CProtons (H+) are highly reactive, they arboxylic ae always solvated or disolvated. In presence of water acids dissociate in water to carboxylates (R-COO-) and oxonium (an exothermic reaction into the acid-anion and H+[H2O]n) ions. Then. Proton Ttransport Cchain (PTC) hypothesis asserts that was developed for enzyme -complexes bridge nascent acids and ensure. The assumption that the enzyme-enzyme interaction is water-free transfer of the intermediate substrateentails that an acid synthesized from enzyme A is transferred as acid to enzyme B. The PTC -hypothesis entails that the concentration of thwas first discussed for the GAPDH-LDHm complex. GAPDH formed NADH-H+ is transferred to LDHm. The consequence of water-free transferred acid is mathematicallyr is that the concentration of NADH-H+ is infinite and a. An infinite concentration drives enzymatic reactions unidirectionally. In support of this, a number of enzymes, such as proton-linked monocarboxylate transporters (MCTs), lactate dehydrogenases (LDHs) or sodium/hydrogen exchangers have been experimentally determined to catalyse unidirectionally. In addition, enzyme complexes, such as the pyruvate dehydrogenase complex (PDHc), are also known to catalyse unidirectionally. Scientific drives the LDHm catalysed reaction. LDHm activity strictly depends on delivery (mol/s) of NADH-H+. The PTC hypothesis replaces the well-established concepts, such as the original Citric Acid Cycle proposed that acids are metabolized in a clockwise direction. The PTC hypothesis provides mechanisms, mathematics and law of nature for biological processes of (single) enzyme-kinetics by enzyme-complex kinetics. Quite well-known proteins complexes driven by PTC are: the pyruvatedehydrogenase complex (PDHc) or the citric acid cycle.
- PTC
- Citric Acid Cycle
- PDHc
- LDH
- GAPDH
- MCT
1. Background
Protons (H+) are completely removed from didactic biochemistry. A century ago, O. F. Meyerhof set glycolysis as the degradation of glucose (C
Protons (H+) are vanished from didactic biochemistry. A century ago, O.F. Meyerhof set glycolysis as the degradation of glucose (C
6
H
12
O
6) to two molecules lactic acid (2x C
) to two molecules lactic acid 2 (C
3
H
6
O
3)[1]. Today, the stoichiometry in the metabolism of one molecule of glucose to two molecules of lactic acid (lacH) has vanished from common understanding. In 1953, H. A. Krebs was honoured with the Noble Prize for the discovery of the Citric Acid Cycle. Krebs presented lacH as substrate of a cycle unidirectionally cycling carboxylic acids[2]. Today, the carboxylate pyruvate (pyr-) is considered as the substrate of a “Krebs citrate cycle”. The change from Meyerhof’s and Krebs’ scientifically based concepts to an alchemistic model of glucose metabolism started soon after their discoveries. In 1949 Kennedy and Lehninger investigated mitochondrial function on isolated mitochondria[3]. They used the term carboxylates and determined carboxylates such as, pyr-, malate, a-ketoglutarate and citrate. They discussed their data on basis of Krebs’ Citric Acid Cycle, but phrases such as: “…oxidation of pyruvate and other intermediates of the Krebs cycle.” and “…aerobic citrate formation from pyruvate when malate served as a source of oxaloacetate.” misquoted Krebs’ acid cycle. But, to say misquote is incorrect, because Kennedy and Lehninger failed to cite one Krebs’ manuscripts. Yet somehow, Lehningers’ models of ‘citrate cycles’ and ‘glycolysis’ have conquered the world.
) [1]. Today, the stoichiometry to metabolize glucose to two lactic acid is generally unknown. In 1953, H.A. Krebs was honoured with the Noble Prize for the discovery of the citric acid cycle. Krebs presented lactic acid as substrate of a cycle unidirectionally cycling acids [2]. Today, the acid-anion pyruvate was considered as substrate of Krebs “citrate cycle”. The change from Krebs scientifically based model to an alchemistic citrate cycle started quite early. In 1949 Kennedy and Lehninger investigated mitochondrial function on isolated mitochondria [3]. Kennedy and Lehninger used and determined carboxylates such as, pyruvate, malate, a-ketoglutarate and citrate. They discussed their data on basis of Krebs citric acid cycle, but phrases such as: “.oxidation of pyruvate and other intermediates of the Krebs cycle.” and “…aerobic citrate formation from pyruvate when malate served as a source of oxaloacetate.” misquoted Krebs acid cycle. But, misquote is incorrect, because Kennedy and Lehninger missed to quote one manuscript of Krebs. Lehningers “citrate cycle” has conquered the world.
During formulation of the PTC hypothesis, the knowledge of glucose metabolism was reset to the original work[4].
Kennedy and Lehninger also mentioned “Fluoride was added to inhibit enolase and the end-point measured manometrically indicated the formation of 3-phoshoglyceric acid, which causes CO2 liberation from bicarbonate”. In Lehningers textbooks 3-phoshoglyceric acid as product of phosphoglycerate kinase (PGK) catalysed reaction is simplified to 3-phosohoglycerate
2. Enzyme Complexes Driven by PTCs
The PTC hypothesis discussed plasma membrane located PGK in complex with proton-linked monocarboxylate transporter 4 (MCT4). Permanent delivery of 3-phoshoglyceric acid drives MCT4 to unidirectionally export monocarboxylic acids [4] [5] [6] [7].
PTCs have been investigated for decades. Unfortunately, by not adding H+ into the chemical formula and giving enzymes incorrect names, such as pyruvate dehydrogenase or pyruvate carboxylase, the concept of PTCs was largely hidden from the mainstream.
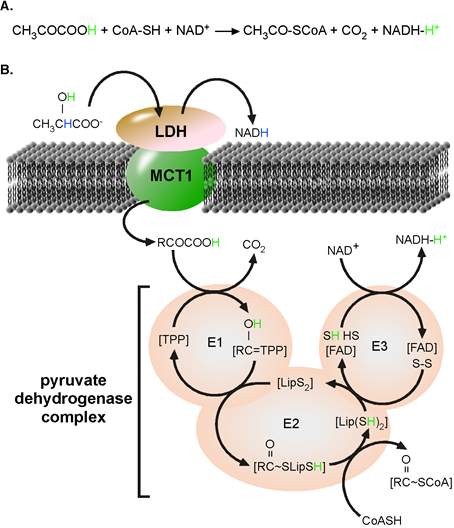
Pyruvate dehydrogenase complex (PDHc): PDHc together with a-ketoglutarate dehydrogenase belongs to the family of a-ketoacid dehydrogenases[5]. Pyruvic acid (pyrH) is the substrate of PDHc and proton-linked MCT1 is one transporter catalysing the transfer of pyr- and H+, pyrH to PDHc. The functional proton-linked MCT1•heart LDH (LDH-h) complex is located at the inner mitochondrial membrane[6]. The kinetics and reaction catalysed the proton-linked MCT has been investigated and published many times[7]. Similarly, PDHc catalyses a well-known chemical reaction[8]. In form of an acid, the a-keto group of pyrH is partially positively charged allowing a nucleophile substitution. The net reaction and mechanism of the unidirectional acting PDHc is depicted in Figure 1.
Figure 1. PDHc complex catalysed reaction. (A) Chemical formula for the overall reaction. (B) Mechanism of reaction catalyzed by the pyruvate dehydrogenase complex. LDH catalyzes the oxidation lactate to pyruvate. Nicotinamide adenine dinucleotide (NAD+) is reduced to NADH and a reactive H+, originally from the α-hydroxyl group of lactate (green). In the absence of water, pyruvate disolvates the new reactive H+ to form pyruvic acid. Monocarboxylate transporter 1 (MCT1) promotes the charge-neutral membrane transfer of pyruvic acid. Within the PDH complex, H+ from the α-hydroxyl group of lactate (green) takes active part in all steps of the reaction and finally ends disolvated by NADH. E1, pyruvate dehydrogenase; E2, dihydrolipoamide acetyltransferase; E3, dihydrolipoamide dehydrogenase; FAD, flavin adenine dinucleotide; Lip, Lipoyl; TPP, thiamine pyrophosphate. Image adapted from Roosterman et al. 2018[4].
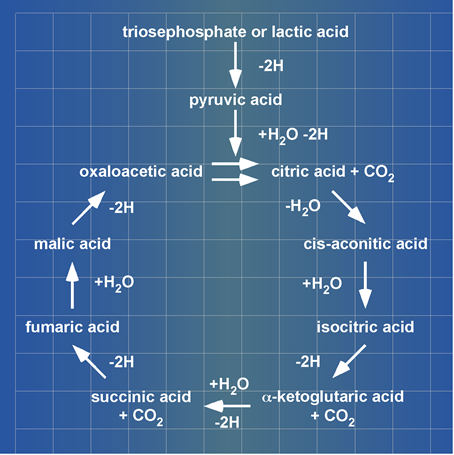
The Citric Acid Cycle enzyme complex: H. A. Krebs presented at his Noble Prize lecture lactic acid (lacH) as substrate and carboxylic acids being unidirectionally (clockwise) cycled (Figure 2).
Figure 2. A blueprint of Krebs’ Citric Acid Cycle. Krebs presented this cycle of carboxylic acids at his Noble Prize lecture in 1953. ©The Nobel Foundation[2].
Proton-linked MCT1•carbonic anhydrase II (CAII) enzyme complex: Proton-linked MCT1 has been characterized as a unidirectional importer of monocarboxylic acids and the PTC hypothesis provides the mechanism and rationale for this unidirectional transport[9]. The proton-linked MCT1•CAII complex functionally links the energy of permanently emitted carbonic acid (H
Plasma membrane located MCT1 is discussed in complex with carbonic anhydrase II (CAII). Permanent export of carbonic acid (H
2
CO
3) (the end product of oxidative phosphorylation) with the import of the substrate of oxidative phosphorylation, lacH[4]. Thus, the complex acts as a carboxylic acid/carbonic acid antiporter.
) drives MCT1 to unidirectionally import monocarboxylic acids [4] [5] [6] [7].
Proton-linked MCT4•phosphoglycerate kinase complex: Proton-linked MCT4 is also a unidirectional transporter, but acts as an exporter of monocarboxylic acids[10]. Similarly, here, the transfer of the energy entity H+, initiates the monocarboxylic acid transfer. As yet the definitive proton-donor enzyme producing the acid is unknown. However, the proton-donor enzyme must be located at the plasma membrane for direct (water-free) H+-transfer. An increase in the rate of glycolysis (metabolism of glucose to two molecules of lacH) is linked with an increased export of lacH, indicating that at least one product of the glycolytic enzymes has to be an acid. The development of the PTC hypothesis provoked reconsideration of not only the well-established chemical formula of glycolytic enzymes, but also the subcellular location of glycolytic enzymes. Intriguingly, phosphoglycerate kinase (PGK) can be located at the plasma membrane and the product of the PGK catalysed reaction is 3-phosphoglyceric acid[4]. Thus, a proton-linked MCT4•PGK complex was postulated to link the unidirectional export of monocarboxylic acids with the rate of glycolysis rate.
Mitochondrial membrane located LDHh-MCT1 was discussed to catalyse the first reaction of Krebs citric acid cycle: oxidation of lactate to pyruvate and membrane transfer of pyruvic acid to the PDHc complex.
Glyceraldehyde-3 phosphate dehydrogenase•muscle LDH complex: The unidirectional nature of this enzyme complex was discussed in the PTC hypothesis. The enzyme complex always catalyses the unidirectional transfer of NADH-H+ from GAPDH to muscle LDH (LDH-m) and reduces pyr- to lactate (lac-)[4][11]. Thus, lac- is always a product of glycolysis.
Mitochondrial membrane located LDHh-MCT1 was also introduced to pyruvic acid to pyruvate carboxylase as substrate of oxaloacetic acid synthesis [4] [5] [6] [7].
3. Proton Transport Chains Provide an Alternative View on Biological Processes
The PTC hypothesis questions and replaces most of what is considered to be well established in biochemistry:
Mathematics: The mathematics of enzyme kinetics understands enzymes to reversibly catalyse an equilibrium depending on concentration [mol/L]. The deduced mathematics of enzyme complex kinetics understands enzyme complexes to catalyse unidirectionally depending on the provision of the acid (mol/s).
Laws of nature: Maximal entropy, or random distribution and movement of enzymes, substrates and products are the premise to apply the mathematics of enzyme kinetics[12]. The PTC hypothesis is based on the diametrical opposite of maximal entropy; ideal organized systems. The PTC hypothesis inspired the transfer of the 4
The proposed citric acid cycle 1.1 balances burning of lactic acid and malic acid synthesis. Citric acid cycle 1.1 provides the mechanism of the data O.F. Meyerhof presented at his Noble Prize lecture: up-taken lactic acid is burned as well used as building block [8]. Citric acid cycle 1.1 illustrates the molecular mechanism for: synthesis is the product of degradation and thereby the transfer of the 4
th law of thermodynamics from physics to biological processes[13]. The 4th law asserts that the flow of energy and material is sufficient to form ordered structures[14]. Whereas maximal entropy ignores ordered structures such as enzyme complexes, metabolons, organelles, membranes, cells, organism, biology in general; the 4th law incorporates these and provides a path to self-organization.
law of thermodynamics to biologic process [9]. Textbooks, such as A. Lehningers Biochemistry, propagate maximal entropy and thereby exclude ordered structures, such as membranes, enzyme-complexes, metabolons, organelles, cells, brief biologic systems, the PTC hypothesis .
Definitions of glucose metabolism: Glycolysis was defined as cytosolic process, starting with hexokinase II as first enzyme and pyr- as final product. This definition was replaced by considering glycolysis as a flow of energy and material (mol/s). Glucose transporters (GLUTs) are set as first enzymes and proton-linked MCT4•PGK as final enzyme complex. The definition that glucose metabolism provides metabolites to be “burned” and used as building blocks was expanded by setting glucose metabolism as the operating system of the cell and ‘major in command’. Metabolites are not simply intermediates but are defined as signalling molecules. Glucose and its metabolites not only promote the release of insulin, but also the release of a variety of cytokines, interleukins and growth factors[15]. Thus, hormones and transmitters are set as ‘second in command’. Unidirectionally importing or exporting proton-linked MCT complexes are classified as metabolic receiver- or sender-complexes, respectively[16]. Glycogen stores are considered not only as stores of energy, but also as stores of metabolic signalling molecules.
4. History of Deleting Protons in Biochemistry
As mentioned above misquoting pioneering work on glucose metabolism and consequently refusing to integrate scientific progress in a didactic model of glucose metabolism has a long history. The huge variety of ‘Krebs’ Citrate Cycles’ propagated in textbooks and learned by rota have nothing in common with Krebs’ scientifically based concept of a Citric Acid Cycle. The high similarity between Kennedy and Lehninger’s changes of Krebs’ work and the actually propagated models of “Krebs’ citrate cycles” strongly suggests that Krebs’ work belongs to the most often quoted, but never read and never referred to publications in history[3]. The 70-year-old original Citric Acid Cycle merged the PDHc-catalysed formation of acetyl-SCoA and the citrate synthase-catalysed reaction into one step. Krebs formulated the reaction as follows (see Figure 2): pyruvic acid + oxaloacetic acid + water ® citric acid + 2H + CO2. A. Lehninger’s textbook Biochemistry also mentioned this point, but detailing the reaction as: pyruvate + oxaloacetate ® citrate + CO2. [12].
It must be mentioned, that both Krebs and Meyerhof experimentally determined carboxylates and both knew that acids dissociate in water and enzymes act reversibly, but interestingly both created concepts with carboxylic acids and unidirectionally acting enzymes. The key to their scientifically based concepts, which is different to the experimentally gained data, is applying scientific rules, such as the principle of mass conservation or stoichiometry.
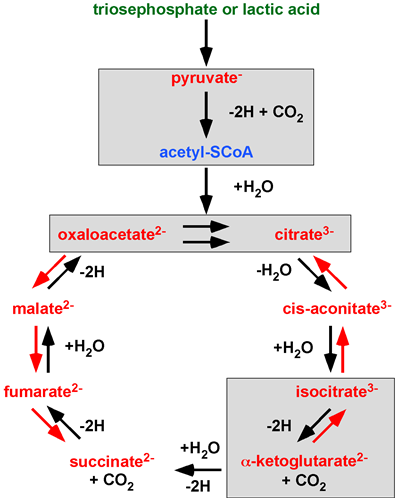
Actually, every single chemical reaction published by H. A. Krebs is incorrectly transferred when a ‘Krebs’ citrate cycle’ is introduced. Krebs aimed to investigate and understand the scientific field of metabolism. All of Krebs’ work misunderstood by A. Lehninger is highlighted in Figure 3. The PTC hypothesis works with the original concept of the Citric Acid Cycle, a unidirectional cycle of carboxylic acids. The PTC hypothesis, by providing the missing rationale (enzyme complexes and water-free transfer), mathematics (infinite concentration) and the law of nature the PTC hypothesis gives Krebs’ Citric Acid Cycle a second chance.
Figure 3. A ‘Krebs’ citrate cycle’ variant found in textbooks, scientific publications and metabolic databanks. 70 years of deletions (green), additions (blue) and integrated changes (red) to Krebs’ Citric Acid Cycle. Chemical reactions showing alchemy or incorrect stoichiometry are highlighted in grey boxes. Image adapted from Krebs’ Noble Prize lecture in 1953. ©The Nobel Foundation.
Lehninger has changed the work of O. F. Meyerhof and H. A. Krebs and the work of many more scientists into bio-alchemy. Kennedy and Lehninger wrote: “…it has been a general finding that the more highly organized enzyme systems of animal tissue responsible for oxidation of metabolites by molecular oxygen.”[3]. Thus, Lehninger published ‘the general finding of highly organized enzyme system’, but in his textbook Biochemistry, the diametral opposite is presented. The advantage of disorganization is propagated and stressed in the box “Entropy” [12]. The propagated mathematics/understanding of Lehninger’s citrate cycle, based on changes in concentration, allowed the mis-incorporation of reversibly acting enzymes. In 1951, D. E. Green summarized “…intermediates do not accumulate during the normal activity of the cyclophorase system… (Citric Acid Cycle)”[17]. In other words, 70 years ago, it was known that the amount of carboxylic acids in the Citric Acid Cycle complex is constant and that a mathematics based on equilibration reaction and changes in concentration is not fit for biological processes, such as the Citric Acid Cycle. On the basis of the PTC hypothesis, the redox-mechanism “metabolic traffic jam” was developed[18]. The metabolic traffic jam ‘pushes’ carboxylic acids out of the complex when a new carboxylic acid is actively transferred into the complex. By doing so, intermediates do not accumulate.
In order to regain stoichiometry in the well-established chemical formulae of glycolysis, it was postulated that 3-phosphoglyceric acid is the product of PGK. 70 years ago, the stoichiometry to metabolize glucose to two molecules lacH was known. Even Kennedy and Lehninger wrote: “Fluoride was added to inhibit enolase and the end-point measured manometrically indicated the formation of 3-phosphoglyceric acid, which causes CO2 liberation from bicarbonate”. Yet, in Lehninger’s textbook Biochemistry the product of the phosphoglycerate kinase (PGK) catalysed reaction was set 3-phosphoglycerate, a deliberate change from science to alchemy.
5. Integrating the PTC Hypothesis in Actual Bioscience
Maps of metabolic pathways are reminiscent of a railway map. Every molecule is connected to metabolic pathways. Point to one molecule on the map, and we know how the molecule is degraded and synthesized. Such maps give the impression that metabolism is well understood. For example, the effects of insulin on an organism and the signalling pathways of insulin have been investigated for a century; these are well understood. Insulin accelerates the processing of glucose to glucose-1 phosphate and lacH to store excess energy as glycogen and lipids, respectively.
Metabolic maps provide the destination and insulin is the ticket for this route. The PTC hypothesis introduced metabolic switches (enzyme complexes) setting the route and the energy (H+), driving material in one direction.
The PTC hypothesis defined nascent acids as energy carriers driving one metabolic pathway. Enzyme complexes are metabolic switches. The switch-stand GAPDH•LDH-m drives the material in one metabolic pathway, the switch-stand GAPDH•glycerol-3 phosphate dehydrogenase in another metabolic pathway. The same is true for the plasma membrane-located PGK and cytosolic-located PGK.
Malfunctioning switch-stands are linked to obesity, heart failure, major depression, chronic inflammation and many more conditions. Genetic variation and/or environmental predisposition are linked to the inaccurate building of the metabolic flow of energy and material between cells. Briefly, the PTC hypothesis provides mechanisms, mathematics and law of nature for biological processes.
- Meyerhof O. RECENT INVESTIGATIONS ON THE AEROBIC AND AN-AEROBIC METABOLISM OF CARBOHYDRATES. J Gen Physiol. 1927;8: 531–542. doi:10.1085/jgp.8.6.531
- The Nobel Prize in Physiology or Medicine 1953. In: NobelPrize.org [Internet]. [cited 11 Nov 2020]. Available: https://www.nobelprize.org/prizes/medicine/1953/krebs/lecture/
- Kennedy EP. and Lehninger AL. Oxidation of fatty acids and tricarboxylic acid intermediates by isolated rat mitochondria. JBC 1949 Available: https://www.jbc.org/content/179/2/957.full.pdf
- Roosterman D, Meyerhof W, Cottrell GS. Proton Transport Chains in Glucose Metabolism: Mind the Proton. Front Neurosci. 2018;12. doi:10.3389/fnins.2018.00404
- Roosterman D, Cottrell GS. The two-cell model of glucose metabolism: a hypothesis of schizophrenia. Mol Psychiatry. 2021. doi:10.1038/s41380-020-00980-4
- Roosterman D, Cottrell GS. Rethinking the Citric Acid Cycle: Connecting Pyruvate Carboxylase and Citrate Synthase to the Flow of Energy and Material. Int J Mol Sci. 2021;22. doi:10.3390/ijms22020604
- Roosterman D, Cottrell GS. Astrocytes and neurons communicate via a monocarboxylic acid shuttle. AIMS Neurosci. 2020;7: 94–106. doi:10.3934/Neuroscience.2020007
- The Nobel Prize in Physiology or Medicine 1922. In: NobelPrize.org [Internet]. [cited 25 Dec 2020]. Available: https://www.nobelprize.org/prizes/medicine/1922/summary/
- Glansdorff P, Prigogine I. Thermodynamic Theory of Structure, Stability and Fluctuations. London, New York: John Wiley & Sons Ltd; 1971.