyde fixed rabbit RBC, which present multiple α-gal epitopes) prior to the assay. Anti-non gal antibody titers peaked 3–6 months post transplantation, and subsequently decreased with the increased replacement of porcine tissue with the monkey fibroblasts and the ECM they produced (). Anti-non gal antibody activity did not return to the pre-implantation level, even at 12 months post implantation, suggesting that not all porcine soft tissue was eliminated at that time point. As described below, near complete replacement of porcine BTB with human tissue in implanted patients, was observed by the anti-non gal ELISA assay ~2 years post implantation.
5. Implantation of Porcine BTB Bioprosthesis in Patients with Torn ACL
The studies in monkeys indicated that treatment with porcine BTB bioprosthesis is safe and results in remodeling and regeneration of the implant into a functional autologous monkey tissue, as hypothesized in . Thus, the study of possible humanization of porcine BTB bioprostheses progressed to a clinical trial, performed in patients with torn ACL. This clinical trial was a non-randomized FDA and Institutional Review Board (IRB) approved Phase 1, single-center feasibility study, and included 10 consenting subjects [
71]. The study group was a highly active athletic subject population. The average age was 41 years (range of 21 to 51). In each of the patients, the damaged ACL was replaced with a porcine BTB bioprosthesis. The bone-plugs of the bioprosthesis were fixed to drilled femoral and tibial tunnels with interference fit screws. Patients underwent periodic clinical examinations, radiographic and MRI examinations, and blood and urine analysis for each subject for a two-year period following surgery.
Of the six evaluable subjects, five presented with functional humanized ACL at the 24-month post-operative time-point and satisfied all study success criteria including functional return to a high level versus the unoperated knee at 12 and 24 months after surgery. Athletes undergoing this surgery returned to regular training activity within 6–12 months. In all these patients, the humanized ACLs have continued and are continuing to function for ~17 years, and thus seem to be permanently functional. The sixth evaluable subject presented with tibial bone-plug loosening at 15-months post ACL reconstruction, had the implant removed, and was grafted with an allograft patellar tendon. The remaining four patients were non-evaluable subjects, who ruptured their porcine BTB bioprostheses due to sport injuries within the first-year post implantation, in accidents that usually cause the rupture of autologous ACL, as well. Their implants were explanted in secondary surgical interventions. Histologic examination of the explanted porcine BTB implants in the four non-evaluable subjects provided insight into the cellular events within the bioprosthesis in course of its humanization into an autologous ACL () [
71].
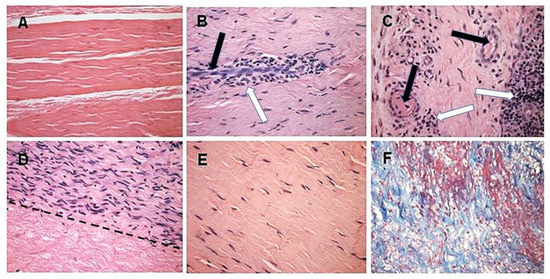
Figure 5. Histopathology demonstrating humanization stages in patients with implanted porcine BTB bioprostheses. Black arrows: blood vessels, white arrows: macrophages. (
A) Pre-implantation porcine BTB bioprosthesis. (
B) Infiltration of macrophages into the implanted bioprosthesis by extravasation. Elongated cells are endothelial cells of a blood vessel. (
C) Vascularization of the implanted BTB in a region near macrophage infiltrates. (
D) Repopulation of a section of the bioprosthesis by the recipient’s fibroblasts that aligned with the porcine collagen fiber scaffold (above the dashed line). Porcine collagen fibers and no cells, seen under the dashed line. (
E) An advanced stage of humanization, with repopulating fibroblasts secreting their own ECM. (
F) De novo produced collagen fibers, stained blue in Mason-trichrome staining. H&E, (×200) (modified from [
71]).
The initial recruitment of macrophages (illustrated in Stage 2 of ) and the start of neo-vascularization, which enables additional infiltration of macrophages, are shown in B, in which endothelial cells of a small blood vessel are surrounded by infiltrating mononuclear cells, many of which are macrophages. The continuing migration of macrophages through the blood vessels is further shown in C. The right section of this figure demonstrates an area with a high concentration of the infiltrating macrophages. The blood vessels also enable infiltration of the recipient’s fibroblasts, which align with the porcine collagen fibers scaffold (D). This figure suggests that the humanization process occurs in different stages at various areas of the implant. In the upper half of the figure, multiple fibroblasts align with the porcine collagen fibers scaffold, whereas in the lower part, this scaffold is devoid of infiltrating cells. The aligned infiltrating fibroblasts secrete their own collagen fibers, and thus humanize the porcine patellar-tendon into an autologous functional, viable ACL (E). The newly formed collagen fibers are stained blue by Mason-trichrome staining (F). The blood vessels in B,C are likely to be the result of neo-vascularization, since the porcine blood vessels were crosslinked by glutaraldehyde, and no anastomoses were made between the recipient blood vessels and the implanted bioprostheses. Overall, the neo-vascularization and macrophage infiltration observed in B,C, the infiltrating fibroblasts in D, and the newly formed collagen fibers in F, all strongly suggest the occurrence of an active humanization process within the first year post-operatively. This suggestion is supported by the observation of the peak anti-non gal antibody production at 6 months post-operatively (), implying ongoing degradation of the bioprostheses at that time.
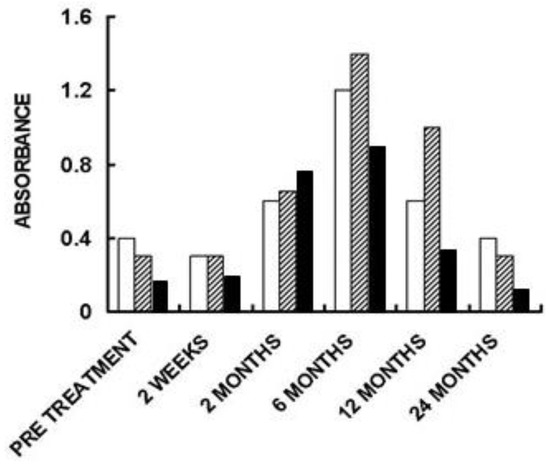
Figure 6. Anti-non gal IgG antibody response in three of the patients implanted with processed porcine BTB bioprosthesis for the reconstruction of torn ACL. Anti-non gal antibody activity at various time-points post-implantation was determined with anti-Gal depleted sera, by ELISA. Homogenate of fragmented porcine tendon was used as solid-phase antigen. The figure describes antibody binding at serum dilution of 1:640 (based on data from [
71]).
The humanization of porcine BTB into autologous ACL appears to complete within ~2 years, as indicated by the anti-non gal antibody production at various time-points. The titer of these antibodies in pre-implantation sera is usually very low (~1:20) and reflects a background level (). At the low level of pre-implantation anti-non gal antibody activity, no significant antibody binding to porcine tendon proteins was observed in Western blots (). Anti-non gal antibody production, as measured by ELISA with homogenate of porcine tendon as solid-phase antigen, and with sera depleted of anti-Gal, peaked at ~6 months post-implantation (). This peak anti-non gal antibody activity reflects the immune response to the multiple porcine antigens released from the porcine BTB that is gradually degraded by macrophages and is further shown in . After 12 months, anti-non gal antibody titers decreased because of diminishing amounts of released porcine antigens. By 24 months, this antibody production decreased to a level that was within the range of the pre-implantation level, because of diminished, or absence of stimulating porcine antigens (). This absence of anti-non gal antibodies at 24 months, strongly suggests that most or all of the original porcine tissue was replaced by permanently functioning human ACL tissue, thus completing the humanization process.
Porcine BTB bioprostheses processed for elimination of α-gal epitopes and partial crosslinking, as described above, were also used in an international double blinded, randomized controlled clinical trial in clinical centers in Italy, Denmark, Belgium, Spain, the Netherlands, and South Africa, for the reconstruction of torn ACL [
79]. That study was initiated ~10 years ago with a second group of patients that included 61 subjects with ruptured ACL, of which 32 were grafted with cadaveric allografts and 29 were implanted with porcine BTB bioprostheses. The processing of these bioprostheses was the same as that of the bioprostheses described above, and in and [
53,
71], but included an additional step of decellularization prior to treatment with rα-galactosidase. Six additional subjects in the BTB bioprosthesis implanted group got a deep infection in the bioprostheses, attributed to a water-based pathogen (
Ralstonia pickettii) bioprostheses contamination that occurred during the processing. By changing the water filter from 0.2 μm to 0.05 μm, this contamination was prevented in subsequent processed bioprostheses [
79]. Similar to the patients in the first group described above [
71], the patients in the second group [
79] presented with functional reconstructed ACL at the 24-month post-operative time-point. Moreover, at 24 months, functional performance assessment in the bioprosthesis implanted subjects satisfied all study success criteria, and did not reveal significant functional performance differences between subjects implanted with the BTB bioprosthesis and those with cadaveric allograft. In addition, anti-Gal production increased above the pre-surgery level at 2 weeks post surgery, and returned to the natural level after ~12 months. Anti-non gal antibody production peaked at 3–6 months at 100 fold the background level, and subsequently decreased back, close to the background level after 24 months [
79]. Taken together, the lack of differences in functional performance between recipients of the porcine BTB bioprosthesis and the recipients of cadaveric allograft tendons, and the return of anti-Gal and anti-non gal antibody levels close to the pre-surgery level, strongly suggest that in the second group of bioprosthesis recipients, the implants also underwent humanization into autologous, functional, viable ACL. These humanized ACL have continued to function with no failure for >8 years post surgery (personal communication). The similarities in results between the study of patients in the first group [
71] and those in the study of the second group [
79] further suggest that the addition of the decellularization step is not required for successful humanization of the porcine bioprosthesis into viable human tissue.
Overall, the studies above imply that elimination of α-gal epitopes and partial crosslinking of the porcine BTB to slow macrophage infiltration and degradation of the implant, enable neo-vascularization, fibroblasts infiltration, and alignment with the porcine collagen fiber scaffold. Continuous degradation of this scaffold by macrophages and its concomitant replacement with human fibroblasts, collagen fibers and other ECM components, result in humanization of the bioprosthesis into viable and permanently functional autologous ACL.
7. Conclusions
Partially crosslinked porcine bone-patellar-tendon-bone (BTB) bioprostheses, devoid of α-gal epitopes and implanted in patients with torn ACL, undergo humanization into autologous, viable, permanently functional ACL. In that process, anti-non gal antibodies contribute to the recruitment of macrophages that infiltrate into the implanted bio- prostheses. This infiltration is slowed, but not prevented, by partial crosslinking with glutaraldehyde molecules that function as “speed bumps” within the bioprosthesis. The infiltrating macrophages induce neo-vascularization, which enables recruitment of many more macrophages that degrade and debride the bioprosthesis with the help of anti-non gal antibodies binding to the BTB. Fibroblasts, following the recruited macrophages, align with the porcine collagen fibers scaffold and secrete their own collagen and other ECM components. The humanization process, which includes gradual degradation of the bioprosthesis and the concomitant replacement of the destroyed porcine tissue with human fibroblasts and ECM, is completed within ~2 years, and results in the formation of an autologous ACL that conserves permanently the biomechanical function even in athletic patients.
Porcine bioprostheses of heart valves (BHV) contain ECM and cellular components similar to those in tendons. Thus, studies on the possible humanization of BHV implants that are processed by elimination of α-gal epitopes and partial crosslinking by glutaraldehyde, should be considered. Successful conversion of such porcine or bovine BHV into viable, autologous, functional heart valves in experimental animal models may be followed by studies on BHV replacing impaired heart valves in young patients. Presently, these patients are implanted only with mechanical heart valves that require anticoagulation therapy. In addition, porcine, bovine, or equine (and possibly other mammalian) dermis, intestinal submucosa, pericardium, urinary bladder, blood vessels, ligaments, and other soft tissues processed to lack α-gal epitopes, and which are partially crosslinked, should be considered for studying as bioprostheses with high biomechanical integrity, and which undergo gradual humanization for conversion into functioning autologous viable tissues.
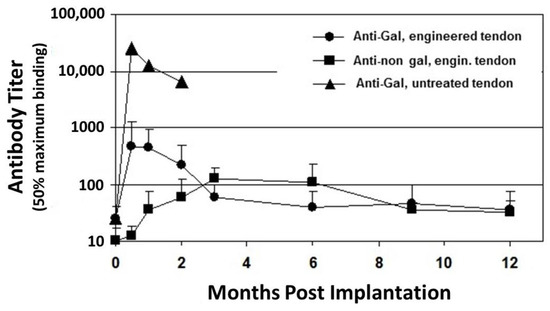
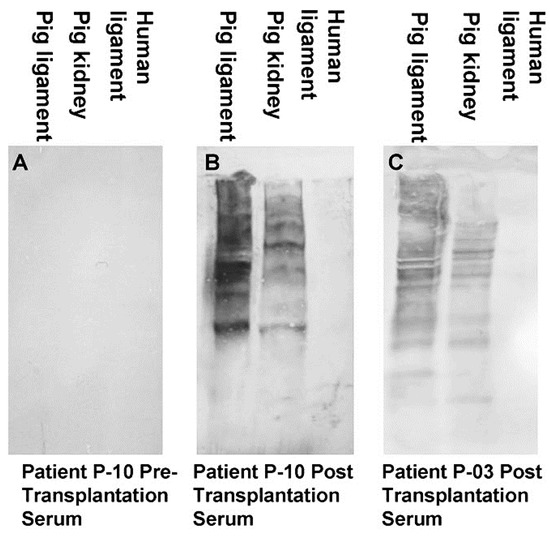 Figure 2. Anti-non gal antibody analysis by Western blots with porcine patellar-tendon and kidney proteins, or human patellar-tendon proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). (A) Pre-implantation serum of patient P-10. (B) Serum of patient P-10, six months post-implantation of porcine BTB. (C) Serum of patient P-03, six months post-implantation of porcine BTB. In this analysis, the sera were diluted 1:10, and depleted of anti-Gal by adsorption on glutaraldehyde fixed rabbit red blood cells (RBC) (modified from [71]).
Figure 2. Anti-non gal antibody analysis by Western blots with porcine patellar-tendon and kidney proteins, or human patellar-tendon proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). (A) Pre-implantation serum of patient P-10. (B) Serum of patient P-10, six months post-implantation of porcine BTB. (C) Serum of patient P-03, six months post-implantation of porcine BTB. In this analysis, the sera were diluted 1:10, and depleted of anti-Gal by adsorption on glutaraldehyde fixed rabbit red blood cells (RBC) (modified from [71]).