Absolute uterine factor infertility (AUFI) includes congenital uterine malformation and defects, such as Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome, which occurs in one in 5000 women; acquired uterine defects caused by treatment of uterine cancers or hysterectomy due to puerperal bleeding; extended uterine myomatosis; and Asherman’s syndrome, in which the endometrium is adhered.
- uterus transplantation
- living donor surgery
- laparotomy
- laparoscopy
- robot assisted
- uterine vein
- ovarian vein
- utero-ovarian vein
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Absolute uterine factor infertility (AUFI) includes congenital uterine malformation and defects, such as Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome [1], which occurs in one in 5000 women; acquired uterine defects caused by treatment of uterine cancers or hysterectomy due to puerperal bleeding; extended uterine myomatosis; and Asherman’s syndrome, in which the endometrium is adhered [2].
A new transplantation technique, uterine transplantation (UTx), has been clinically applied in recent years for the treatment of AUFI. UTx was first performed in Saudi Arabia in 2000 [3]. Although the world’s first UTx failed with the removal of a transplanted uterus, basic research using animal models was continued, and in 2014, a Swedish team reported the first live birth after UTx [4]. Since then, UTx has been applied clinically in many countries, and there have been some reports of live births from women who have undergone UTx [5].
However, there are medical, ethical, and social challenges to UTx. One of the medical challenges is the highly invasive procedure for living donors. In UTx living-donor surgery, the uterine artery is usually used for the arterial vessel, but there are several venous options. The uterine vein (UV), a branch of the internal iliac vein, is widely used [6], as by the Swedish team that obtained the first live birth after UTx. When the UV is used, the surgical operation is similar to radical hysterectomy. As the surgical isolation of the UV is performed in a narrow and deep area of the pelvis and there is a complex network of vessels, the procedure is sometimes difficult, resulting in longer surgical time and massive hemorrhage. In addition, as the procedure is performed near the hypogastric nerve, there is a risk of postoperative complications such as dysuria in the living donor [7].
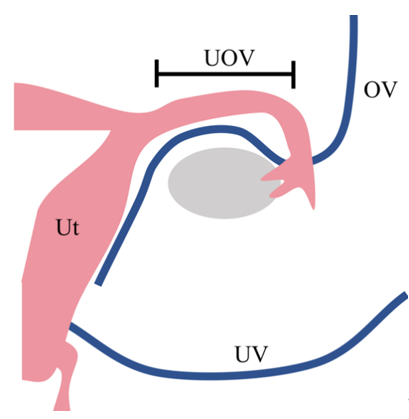
To solve this problem, the use of ovarian veins (OV) and utero-ovarian veins (UOV) as drainage veins has been investigated (Figure 1) [8]. When these veins are used, the surgical technique is easier because the vessels to be preserved are in a more superficial layer than when the UV is preserved. In addition, UTx living-donor surgery was initially performed using an open approach, but recently there have been reports of laparoscopic [9] and robot-assisted approaches [10] for donor surgery.
Figure 1. Drainage vein options for uterus transplantation.
In many cases of uterus transplantation performed to date, the uterine veins from the internal iliac vein are used as drainage veins. However, this surgery is challenging because these vessels are located in the deep pelvic floor and surround the ureter. To minimise the invasiveness of living-donor surgery, the use of the ovarian vein or the utero-ovarian vein—which runs continuously from the ovarian vein through the mesosalpinx—as the drainage vein, has been considered as an alternative to the use of the uterine vein. Ut, uterus; UV, uterine vein; UOV, utero-ovarian vein; OV, ovarian vein
In many cases of UTx performed to date, the uterine veins from the internal iliac vein are used as drainage veins. However, this surgery is challenging because these vessels are located in the deep pelvic floor and surround the ureter. To minimize the invasiveness of living-donor surgery, the use of the ovarian vein or the utero-ovarian vein—which runs continuously from the ovarian vein through the mesosalpinx—as the drainage vein, has been considered as an alternative to the use of the uterine vein (Ut, uterus; UV, uterine vein; UOV, utero-ovarian vein; OV, ovarian vein).
2. Materials and Methods
2.1. Search Strategy
A thorough search of the PubMed database was conducted. The search was not limited by language or date of publication. The search strategies were as follows: (uterus[Title/Abstract] OR uterine[Title/Abstract] OR womb[Title/Abstract]) AND (transplantation OR transplant) AND (“surgery”[Title/Abstract] OR operation[Title/Abstract] OR laparoscopy[Title/Abstract] OR laparoscopic[Title/Abstract] OR robot[Title/Abstract] OR robotic[Title/Abstract] OR laparotomy[Title/Abstract] OR vein[Title/Abstract] OR veins[Title/Abstract] OR venous[Title/Abstract] OR anastomosis[Title/Abstract] OR ovarian[Title/Abstract] OR utero-ovarian [Title/Abstract] OR utero-ovarian[Title/Abstract] OR living[Title/Abstract] OR donor[Title/Abstract] OR livebirth[Title/Abstract] OR live-birth[Title/Abstract] OR human). The data were collected on 13 October 2020.
2.2. Eligibility Assessment
Two reviewers (Y.M. and I.K.) independently assessed each article and determined eligibility for inclusion in the review article. Inclusion criteria were English peer-reviewed articles reporting one of the following: (i) surgical information (operative approach, surgical time, blood loss, types and numbers of veins, and operative complications); or (ii) postoperative course (discharge timing, graft failure, and live birth after UTx). Articles regarding animal research on UTx, UTx on deceased donors, not original articles (video article, review, letter to the editor, commentary, and editorial), not written in English, or that did not report the information above were excluded.
2.3. Data Extraction and Analysis
The included studies were reviewed by two independent reviewers (Y.M. and I.K.), and relevant data were extracted including the number of performed human UTx cases, surgical approach of living-donor surgery (open approach, laparoscopic approach, or robot-assisted approach), surgical time, blood loss during donor surgery, the types and numbers of removed veins (UV, UOV, or OV), operative complications, discharge timing, and live birth after UTx.
The data were classified into open, laparoscopic, and robot-assisted approaches for analysis.
The data were also classified and analyzed according to whether the UV was removed within each approach.
3. Results
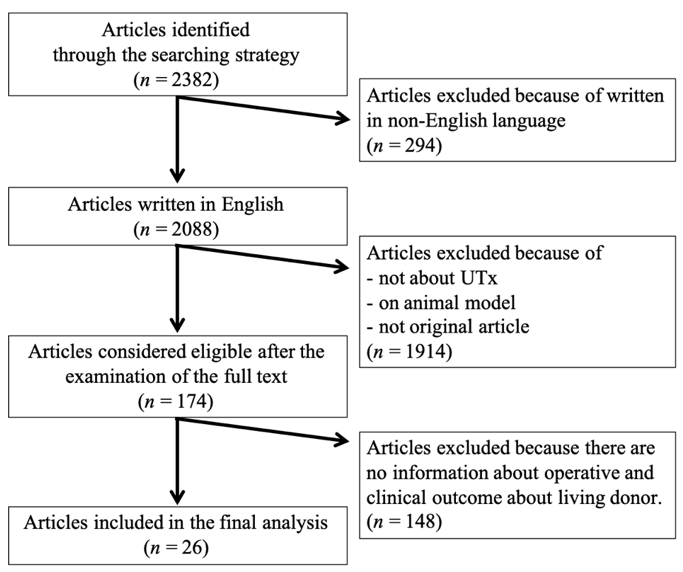
This review included 26 original articles (Figure 2). Reports of living-donor uterus transplants from Saudi Arabia [3], Sweden [4][6][11][12][13][14][15][16][17][18][19][4,6,11–19], China [10][20][10,20], USA (Dallas) [8][21][22][23][24][8,21–24], Czech Republic [7][25][26][7,25,26], Germany [27][28][27,28], and India [9][29][9,29] were identified, and 51 living-donor UTx were incorporated. The surgical information and clinical data for each case are shown in Table 1. In one case in Germany, the uterus was removed from a donor, but was found to be unsuitable for transplantation during back table processing, and the transplant was not performed. In another case, the uterine veins were not used for transplantation, even though they were preserved and removed from the donor in a Czech case.
Figure 2. Flowchart of article selection.
On 13 October 2020, an article search was conducted on PubMed according to the search strategy. Of 2382 articles, 26 original articles were finally included in the review. They include the operative and clinical outcome data of the UTx living donor. UTx, uterine transplantation
Table 1.
Reported operative and clinical data of living-donor surgery for uterus transplantation.
|
Country |
Operation |
No. |
Surgical Time (h:min) |
Blood Loss (mL) |
Preserved Vein |
Graft Failure |
Operative Complications (Grade*) |
Discharge |
Live Birth |
Remarks |
|
Saudi Arabia [3] |
OPEN |
1 |
N/R |
N/R |
2×UV |
Yes |
Intraoperative ureteric injury (N/R) |
N/R |
N/A |
|
|
Sweden [4][6][11][12][13][14][15][16][17][18][19] |
OPEN |
1 |
10:54 |
300 |
2×UV, 1×UOV |
No |
Nocturia (1) |
6POD |
Yes×2 |
|
|
OPEN |
2 |
12:37 |
2400 |
2×UV, 1×UOV |
Yes |
Wound infection (2) |
6POD |
N/A |
||
|
OPEN |
3 |
12:53 |
800 |
2×UV, 1×UOV |
No |
None |
6POD |
no |
||
|
OPEN |
4 |
10:34 |
600 |
2×UV, 1×UOV |
No |
Unilateral sensibility impairment of the thigh (1) |
6POD |
Yes×2 |
||
|
OPEN |
5 |
10:17 |
600 |
2×UV |
No |
None |
6POD |
Yes×1 |
||
|
OPEN |
6 |
10:52 |
700 |
2×UV, 1×UOV |
No |
None |
6POD |
Yes×2 |
||
|
OPEN |
7 |
10:17 |
400 |
2×UV, 1×UOV |
No |
None |
6POD |
Yes×1 |
||
|
OPEN |
8 |
11:23 |
400 |
2×UV |
No |
None |
6POD |
Yes×1 |
||
|
OPEN |
9 |
13:08 |
2100 |
2×UV |
Yes |
None |
6POD |
N/A |
||
|
ROBOT |
1 |
13:00 |
600 |
2×UV, 1×UOV |
No |
None |
N/R |
N/R |
||
|
ROBOT |
2 |
12:30 |
400 |
2×UV, 1×UOV |
No |
Gluteal light pain when walking (N/R) |
5POD |
Yes |
||
|
ROBOT |
3 |
11:30 |
N/R |
2×UV, 2×UOV |
Yes |
N/R |
N/R |
N/A |
||
|
ROBOT |
4 |
12:30 |
N/R |
2×UV, 1×UOV |
No |
Pressure alopecia (2) |
N/R |
N/R |
||
|
ROBOT |
5 |
11:30 |
N/R |
2×UV, 2×UOV |
No |
N/R |
N/R |
N/R |
||
|
ROBOT |
6 |
11:30 |
N/R |
2×UV, 2×UOV |
No |
N/R |
N/R |
N/R |
||
|
ROBOT |
7 |
11:30 |
N/R |
2×UV, 2×UOV |
No |
N/R |
N/R |
N/R |
||
|
ROBOT |
8 |
10:00 |
N/R |
2×UV, 1×UOV |
Yes |
Pyelonephritis (3b) |
N/R |
N/A |
||
|
ROBOT |
1 |
6:00 |
100 |
2×OV |
No |
None |
5POD |
Yes |
||
|
US (Dallas) [8][21][22][23][24] |
OPEN |
1 |
5:45 |
400 |
1×UV, 1×UOV |
Yes |
Leg/buttocks pain (1) |
6POD |
N/A |
|
|
OPEN |
2 |
7:21 |
1000 |
1×UV, 1×UOV |
Yes |
UTI (2) |
6POD |
N/A |
||
|
OPEN |
3 |
6:41 |
1300 |
1×UV, 1×UOV |
Yes |
Vaginal cuff dehiscence (3b) Depression (2), UTI (2) |
6POD |
N/A |
||
|
OPEN |
4 |
6:40 |
1700 |
2×UOV |
No |
UTI (2) |
5POD |
Yes |
||
|
OPEN |
5 |
6:34 |
250 |
2×UOV |
No |
Faecal impaction (3b) |
7POD |
Yes |
||
|
OPEN |
6 |
7:07 |
1100 |
1×UV, 1×UOV |
No |
Acute blood loss anaemia (2) |
5POD |
Yes |
||
|
OPEN |
7 |
6:38 |
600 |
2×UV |
No |
UTI (2) |
5POD |
Yes |
||
|
OPEN |
8 |
6:12 |
400 |
2×UOV |
Yes |
None |
6POD |
N/A |
||
|
OPEN |
9 |
7:34 |
750 |
1×UV, 1×UOV |
No |
Symptomatic anaemia (2), UTI (2) |
5POD |
Yes |
||
|
OPEN |
10 |
6:27 |
1500 |
2×UV |
No |
Acute blood loss anaemia (4a) Prolonged intubation (4a), UTI (2) |
8POD |
N/R |
||
|
OPEN |
11 |
5:33 |
600 |
2×UOV |
No |
None |
5POD |
Yes |
||
|
OPEN |
12 |
5:13 |
950 |
1×UV, 2×UOV |
Yes |
Haemorrhage (N/R) |
4POD |
N/A |
||
|
OPEN |
13 |
6:10 |
800 |
1×UV, 1×UOV |
No |
UTI (2) |
6POD |
Yes |
Not anastomosed UV |
|
|
ROBOT |
1 |
9:25 |
150 |
1×UV, 2×UOV |
No |
Temporary alopecia (1) |
4POD |
N/R |
Not anastomosed UV |
|
|
ROBOT |
2 |
10:48 |
100 |
1×UV, 2×UOV |
N/R |
Ureteral blood clot (3b) |
6POD |
N/R |
||
|
ROBOT |
3 |
12:10 |
200 |
1×UV, 2×UOV |
N/R |
Bilateral ureteral injury (3b) |
3POD |
N/R |
Not anastomosed UV |
|
|
ROBOT |
4 |
9:27 |
20 |
2×UV, 2×UOV |
N/R |
None |
4POD |
N/R |
There were 2 left UOV |
|
|
ROBOT |
5 |
12:03 |
100 |
3×UOV |
N/R |
None |
3POD |
N/R |
||
|
OPEN |
1 |
5:20 |
100 |
2×UV, 2×OV |
No |
None |
7POD |
N/R |
Not anastomosed UV |
|
|
OPEN |
2 |
6:10 |
800 |
2×UV, 2×OV |
No |
None |
7POD |
N/R |
Not anastomosed UV |
|
|
OPEN |
3 |
7:10 |
100 |
2×UV, 2×OV |
No |
Climacteric symptoms (N/R) |
6POD |
N/R |
Not anastomosed UV |
|
|
OPEN |
4 |
5:30 |
100 |
2×UV, 2×OV |
Yes |
Bladder hypotonia (3a) |
11POD |
N/A |
Not anastomosed OV |
|
|
OPEN |
5 |
5:30 |
1000 |
2×UV, 2×OV |
No |
Ureter laceration (3a) |
9POD |
Yes |
||
|
OPEN |
1 |
12:07 |
100 |
2×UV |
No |
None |
11days† |
Yes |
||
|
OPEN |
2 |
13:06 |
N/R |
UV‡ |
N/A |
Hydronephrosis (3b) |
N/R |
N/A |
No transplantation performed |
|
|
OPEN |
3 |
9:03 |
100 |
1×UV, 1×OV |
No |
None |
12days† |
Yes |
||
|
OPEN |
4 |
10:24 |
100 |
2×UV, 1×UOV |
No |
None |
14days† |
N/A |
||
|
OPEN |
5 |
9:11 |
100 |
2×UV, 2×UOV |
No |
None |
14days† |
N/A |
||
|
LAP |
1 |
4:00 |
100 |
1×or 2×UV, 2×OV§ |
No |
None |
7POD |
N/R |
||
|
LAP |
2 |
4:00 |
100 |
1×or 2×UV, 2×OV§ |
No |
None |
7POD |
N/R |
||
|
LAP |
3 |
2:40 |
100 |
2×OV |
No |
None |
6POD |
N/R |
||
|
LAP |
4 |
3:20 |
100 |
2×OV |
No |
None |
6POD |
N/R |
|
Country |
Operation |
No. |
Surgical Time (h:min) |
Blood Loss (mL) |
Preserved Vein |
Graft Failure |
Operative Complications (Grade*) |
Discharge |
Live Birth |
Remarks |
|
Saudi Arabia [3] |
OPEN |
1 |
N/R |
N/R |
2×UV |
Yes |
Intraoperative ureteric injury (N/R) |
N/R |
N/A |
|
|
Sweden [4,6,11–19] |
OPEN |
1 |
10:54 |
300 |
2×UV, 1×UOV |
No |
Nocturia (1) |
6POD |
Yes×2 |
|
|
OPEN |
2 |
12:37 |
2400 |
2×UV, 1×UOV |
Yes |
Wound infection (2) |
6POD |
N/A |
||
|
OPEN |
3 |
12:53 |
800 |
2×UV, 1×UOV |
No |
None |
6POD |
no |
||
|
OPEN |
4 |
10:34 |
600 |
2×UV, 1×UOV |
No |
Unilateral sensibility impairment of the thigh (1) |
6POD |
Yes×2 |
||
|
OPEN |
5 |
10:17 |
600 |
2×UV |
No |
None |
6POD |
Yes×1 |
||
|
OPEN |
6 |
10:52 |
700 |
2×UV, 1×UOV |
No |
None |
6POD |
Yes×2 |
||
|
OPEN |
7 |
10:17 |
400 |
2×UV, 1×UOV |
No |
None |
6POD |
Yes×1 |
||
|
OPEN |
8 |
11:23 |
400 |
2×UV |
No |
None |
6POD |
Yes×1 |
||
|
OPEN |
9 |
13:08 |
2100 |
2×UV |
Yes |
None |
6POD |
N/A |
||
|
ROBOT |
1 |
13:00 |
600 |
2×UV, 1×UOV |
No |
None |
N/R |
N/R |
||
|
ROBOT |
2 |
12:30 |
400 |
2×UV, 1×UOV |
No |
Gluteal light pain when walking (N/R) |
5POD |
Yes |
||
|
ROBOT |
3 |
11:30 |
N/R |
2×UV, 2×UOV |
Yes |
N/R |
N/R |
N/A |
||
|
ROBOT |
4 |
12:30 |
N/R |
2×UV, 1×UOV |
No |
Pressure alopecia (2) |
N/R |
N/R |
||
|
ROBOT |
5 |
11:30 |
N/R |
2×UV, 2×UOV |
No |
N/R |
N/R |
N/R |
||
|
ROBOT |
6 |
11:30 |
N/R |
2×UV, 2×UOV |
No |
N/R |
N/R |
N/R |
||
|
ROBOT |
7 |
11:30 |
N/R |
2×UV, 2×UOV |
No |
N/R |
N/R |
N/R |
||
|
ROBOT |
8 |
10:00 |
N/R |
2×UV, 1×UOV |
Yes |
Pyelonephritis (3b) |
N/R |
N/A |
||
|
China [10,20] |
ROBOT |
1 |
6:00 |
100 |
2×OV |
No |
None |
5POD |
Yes |
|
|
US (Dallas) [8,21–24] |
OPEN |
1 |
5:45 |
400 |
1×UV, 1×UOV |
Yes |
Leg/buttocks pain (1) |
6POD |
N/A |
|
|
OPEN |
2 |
7:21 |
1000 |
1×UV, 1×UOV |
Yes |
UTI (2) |
6POD |
N/A |
||
|
OPEN |
3 |
6:41 |
1300 |
1×UV, 1×UOV |
Yes |
Vaginal cuff dehiscence (3b) Depression (2), UTI (2) |
6POD |
N/A |
||
|
OPEN |
4 |
6:40 |
1700 |
2×UOV |
No |
UTI (2) |
5POD |
Yes |
||
|
OPEN |
5 |
6:34 |
250 |
2×UOV |
No |
Faecal impaction (3b) |
7POD |
Yes |
||
|
OPEN |
6 |
7:07 |
1100 |
1×UV, 1×UOV |
No |
Acute blood loss anaemia (2) |
5POD |
Yes |
||
|
OPEN |
7 |
6:38 |
600 |
2×UV |
No |
UTI (2) |
5POD |
Yes |
||
|
OPEN |
8 |
6:12 |
400 |
2×UOV |
Yes |
None |
6POD |
N/A |
||
|
OPEN |
9 |
7:34 |
750 |
1×UV, 1×UOV |
No |
Symptomatic anaemia (2), UTI (2) |
5POD |
Yes |
||
|
OPEN |
10 |
6:27 |
1500 |
2×UV |
No |
Acute blood loss anaemia (4a) Prolonged intubation (4a), UTI (2) |
8POD |
N/R |
||
|
OPEN |
11 |
5:33 |
600 |
2×UOV |
No |
None |
5POD |
Yes |
||
|
OPEN |
12 |
5:13 |
950 |
1×UV, 2×UOV |
Yes |
Haemorrhage (N/R) |
4POD |
N/A |
||
|
OPEN |
13 |
6:10 |
800 |
1×UV, 1×UOV |
No |
UTI (2) |
6POD |
Yes |
Not anastomosed UV |
|
|
ROBOT |
1 |
9:25 |
150 |
1×UV, 2×UOV |
No |
Temporary alopecia (1) |
4POD |
N/R |
Not anastomosed UV |
|
|
ROBOT |
2 |
10:48 |
100 |
1×UV, 2×UOV |
N/R |
Ureteral blood clot (3b) |
6POD |
N/R |
||
|
ROBOT |
3 |
12:10 |
200 |
1×UV, 2×UOV |
N/R |
Bilateral ureteral injury (3b) |
3POD |
N/R |
Not anastomosed UV |
|
|
ROBOT |
4 |
9:27 |
20 |
2×UV, 2×UOV |
N/R |
None |
4POD |
N/R |
There were 2 left UOV |
|
|
ROBOT |
5 |
12:03 |
100 |
3×UOV |
N/R |
None |
3POD |
N/R |
||
|
Czech [7,25,26] |
OPEN |
1 |
5:20 |
100 |
2×UV, 2×OV |
No |
None |
7POD |
N/R |
Not anastomosed UV |
|
OPEN |
2 |
6:10 |
800 |
2×UV, 2×OV |
No |
None |
7POD |
N/R |
Not anastomosed UV |
|
|
OPEN |
3 |
7:10 |
100 |
2×UV, 2×OV |
No |
Climacteric symptoms (N/R) |
6POD |
N/R |
Not anastomosed UV |
|
|
OPEN |
4 |
5:30 |
100 |
2×UV, 2×OV |
Yes |
Bladder hypotonia (3a) |
11POD |
N/A |
Not anastomosed OV |
|
|
OPEN |
5 |
5:30 |
1000 |
2×UV, 2×OV |
No |
Ureter laceration (3a) |
9POD |
Yes |
||
|
Germany [27,28] |
OPEN |
1 |
12:07 |
100 |
2×UV |
No |
None |
11days† |
Yes |
|
|
OPEN |
2 |
13:06 |
N/R |
UV‡ |
N/A |
Hydronephrosis (3b) |
N/R |
N/A |
No transplantation performed |
|
|
OPEN |
3 |
9:03 |
100 |
1×UV, 1×OV |
No |
None |
12days† |
Yes |
||
|
OPEN |
4 |
10:24 |
100 |
2×UV, 1×UOV |
No |
None |
14days† |
N/A |
||
|
OPEN |
5 |
9:11 |
100 |
2×UV, 2×UOV |
No |
None |
14days† |
N/A |
||
|
India [9,29] |
LAP |
1 |
4:00 |
100 |
1×or 2×UV, 2×OV§ |
No |
None |
7POD |
N/R |
|
|
LAP |
2 |
4:00 |
100 |
1×or 2×UV, 2×OV§ |
No |
None |
7POD |
N/R |
||
|
LAP |
3 |
2:40 |
100 |
2×OV |
No |
None |
6POD |
N/R |
||
|
LAP |
4 |
3:20 |
100 |
2×OV |
No |
None |
6POD |
N/R |
*Clavien-Dindo classification; †Hospital stay; ‡Number of removed UV not reported; §Used 1 x UV on No. 1 or No. 2 case. OPEN, open approach; N/R, not reported; UV, uterine vein; N/A, not applicable; UOV, utero-ovarian vein; POD, postoperative day; ROBOT, robot-assisted approach; OV, ovarian vein; UTI, urinary tract infection; LAP, laparoscopic approach.
Of the 51 living-donor UTx cases, the open approach was used in 33 cases, the laparoscopic approach in four cases, and the robot-assisted approach in 14 cases. The data of each approach are summarized in Table 2. The average operative time was 8 h 26 min ± 2 h 47 min for the open approach, 3 h 30 min ± 0 h 33 min for the laparoscopic approach, and 10 h 59 min ± 1 h 45 min for the robot-assisted approach, with a trend toward shorter operative times for the laparoscopic approach and longer operative times for the robot-assisted approach. The mean blood loss was 715 ± 584 mL with the open approach, 100 ± 0 mL with the laparoscopic approach, and 209 ± 182 mL with the robot-assisted approach, with a trend toward less blood loss with minimally invasive procedures, such as the laparoscopic and robot-assisted approaches. The day of discharge was 6.2 ± 1.3 postoperative days on average with the open approach, 6.5 ± 0.5 days postoperatively with the laparoscopic approach, and 4.3 ± 1.0 days postoperatively with the robot-assisted approach. There were 19 surgical complications with the open approach (57.6%), zero with the laparoscopic approach (0.0%), and six with the robot-assisted approach (42.9%). There were nine cases (28.1%) of graft failure in open approach, zero cases (0.0%) on the laparoscopic approach, and two cases (14.3%) in the robot-assisted approach. Live birth after living-donor UTx was reported in 16 cases (48.5%) with the open approach, zero cases (0.0%) with the laparoscopic approach, and two cases (14.3%) with the robot-assisted approach.
Table 2.
Operative and clinical data for each operative approach with or without using the uterine vein.
|
OPEN |
LAP |
ROBOT |
|||||||
|
UV (+) |
UV (-) |
Total |
UV (+) |
UV (-) |
Total |
UV (+) |
UV (-) |
Total |
|
|
n |
29 |
4 |
33 |
2 |
2 |
4 |
12 |
2 |
14 |
|
Surgical time (h:min)* |
8:45 ± 2:39 |
6:14 ± 0.26 |
8:26 ± 2:47 |
4:00 ± 0:00 |
3:00 ± 0.20 |
3:30 ± 0.33 |
11:19 ± 1:08 |
9:01 ± 3:01 |
10:59 ± 1:45 |
|
Blood loss (mL)* |
711 ± 586 |
738 ± 569 |
715 ± 584 |
100 ± 0 |
100 ± 0 |
100 ± 0 |
245 ± 197 |
100 ± 0 |
209 ± 182 |
|
Discharge (POD) |
6.3 ± 1.4 |
5.8 ± 0.8 |
6.2 ± 1.3 |
7.0 ± 0.0 |
6.0 ± 0.0 |
6.5 ± 0.5 |
4.4 ± 1.0 |
4.0 ± 0.0 |
4.3 ± 1.0 |
|
Complications (n,%) |
17 (58.6%) |
2 (50.0%) |
19 (57.6%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
6 (50.0%) |
0 (0.0%) |
6 (42.9%) |
|
Graft failure (n,%) |
8 (28.6%)† |
1 (25.0%) |
9 (28.1%)† |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
2 (16.7%) |
0 (0.0%) |
2 (14.3%) |
|
Live birth (n,%) |
13 (46.4%) |
3 (75.0%) |
16 (48.5%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (8.3%) |
1 (50.0%) |
2 (14.3%) |
*Mean ± SD; †Not including 1 case in which uterine transplantation was not performed. OPEN, open approach; LAP, laparoscopic approach; ROBOT, robot-assisted approach; UV, uterine vein; POD, postoperative day.
Clinical data for each operative approach with or without the uterine veins are also shown in Table 2. In the open approach, the mean operative time was 8 h 45 min ± 2 h 39 min and the mean blood loss was 711 ± 586 mL in the cases where UVs were preserved (n = 29), and in the cases where UVs were not preserved (n = 4), the mean operative time was 6 h 14 min ± 0 h 26 min, and the mean blood loss was 738 ± 569 mL. In the laparoscopic approach, the mean operative time was 4 h 0 min ± 0 h 0 min and the mean blood loss was 100 ± 0 mL in the UVs preserved cases (n = 2), and the mean operative time was 3 h 0 min ± 0 h 20 min and the mean blood loss was 100 ± 0 mL in the non-UVs preserved cases (n = 2). In the robot-assisted approach, the mean operative time and mean blood loss were 11 h 19 min ± 1 h 8 min and 245 ± 197 mL in the UVs preserved cases (n = 12), respectively, and the mean operative time was 9 h 1 min ± 3 h 1 min and the mean blood loss was 100 ± 0 mL in the non-UVs preserved cases (n = 2). In each approach, the operative time was reduced in the non-UVs preserved cases. The discharge time was 6.3 ± 1.4 postoperative days for the open approach in the UVs preserved cases and 5.8 ± 0.8 days in the non-UVs preserved cases, and was 7.0 ± 0.0 postoperative days for the laparoscopic approach in the UVs preserved cases and 6.0 ± 0.0 postoperative days in the non-UVs preserved cases. In the robot-assisted approach, the postoperative discharge time was 4.4 ± 1.0 days in the UVs preserved cases and 4.0 ± 0.0 days in the non-UVs preserved cases. There was little difference between patients with and without UVs preserved. Operative complications were found in 17 (58.6%) cases for the open approach with UVs preserved, and in two (50.0%) cases for non-UVs preserved. No complications were reported with the laparoscopic approach in both of the UVs preserved and non-UVs preserved cases. Complications tended to occur more frequently in the robot-assisted approach, with six cases (50.0%) observed solely in the UVs preserved cases, with none in the non-UVs preserved cases. Complications were more frequent in the UVs preserved cases. In the robot-assisted approach, graft failure was reported in two patients (16.7%) with UVs preserved. Live births after UTx utilizing the laparoscopic approach were not reported in any of the papers included in this review. In the robot-assisted approach, one case (8.3%) of a live birth was reported from the UVs preserved cases, and one (50.0%) was reported from the non-UVs preserved cases.
References
- Herlin, M.; Petersen, M.B.; Brännström, M. Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome: A comprehensive update. Orphanet J. Rare Dis. 2020, 15, 1–16, doi:10.1186/s13023-020-01491-9.
- Hur, C.; Rehmer, J.; Flyckt, R.; Falcone, T. Uterine Factor Infertility: A Clinical Review. Obstet. Gynecol. 2019, 62, 257–270.
- Fageeh, W.; Raffa, H.; Jabbad, H.; Marzouki, A. Transplantation of the human uterus. J. Gynecol. Obstet. 2002, 76, 245–251, doi:10.1016/s0020-7292(01)00597-5.
- Brannstrom, M.; Johannesson, L.; Bokstrom, H.; Kvarnstrom, N.; Molne, J.; Dahm-Kahler, P; Enskog, A.; Milenkovic, M.; Ekberg, J.; Diaz-Garcia, C.; et al. Livebirth after uterus transplantation. Lancet 2015, 385, 607–616.
- Daolio, J.; Palomba, S.; Paganelli, S.; Falbo, A.; Aguzzoli, L. Uterine transplantation and IVF for congenital or acquired uterine factor infertility: A systematic review of safety and efficacy outcomes in the first 52 recipients. PLoS ONE 2020, 15, e0232323, doi: 10.1371/journal.pone.0232323.
- Brännström, M.; Johannesson, L.; Dahm-Kähler, P.; Enskog, A.; Mölne, J.; Kvarnström, N.; Diaz-Garcia, C.; Hanafy, A.; Lundmark, C.; Marcickiewicz, J.; et al. First clinical uterus transplantation trial: A six-month report. Steril. 2014, 101, 1228–1236, doi: 10.1016/j.fertnstert.2014.02.024.
- Chmel, R.; Novackova, M.; Janousek, L.; Matecha, J.; Pastor, Z.; Maluskova, J.; Cekal, M.; Kristek, J.; Olausson, M.; Fronek, J. Revaluation and lessons learned from the first 9 cases of a Czech uterus transplantation trial: Four deceased donor and 5 living donor uterus transplantations. J. Transplant. 2019, 19, 855–864, doi:10.1111/ajt.15096.
- Testa, G.; McKenna, G.J.; Gunby, R.T.; Anthony, T.; Koon, E.C.; Warren, A.M.; Putman, J.M.; Zhang, L.; DePrisco, G.; Mitchell, J.M.; et al. First live birth after uterus transplantation in the United States. J. Transplant. 2018, 18, 1270–1274, doi:10.1111/ajt.14737.
- Puntambekar, S.; Telang, M.; Kulkarni, P.; Puntambekar, S.; Jadhav, S.; Panse, M.; Sathe, R.; Agarkhedkar, N.; Warty, N.; Kade, S.; et al. Laparoscopic-Assisted Uterus Retrieval from Live Organ Donors for Uterine Transplant: Our Experience of Two Patients. Minim. Invasive Gynecol. 2018, 25, 622–631, doi: 10.1016/j.jmig.2018.01.009.
- Wei, L.; Xue, T.; Tao, K.-S.; Zhang, G.; Zhao, G.-Y.; Guang-Yue, Z.; Cheng, L.; Yang, Z.-X.; Zheng, M.-J.; Bi-Liang, C.; et al. Modified human uterus transplantation using ovarian veins for venous drainage: The first report of surgically successful robotic-assisted uterus procurement and follow-up for 12 months. Steril. 2017, 108, 346–356, doi: 10.1016/j.fertnstert.2017.05.039.
- Järvholm, S.; Johannesson, L.; Clarke, A.; Brännström, M. Uterus transplantation trial: Psychological evaluation of recipients and partners during the post-transplantation year. Steril. 2015, 104, 1010–1015, doi: 10.1016/j.fertnstert.2015.06.038.
- Brännström, M.; Bokström, H.; Dahm-Kähler, P.; Díaz-García, C.; Ekberg, J.; Enskog, A.; Hagberg, H.; Johannesson, L.; Kvarnström, N.; Mölne, J.; et al. One uterus bridging three generations: First live birth after mother-to-daughter uterus transplantation. Steril. 2016, 106, 261–266, doi:10.1016/j.fertnstert.2016.04.001.
- Mölne, J.; Broecker, V.; Ekberg, J.; Nilsson, O.; Dahm-Kähler, P.; Brännström, M. Monitoring of Human Uterus Transplantation With Cervical Biopsies: A Provisional Scoring System for Rejection. J. Transplant. 2017, 17, 1628–1636, doi:10.1111/ajt.14135.
- Kvarnstrom, N.; Jarvholm, S.; Johannesson, L.; Dahm-Kahler, P.; Olausson, M.; Brannstrom, M. Live Donors of the Initial Obser-vational Study of Uterus Transplantation-Psychological and Medical Follow-Up Until 1 Year After Surgery in the 9 Cases. Transplantation 2017, 101, 664–670.
- Brännström, M.; Dahm‐Kähler, P.; Kvarnström, N.; Akouri, R.; Rova, K.; Olausson, M.; Groth, K.; Ekberg, J.; Enskog, A.; Sheikhi, M.; et al. Live birth after robotic‐assisted live donor uterus transplantation. Acta Obstet. Gynecol. Scand. 2020, 99, 1222–1229, doi:10.1111/aogs.13853.
- Brännström, M.; Dahm-Kähler, P.; Ekberg, J.; Akouri, R.; Groth, K.; Enskog, A.; Broecker, V.; Mölne, J.; Ayoubi, J.M.; Kvarnström, N. Outcome of Recipient Surgery and 6-Month Follow-Up of the Swedish Live Donor Robotic Uterus Transplantation Trial. Clin. Med. 2020, 9, 2338, doi:10.3390/jcm9082338.
- Järvholm, S.; Dahm-Kähler, P.; Kvarnström, N.; Brännström, M. Psychosocial outcomes of uterine transplant recipients and partners up to 3 years after transplantation: Results from the Swedish trial. Steril. 2020, 114, 407–15, doi: 10.1016/j.fertnstert.2020.03.043.
- Brännström, M.; Kvarnström, N.; Groth, K.; Akouri, R.; Wiman, L.; Enskog, A.; Dahm-Kähler, P. Evolution of surgical steps in robotics-assisted donor surgery for uterus transplantation: Results of the eight cases in the Swedish trial. Steril. 2020, 114, 1097–1107, doi: 10.1016/j.fertnstert.2020.05.027.
- Broecker, V.; Brännström, M.; Ekberg, J.; Dahm-Kähler, P.; Mölne, J. Uterus transplantation: Histological findings in explants at elective hysterectomy. J. Transplant. 2020, doi:10.1111/ajt.16213.
- Huang, Y.; Ding, X.; Chen, B.; Zhang, G.; Li, A.; Hua, W.; Zhou, D.; Wang, X.; Liu, D.; Yan, G.; et al. Report of the first live birth after uterus transplantation in People’s Republic of China. Steril. 2020, 114, 1108–1115, doi: 10.1016/j.fertnstert.2020.06.007.
- Testa, G.; Koon, E.C.; Johannesson, L.; McKenna, G.J.; Anthony, T.; Klintmalm, G.B.; Gunby, R.T.; Warren, A.M.; Putman, J.M.; DePrisco, G.; et al. Living Donor Uterus Transplantation: A Single Center’s Observations and Lessons Learned from Early Setbacks to Technical Success. J. Transplant. 2017, 17, 2901–2910, doi:10.1111/ajt.14326.
- Ramani, A.; Testa, G.; Ghouri, Y.; Koon, E.C.; Di Salvo, M.; McKenna, G.J.; Bayer, J.; Warren, A.M.; Wall, A.; Johannesson, L. DUETS (Dallas UtErus Transplant Study): Complete report of 6‐month and initial 2‐year outcomes following open donor hysterectomy. Transplant. 2020, 34, e13757, doi:10.1111/ctr.13757.
- Johannesson, L.; Koon, E.C.; Bayer, J.; McKenna, G.J.; Wall, A.; Fernandez, H.; Martinez, E.J.; Gupta, A.; Ruiz, R.; Onaca, N.; et al. DUETS (Dallas UtErus Transplant Study): Early Outcomes and Complications of Robot-Assisted Hysterectomy for Living Uterus Donors. Transplantation 2021, 105, 225–230.
- Testa, G.; McKenna, G.J.; Bayer, J.; Wall, A.; Fernandez, H.; Martinez, E.; Gupta, A.; Ruiz, R.; Onaca, N.; Gunby, R.T.; et al. The Evolution of Transplantation from Saving Lives to Fertility Treatment: DUETS (Dallas UtErus Transplant Study). Surg. 2020, 272, 411–417.
- Chmel, R.; Cekal, M.; Pastor, Z.; Chmel, R., Jr.; Paulasova, P.; Havlovicova, M.; Macek, M., Jr.; Novackova, M. Assisted Reproductive Techniques and Preg-nancy Results in Women with Mayer-Rokitansky-Kuster-Hauser Syndrome Undergoing Uterus Transplantation: The Czech Experience. Pediatr. Adolesc. Gynecol. 2020, 33, 410–414.
- Chmel, R.; Novackova, M.; Pastor, Z. Lessons learned from the Czech uterus transplant trial related to surgical technique that may affect reproductive success. New Zealand J. Obstet. Gynaecol. 2020, 60, 625–627, doi:10.1111/ajo.13184.
- Brucker, S.Y.; Brannstrom, M.; Taran, F.A.; Nadalin, S.; Konigsrainer, A.; Rall, K.; Schöller, D.; Henes, M.; Bösmüller, H.; Fend, F.; et al. Selecting living donors for uterus trans-plantation: Lessons learned from two transplantations resulting in menstrual functionality and another attempt, aborted after organ retrieval. Arch Gynecol Obstet. 2018, 297, 675–684.
- Brucker, S.Y.; Strowitzki, T.; Taran, F.-A.; Rall, K.; Schöller, D.; Hoopmann, M.; Henes, M.; Guthoff, M.; Heyne, N.; Zipfel, S.; et al. Living-Donor Uterus Transplantation: Pre-, Intra-, and Postoperative Parameters Relevant to Surgical Success, Pregnancy, and Obstetrics with Live Births. Clin. Med. 2020, 9, 2485, doi:10.3390/jcm9082485.
- Puntambekar, S.; Puntambekar, S.; Telang, M.; Kulkarni, P.; Date, S.; Panse, M.; Sathe, R.; Agarkhedkar, N.; Warty, N.; Kade, S.; et al. Novel Anastomotic Technique for Uterine Transplant Using Utero-ovarian Veins for Venous Drainage and Internal Iliac Arteries for Perfusion in Two Laparoscopically Harvested Uteri. Minim. Invasive Gynecol. 2019, 26, 628–635, doi: 10.1016/j.jmig.2018.11.021.