Bergamot essential oil (BEO) is the result of the mechanical manipulation (cold pressing) of the exocarp (flavedo) of the hesperidium of Citruslimon Citrus limon (L.) Osbeck Bergamot Group (synonym Citrus × bergamia Risso & Poit.), resulting in the bursting of the oil cavities embedded in the flavedo and the release of their contents. It is chemically dominated by monoterpene hydrocarbons (i.e., limonene), but with significant percentages of oxygenated monoterpenes (i.e., linalyl acetate) and of non-volatile oxygen heterocyclic compounds (i.e., bergapten).
- bergamot
- citrus
- essential oil
- production
- review
1. Introduction
The taxonomy, and consequently the nomenclature, of the genus Citrus, is particularly complicated and has been rapidly changing in recent years (see Table 1). For over 400 years, the centre of origin and biodiversity, along with the evolution and phylogeny of the Citrus species, have all been puzzling problems for botanists and the confusing and changing nomenclature of this taxon over the years can reflect intrinsic reproductive features of the species included in this genus, the cultural and geographical issues, and the rapidly evolving techniques used to clarify its phylogeny [1].
Because of the fragrance of the whole plant (flowers, leaves, fruits) and the delectability of the fruits, Citrus species have been exploited, domesticated, and selected by humankind for millennia. Classical Chinese literature mentions cultivated Mandarins and Pomelos as early as II millennia B.C.E., and citrons were depicted on the walls of the Egyptian Karnak Temple, dating 3000 years ago. Citrus fruits were known in ancient times even in India, Japan, Mesopotamia, Media, Persia, Palestine, and also in the Jewish culture, in Ancient Greece and Rome. In the Medieval period, several Citrus species were cherished and cultivated by the Arabs, who introduced many agronomical innovations and helped spread these plants across known countries [2].
The history of Citrus species distribution in Asia and Europe provides evidence of a complex domestication process, which has led to a high number of artificially obtained interspecific hybrids and cultivars, a fact that makes morphology-based classification very hard, given that fruits with similar morphology may belong to very distant species, and conversely, very similar or genetically almost indistinguishable varieties may give rise to very different morphologies [1][3].
Moreover, Citrus species show a very high frequency of spontaneous mutations and are a very sexually promiscuous taxon, with spontaneous hybridization being extremely common, another factor that makes it very hard to trace a solid and clear genealogy [1], and often these hybrids have been re-crossed with parent species, thus creating a variety of organisms that are sometimes genetically very close but morphologically very different, and vice versa, so much so that most of the Citrus fruits we normally consume are hybrids and not species (see below Table 2) [4]. Many citruses are capable of reproducing without fertilization (apomictic reproduction): specifically, they often present maternal nucellar embryogenesis, where nucellar tissue produces many clonal embryos, resulting in poly-embryonic seeds containing mainly or only nucellar embryos with the sole maternal genetic material. This is opposed to zygotic embryos, which contain genetic material of both parents (in mono-embryonic seeds). This process slows down or even stops the evolutionary process, thus making it again hard for taxonomists using morphological features to understand the true lineage [1][5][6].
Table 1.
Taxonomy of the
Citrus
genus
.
| Angiospermae |
| Eudicots |
| Superrosids |
| Rosids |
| Sapindales Juss. ex Bercht & J.Presl. |
| Rutaceae Juss. |
| ———Rutoideae (120 spp.) |
| ———Spathelipideae (5 spp.) |
| ———Aurantioideae (30 spp.) |
| ——————Clauseneae |
| ——————Citrae (Aurantieae) |
| —————————Triphasinae |
| —————————Balsamocitrinae |
| —————————Citrinae |
| ————————————Primitive Citrus fruits (Severinia, Pleiospermium, Burkillanthus, Limnocitrus, Hesperethusa) |
| ————————————Near Citrus fruits (Atalantia and Citropsis) |
| ————————————True Citrus fruits |
| ———————————————Clymenia |
| ———————————————Eremocitrus |
| ———————————————Microcitrus |
| ———————————————Poncirus |
| ———————————————Fortunella |
| ———————————————Citrus |
| ——————————————————Papeda |
| ——————————————————Eucitrus (Citrus) |
Table 2. Common Citrus Hybrids.
| These hybrids are often treated as separate species. Particularly important is the Mandarin-Pomelo hybridization axis, which leads to species with orange or red peeled fruits when ripe | [1]: | |
| Oranges. The Bitter Orange (Citrus × aurantium L. (sin Citrus × aurantium subsp. amara (Link) Engl)) is a pure Mandarin and Pomelo cross, the Sweet Orange (Citrus sinensis (L.) Osbeck) is a cross between a Mandarin (already crossed with a Pomelo) and a Pomelo. Both hybrids inherit the orange colour of the peel from the Mandarin. | ||
| Grapefruit (Citrus paradisi Macf.). These also derive from the gene pool of Mandarin and Pomelo, but with a higher contribution than the latter one, thanks to a cross between Sweet Orange and Pomelo. | ||
| Mandarins (Citrus reticulata Blanco). Mandarins and Clementine belong to the class of Mandarin hybrids, with genetic contributions from Sweet and Bitter Oranges and Grapefruit. | ||
| Lemons (Citrus limon (L.) Osbeck). “Real” Lemons have a light yellow peel and are derived from a single ancestral hybrid between male Citron and female Bitter Orange (i.e., 50% Citron, 25% Mandarin, 25% Pomelo). | ||
| Bergamot. This is a hybrid whose origin is still debated, but it seems to have been obtained by crossbreeding of some Citrus hybrids (Bitter Orange, Lemon, Lime) with their ancestors (Citron) or with other hybrids. |
1.1. Origins of the Genus Citrus
The first modern classification of the genus Citrus (in the Family Rutaceae) was formulated by Carl von Linné, who, in his “Species Plantarum”, clustered all Citrus fruits under two species: Citrus medica L. (Citron) along with its only variety Citrus medica var. limon (Lemon), and Citrus aurantium L. (Bitter Orange) along with its two varieties, Citrus aurantium var. grandis (Pomelo) and Citrus aurantium var. sinensis (Sweet Orange) [13]. All subsequent classifications in the following centuries were based on morphological and geographical data, and different authors recognized a diverse number of species.
A revolution in the taxonomy of Citrus has come with the development of laboratory techniques involving specific molecular markers, such as Random Amplification of Polymorphic DNA (RAPD), Sequence Characterized Amplified Region (SCAR), cpDNA Simple Sequence Repeats (SSR), Inter-Simple Sequence Repeat (ISSRs), Amplified Fragment Length Polymorphism (AFLP) and Restriction Fragment Length Polymorphisms (RFLPs) markers [3][14], and the triangulation with information from historical literature and contemporary data about the natural distribution of these species [5].
According to one study [15], there are at least 10 progenitor species. Of these, seven are original to Asia: Pomelo (Citrus maxima), the true Mandarin (C. reticulata), Citron (C. medica), Papeda (C. micrantha), Ichang’s Papeda (C. ichangensis), Mangshanyegan (C. mangshanensis), and Oval Kumquat (C. japonica). The remaining three species are Australian: Desert Lime (C. glauca), Round Lime (C. australis), and Lemon Caviar (C. australasica).
Recently, important genomic work [4][16] has provided the most up-to-date evolutionary tree for Citrus, thus challenging previous proposals. The paper by Wu and colleagues [4] that used single nucleotide polymorphism (SNP), proposes that at least three genera (Fortunella, Eremocitrus and Microcitrus) are nested in the Citrus clade, and that of the ten progenitor species, three are to be considered ancestral, namely Citrus maxima, Citrus reticulata and Citrus medica. Four main hybrids are derived from these ancestral species: Lemons, Limes, Oranges and Grapefruits [17][18].
Wu and colleagues [4] state that, according to their analysis of the accessions, “the centre of origin of Citrus species was the southeast foothills of the Himalayas, in a region that includes the eastern area of Assam, northern Myanmar and western Yunnan”, while inferences from modern distribution point towards the central-southern part of China as the primary biodiversity centre, with southeast Himalaya and southern China as two accessory centres [1]. Citrus probably moved from West to East Malaysia and Australia sometime in the Miocene/Pliocene [19].
Because of the intricacy of the taxonomy of the Citrus genus, from an agronomic perspective, when dealing with commercially important species, a pure Linnaean classification could be substituted by just using cultivar names grouped under general agronomic groups. At least eight groups have been recognized so far, the Sweet Orange Group (cultivar: navel, common, blood, acidless), the Mandarin Group (Satsuma, tangerine/clementine, Mediterranean Mandarin and Mandarin), the Lemon Group, the Grapefruit Group, the Lime Group, the Sour Orange Group (cultivar: Seville, Bouquet de fleur, Granita, Chinotto, Bergamot), the Pomelo Group, and the Citron group [13].
1.2. Taxonomy of Bergamot
The phylogeny and taxonomy of Bergamot (Figure 1) are particularly unclear, as it is its exact area of origin [20].
Figure 1.
Bergamot plant and fruit, as illustrated in the traditional Köhler’s Medizinal-Pflanzen
[21]
.
The plant is an interspecific hybrid, and this is testified by the high percentage of heterozygosity [22] and has been clustered with Bitter Orange [23]. The actual progenitor species of Bergamot have been claimed to be Citrus x aurantium (Bitter Orange) and C. x limon (Lemon), or C. x aurantium and C. x aurantifolia (Key Lime) [24], or C. x aurantium and C. medica [25][26].
Bergamot seems to have originated quite late in the phylogeny of the Citrus clade and was unknown to ancient authors. Some sources claim that the hybrid was originated directly in Calabria, or at least in the southern part of Italy [27], while others state that the hybrid first appearance was outside Italy (either in the Antilles or in Greece or the Canary Islands) and that it was brought to Calabria only later [22]. One source hypothesizes that the hybrid was brought to Italy by Columbus via the Spaniard city of Berga, which inspired the fruit name, Bergamot [22]. A more solid hypothesis for the name origin is that the term “Bergamot” may derive from the Turkish word “beg-a-mudi” which means “pears of the Prince”, due to its certain resemblance to Bergamot pears [27].
Some authors also identify four subgroups of Bergamot, namely the “Piccola” group, the “Torulosa” group, the “Melarosa” group, and the “Common” group. All Bergamot fruits that are interesting from an industrial point of view come from the “Common” group, which includes three cultivars:
“Castagnaro”, whose fruits give perhaps the least valuable essential oil (EO), but can be harvested for production for longer than any other cultivar.
“Femminiello”, less vigorous and strong than Castagnaro, with a low yield of a superior, excellent EO.
“Fantastico” (or “Inserto”), supposed to be a hybrid of the first two varieties, certainly introduced more recently (about 1940) but representing now a good part of all the trees in Calabria [22].
Concerning nomenclature, opinions of different authorities diverge, as explained in Table 3.
Table 3. Nomenclature of Bergamot according to different authorities and institutions.
| Authority | Binomial |
|---|
| Integrated Taxonomic Information System | [28] | Citrus aurantium subsp. bergamia (Risso & Poit.) Wight & Arn. ex Engl |
| International Plant Names Index | [29] | Citrus bergamia Risso |
| Tropicos | [30] | Citrus × bergamia Risso & Poit. |
| World Checklist of Selected Plant Families | [31] | Citrus limon (L.) Osbeck |
| U.S. Dep of Agriculture: Agricultural Research Service | [32] | Citrus × limon (L.) Osbeck var. bergamia (Loisel.) ined. |
| Mabberley’s Plant-Book | [12][33] | Citrus × limon, Bergamot group |
1.3. Definition of EO and Peculiarities of Citrus EOs
The International Organization for Standardization (ISO) definition (ISO 2020 document ISO 9235: 2013-2.11) states that an EO is a “product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of Citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes”. Such includes three production methods, one of them exclusively reserved for Citrus EOs, which are therefore clearly separated from other EOs by production method and, therefore, by chemical composition. Distilled EOs contain only volatile molecules, while cold-pressed EOs may contain much heavier molecules and different chemical compounds from those normally found in distilled EOs. The second characteristic of Citrus fruit EOs is that, from an economic point of view, in many cases, the EO is a secondary product, while the fruit juice is the primary product, and other products can be obtained from the fruit (candied peels, “cells”, albedo, etc.). This is true for Lemons, Sweet Oranges, Grapefruits and Mandarins, but not for Bergamots and Bitter Oranges, whose fruit juice is generally produced in small quantities or even not at all. This fact partly explains the higher costs of both EOs [34].
1.4. Anatomy of Citrus fruits
The fruit of Citrus species is a modified berry called “hesperidium”, characterized by an approximately spherical shape, and by a very thick and robust outer rind or peel protecting the fluid-filled trichomes that make up the segments of the fruit juicy part (endocarp). The rind is divided in a superficial, thin, coloured exocarp called flavedo (from classical Latin flavus, or “blonde”) and a deeper and thicker spongy mesocarp called albedo (from the Latin albedo, derivative of albus, or “white”).
The flavedo is surrounded by a thick, waterproof cuticle and by various stomata in the superficial layer of the epidermis, it is made of small, dense collenchyma cells with chromoplasts, and contains many EO-bearing cavities called lumina or lacuna [22]. In the Bergamot fruit, these more or less spherical central cavities are surrounded by four to six layers of secretory cells and are formed in a schizolysigenous way [35], that is first by the separation of parenchymal cells (schizogenesis of the intercellular space), followed by further disintegration of cells in later stages of cavity development (lysogeny). These lacunae have no walls, are not connected, are found at different depths, and fill up with EO as the fruit ripens. They are of different sizes (the range is 0.4–0.6 mm in diameter) and they are conventionally divided in primary, secondary and tertiary cavities [34][36].
It is precisely this superficial distribution of the oil cavities that has made the method of cold-pressing or mechanical treatment the preferred one to obtain Citrus EOs [5][22].
1.5. Distribution and Cultivation of Bergamot
Around 90% of total BEO global production comes from Italy and specifically from a small area of fewer than 1400 hectares in Calabria [37]. Other regions of the world where Bergamot cultivation and BEO production have been tried are the African continent and the Americas, and lately China. Of all these producers, only the Ivory Coast has a commercially important share of the market, with 8–10% of global BEO production, although Brazil and China are emerging as global producers [22].
The introduction of Bergamot in Africa started only in the 1950s, and up until 1959, apart from a few trees in the Botanical Garden of Bingerville close to Abidjan, there was no record of the plants in this region. Other African countries such as Guinea, Liberia, Mali, Algeria, Morocco, and Tunisia only have small industrial productions with no indication of possible expansion [22].
One of the main problems for BEO production in Africa is that it appears to be up to 3–5 times lower per cultivated hectare than that of Calabria [38][39]. The reason for this has been sought in some differences in production technology, in the different climate, but above all in the characteristic composition of the soil. It can be added that a difference of three centuries of cultivation in Italy has allowed a better selection of plants [38][39].
The introduction of Bergamot in the Americas started in the US, specifically in Florida, Louisiana and California, where the tree was imported before 1815 but where it failed to thrive. The cultivations in South America were slightly more successful. Introduced since the 1960s there is only a modest industrial production of a good quality product in Argentina, Brazil and Uruguay [22].
The differences in the chemistry of BEO from various regions of the world are difficult to gauge because of their large variability, due to non-technological parameters according to Dugo and Bonaccorsi [22]. What can be stated is that most of the EOs produced in regions different from Calabria show values of β-pinene and γ-terpinene lower or close to the minima determined for Calabrian EOs and that in terms of enantiomeric composition it seems, from the scarce available chiral data, that EOs from the Ivory Coast and Uruguay agree with those of EOs from Calabria [22].
Distribution and Cultivation of Bergamot in Calabria
The Bergamot tree possibly originated as a seedling in southern Italy but, until the mid-sixteenth century, it was known only as an ornamental plant in Tuscany. It was first officially mentioned as an “aurantium stellatum et roseum” by Ferrari in 1646 in his “Hesperides sive de malorum aureorum cultura et usu”, and first described in 1708 by Volkamer as “gloria limonum fructus inter omnes nobilissimus” in his “Nurnbergische Hesperides”, where the importance of its essence was first reported [27].
It became famous in France by 1686, thanks to Francis Procopius, as the source of a precious perfume ingredient, named “Bergamot water”. It was also introduced in 1676 in Cologne by Paolo Feminis, who used it to create the famous Cologne water (eau de cologne), commercialised by his son-in-law, Gian Maria Farina [27].
This notoriety greatly stimulated the demand for the EO, and as a consequence in 1750 Nicola Parisi planted, in Reggio Calabria, the first Bergamot orchard (Rada Giunchi Estate). From there, then cultivation expanded around the city of Reggio Calabria and along the Straits of the Messina area [27]. The tree seems to be very sensitive to pedoclimatic conditions and hence cultivation was and is mainly limited to the region of Calabria [22]. After periods of expansion and reduction in the cultivated area, today Bergamot cultivation occurs mainly in a stretch of land of 100–140 km from Reggio di Calabria following the Ionian Coast, comprising 1000–1400 hectares between Villa San Giovanni and Monasterace, 800 of which are used by farmers who are part of the organization “Organizzazione Produttori Unionberg” [40].
2. Production Methods of BEO
Production methods of BEO are different and some derive from ancient traditions and have been discussed in previous reviews [13][22][34][41][42][43]. “Expression” or “cold-pressing” is a method of obtaining EOs used only in the production of Citrus EOs (although distillation remains a possible alternative). The term refers to any physical process in which EO glands in the peel are crushed or broken to release the EO (ISO 2020 document ISO 9235: 2013-2.11). The obtained EO will therefore also contain non-volatile molecules, among which there may be furanocoumarins with phototoxic effect [44]. When oil sac walls in the rind are ruptured, the EO spurts out with a force that decreases with the length of fruit maturation and drying. Hence, it is easier to express fresh and immature fruits, while dry material is less elastic and offers more resistance to breaking and puncturing [45].
Cold-pressing was probably introduced before distillation since it is very simple and does not need any specific apparel. The reasons why this method is used are partly dependent on history and tradition, but there are other, less conventional reasons to resort to this method.
The combined effect of temperature, acidic pH, the presence of oxygen and the presence of water (a set of conditions typical of hydro-distillation, and to a lesser extent steam distillation) can facilitate oxidation and hydrolysis [22]. These conditions can cause hydration reactions of the unsaturated monoterpene hydrocarbons such as limonene (the most common compound in Citrus EOs), and the formation of alcohols [13], the degradation of minor sesquiterpenes such as germacrenes A and C [5], and the degradation of aldehydes of both aliphatic (heptanal, octanal, nonanal, decanal, dodecanal) and terpenic origin (neral, geranial, citronellal, perillaldehyde). In particular neral and geranial are amongst the most abundant aldehydes in Citrus EOs, and hence the molecule whose degradation mostly impacts the quality of the EO [46]. Overall, production methods used for Bergamot belong to the same category of methods common to all Citrus fruits, although not all permitted methods are used for Bergamot, as we shall see. According to the Consorzio Unionberg OP, the average annual production of BEO in Calabria is of 70–100.000 kg (data relative to the 2007–2012 period), and the average yield for cold-pressed EOs is of +/−0.550 Kg of EO for 100 Kgs of fruits [38].
2.1. Manual Extraction
One method that was practised a long time ago, particularly in Sicily, was the “spugna” (sponge) method, whereby fruits were halved by expert operators, called “sfumatori”, the pulp was separated from the rind using a special, sharpened spoon-knife called “rastrello” [41], and the EO was extracted from the peel by pressing the peel against a hard object of baked clay (“concolina”) or by bending the peel, with the compression causing oils sacs to burst and eject EO under pressure. In both cases, a large natural sponge was used to absorb the EO emulsion, which, in a later stage, was removed by squeezing it into the concolina or into another container. An improvement of this method that was introduced in later years was to wash the rinds with lime water to buffer the acidity caused by organic acids such as oxalic and malic acid and, then, to let the rinds drain for 24 h before extraction. The system was very time-consuming and gave a poor yield (but apparently of very high quality).
An improvement over the spugna method was the scodella or eucelle method. In this case, the entire fruit was placed in a copper or brass bowl with a hollow central tube at the bottom. The bowl was covered with blunt spikes over which the whole fruit was delicately rolled to burst oil sacs. Released EO was then drained down the funnel stem and collected in a vessel, together with some water content of the rind, which was then separated from the rest by decantation. This method allowed an improved yield of EO and did not require the halving of the fruits, which was a time-consuming process that also possibly led to some EO loss. However, both methods are impractical and very time-consuming and are not used anymore.
2.2. Mechanical Methods
To improve these methods, under the Kingdom of Borbone, a competition was organized for automation of the extraction, which was won in 1844 by Nicola Barilla and Luigi Autieri, with the Calabrese Machine, further improved by Domenico Carbone in 1875, which partially automatized compressing and rasping operations [22][27]. In 1931 an improved version, called “modern Calabrese” machine, or “Polimeni” machine, appeared, that could compress and scrape up to eight fruits at a time, thus reducing processing time maintaining an excellent quality of the product [22][27]. Over the years many other machines were invented and used, all variations on the Calabrese or Pelatrice design (see below for a description), although most of them are not in use any more, and most of today’s production of BEO comes from “Pelatrice Speciale” and “Moscato tipo MP-C3”, plus some production from Brown extractors, “Garozzo” Machine and Food Machinery Corporation (FMC) in-line extractors [22][27] (see Table 4).
Table 4. Mechanical methods for BEO and juice extraction.
| Machine | Type | In Use |
|---|---|---|
| Sfumatrice | Juice/EO | Yes, but not for Bergamot |
| Calabrese machine (1844–1875) | EO/Juice | No |
| Modern Calabrese machine or Polimeni machine (1931) | EO/Juice | No |
| Franco machine (1926) | EO/Juice | No |
| Martorana machine | EO/Juice | No |
| A sbattimento machine, or dry Pelatrice (1944–1945) | EO/Juice | No |
| Pelatrice Speciale | EO/Juice | Yes |
| Indelicato machine | EO/Juice | No |
| Avena Pelatrice (1924) | EO/Juice | No |
| MP-C3 Pelatrice | EO/Juice | Yes, not common |
| Brown extractor | EO/Juice | Yes |
| Garozzo machine | EO/Juice | Yes |
| FMC in-line extractor | EO and Juice | Yes |
Since no Citrus EO extraction can be profitable without exploiting other parts of the fruit, the expression is nowadays part of an integrated system that focuses on zero waste to make the entire chain of production profitable. Hence, modern production methods depend on the use of machinery that can often operate the pressing of the juice and the separation of other parts of the fruit.
We can roughly divide modern, mechanized methods into:
- (1)
-
EO extraction after pulp squeezing (“Sfumatrice”, “Torchiatura”).
- (2)
-
EO production before juice (and other components) extraction (“Pelatrice”, Brown Extractor, “Garozzo”).
- (3)
-
Methods in which the two extractions are performed at the same time (FMC).
It should be noted that, while all these methods are widespread, for BEO extraction the Sfumatrici are not normally used because the Bergamot juice industry is not economically relevant and the EO is of much higher value than other Citrus EOs.
2.3. EO Extracted after Juice Extraction
In the Sfumatrice process, the fruits are halved and the pulp is removed. The rinds are soaked in lime to neutralize acids and then passed through two horizontal ribbed rollers that press and bend the rinds to extract the EO. The EO is washed away from the rollers by fine sprays of water, then it is passed through a separator to remove any solid matter and finally sent to two serial centrifuges to purify the EO. After extraction, winterization can be used to freeze out waxes.
2.4. EO Extracted before Juice Extraction
With these machines, the EO is extracted before squeezing the juice, through a rasping or perforation of the peels (Pelatrice) or the breaking of individual bags (Brown machine, or Garozzo machine). The EO is washed with water spray, and the emulsion between water and EO is filtered and then separated by centrifugation.
In the Pelatrice process, Citrus fruits are put into a slow-moving cochlea surrounded by an abrasive shell. The abrasive, rasping action causes EO sacs to burst and release their EO-water emulsion. The cochlea then transports the fruit into a hopper, in which rollers with abrasive spikes burst the remaining oil sacs. As in the Sfumatrice process, the EO and water emulsion is washed away, the emulsion passes through a separator and then through the two centrifuges. Most BEO and some Lemon EOs are produced this way in Italy. Some methods or machines are today of only historical interest, while others are still used by producers.
In the Brown extractor and the Garozzo machine (a modified and improved version of the Brown extractor) (Figure 2), the fruits, after washing, are moved continuously into a long bed of rolling and shifting cylinders, each bearing thousands of spikes or square teeth, that prick the peel to release the EO, which is collected in a shallow layer of water. Fruits are then moved to a smooth roller section, where the remaining water and EO sticking to the rind are collected. After this passage, the whole fruits are sent to the juicing step. The emulsion is then filtered and sent to the centrifuge to recover the cold-pressed EO. These newer methods have been tested with Bergamot with very interesting results both in terms of quality and in terms of total yield.

Figure 2. The bed of rolling toothed cylinders in a Garozzo machine.
2.5. EO Extracted at the Same Time as the Juice
The only machine that can operate the two extractions at the same time is the FMC in-line extractor, a highly mechanized and automatized contraption in which there are two stainless steel cups, made of interlacing metal fingers, one holding the fruit and the other one descending towards it (Figure 3). The descending cup interlaces with the inferior one, thus squeezing the fruit and sending the juice downwards through a hole made automatically by a circular knife at the bottom of the lower cup. At the same time, the fingers also press the rind and burst oil sacs, thus freeing the EOs which are washed away by a spray. The two operations are physically separated and this ensures that the EO does not mix with the juice and vice-versa, although some contamination is almost inevitable, with some reduction in quality of produced EO.
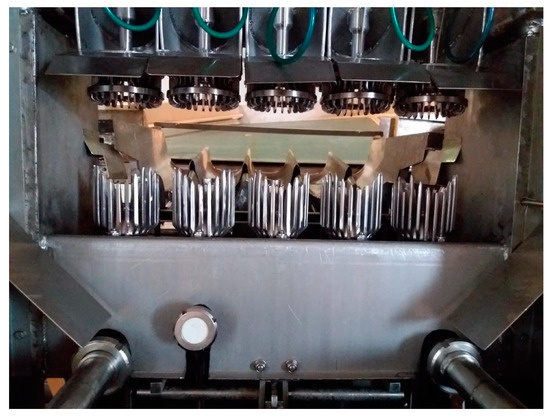
Figure 3. A detail of an FMC in-line extractor showing the upper and lower cups.
2.6. Low-Quality EOs
In the past, those fruits which fell on the ground during the summer season, well before reaching perfect maturation, were still manipulated in a different, dedicated extraction line [41]. The derived EO was named “Nero di bergamotto” (Bergamot Black) because often these fallen fruits lay on the ground for a long time before being collected, and were scorched, blackened by the Sun. The product was of inferior quality, and this practice has been discontinued because of the increasing demand for superior products. However, while the “Nero di bergamotto” is not in production any more, in certain instances fruits that have fallen before maturation but later on in the season, and that have consequently spent less time on the ground before being picked, are extracted with specialised, small machines of the Calabrese Pelatrice type (see above). The EO that results from this process, called “Bergamottella”, has a lighter colour than “Nero di bergamotto” and has a quality that depends on the moment the fruit has fallen and it was processed, but in certain cases, the EO can reach a high percentage of esters, to a maximum of 30% [22][41].
There are also EOs derived from the residues of the normal extracting techniques: “Ricicli” (recycled) EOs are obtained by centrifugation of the waters left at the end of the daily process of extraction, which can amount to about 1% of the total EO; “Torchiati” (screw-pressed) EOs are recovered by pressing the solid residues in the secondary centrifuge, with a yield of about 3.5% of the total EO;
“Pulizia dischi” (disc cleaning) EOs are those obtained by decanting the liquid residues from the secondary centrifuge disk at the end of the daily process, with a yield of about 0.5% of the total EO [13][22].
2.7. Distillation
It should be noted that it is also possible to obtain all Citrus EOs by distillation, both of the juice and of the separated skin, but these distilled EOs are quite rare because they often have a lower organoleptic quality. Distillation is used as a customary method only for Lime and is never used for Bergamot. This is because any Citrus EO obtained through distillation has a reduced olfactory quality, and is more fragile because of the lack of non-volatile molecules which appear to provide some antioxidant activity [47]. In the Bergamot industry, distillation is only used for refining the cold-pressed BEO, or for second-grade products. For the refining step, distillation is adopted to modify the chemistry of cold-pressed EOs, either to reduce the percentage of monoterpenes (deterpenation), or to reduce the percentage of bergapten (bergapten-free BEO), or to reduce the percentage of furocoumarins (furocoumarin-free BEO).
Distillation is also used to produce second-grade derivatives. Distilled EO of “Bergamottella” is obtained by distilling unripe fruits that are too small for the Pelatrice process; “Feccia” EOs are derived from the distillation of the solid and semi-fluids residues of the Pelatrice machines; the “Peratoner EOs” are produced by vacuum distillation of the waters coming out of the centrifuges separating the EOs, or of generic residues of mechanical processing (Figure 4). Because of the lower temperature during distillation, it gives superior product compared to the other methods, and it is slowly supplanting them; and the “distilled juice notes” are EOs obtained from the distillation of the juice [22]. Innovative distillation techniques such as solvent- and water-free microwave extraction have been tested with good results, but they are still at a laboratory or pilot level [48].

Figure 4. Two Peratoner still units.
2.8. Terpeneless BEOs
Distillation under vacuum using a fractionating column is the classic technology to selectively eliminate monoterpene hydrocarbons such as limonene. Most cold-pressed EOs are dominated by monoterpenes (usually between 90% and 98%), mostly (+)-limonene, but also terpinene and β-pinene), with a very low olfactory impact. Selectively eliminating these monoterpenes allows increasing the weight of compounds with a high olfactory impact, such as esters and aldehydes. The deterpenation, therefore, results in two products, a deterpenated EO with a high olfactory impact, and the terpenic fraction (Orange terpenes, Lemon terpenes, Bergamot terpenes, etc.), often used in perfumery, for low-cost aromatisation, or to adulterate other EOs. Terpeneless BEO is produced in smaller volumes if compared to other Citrus EOs because Bergamot has already a high percentage of oxygenated compounds and the need to concentrate them by reducing the monoterpene content is less relevant. The yield of deterpened BEO (folded Bergamot EO) is 33–44% of the original BEO, while the yield for Orange and Lemon EOs is 1–3% and 3.5–6%, respectively. Usually, the EO is distilled under vacuum after a winterization passage. New technologies for deterpenization are available: some examples include alcohol extraction followed by a concentration step (under vacuum distillation of alcohol), counter-current extraction with two immiscible solvents, extraction with a binary mixture of a polar and an apolar solvent, chromatographic separation, SC-CO2 extraction, or membrane extraction. However, none of these methods has reached an industrial level.
2.9. Bergapten-Free and Furocoumarin-Free BEO
Another refining step sometimes used for Bergamot is the production of bergapten-free BEO for the fragrance industry. Bergaptene-free BEO is a reduced-bergapten content EO, with bergapten present at a content level below 30 ppm. In Italy, this step is done using a chemical passage, the mixture of the EO with an alkaline solution of sodium hydroxide (NaOH). This treatment has the effect of hydrolyzing the lactonic ring of bergapten and citropten but does not affect bergamottin and 5-geranyloxy-7-methoxycoumarin, because the lactonic function is sterically protected by the geranyloxy group. The resulting EO has a bergapten content of 0–91 ppm, a bergamottin content of 11,726–16,250 ppm, and a 5-geranyloxy-7-methyl coumarin content of 1539–1975 ppm. The passage is followed by centrifugation and separation, further washing with water and more centrifugation and separation [49].
A similar product is the furanocoumarins free FCF BEO, which is an EO of Bergamot deprived not only of bergapten but of all furocoumarins. This elimination is done by vacuum re-distillation over a fractionating column, and it usually results in the production of a very high-quality EO, with a bergapten content of 0-41ppm, a bergamottin content of 0–3017 ppm, and a 5-geranyloxy-7-methylcoumarin content of 0–349 ppm [49]. A graphical summary of BEO extraction processes is reported in Figure 5.
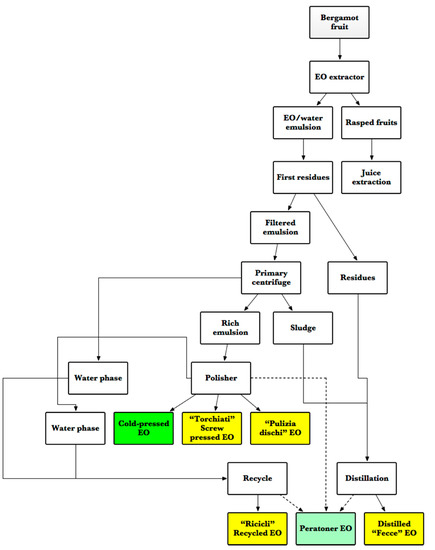
Figure 5. Flowchart of BEO cold-extraction process (adapted from [22]). The box in deep green color indicates the primary product of the process (cold-pressed EO), yellow boxes indicate the product of the extraction of inferior, or second grade, materials, and the box in light green color indicates an alternative (and superior) mode of production of the same inferior materials (Peratoner vacuum distillation).
2.10. Innovative Methods
Although strictly they are not EO-obtaining production methods, because they do not comply with the ISO definition of EOs, some innovative techniques have been proposed to extract EOs from Citrus peels. In most cases, these extracts have different chemical compositions from cold-pressed EOs [50], and most of them are still at a laboratory or pilot level: ultrasound-accelerated solvent extraction, solid-phase microextraction, supercritical CO2 extraction [48], and pervaporation [51].
3. Chemistry of BEO
BEO is a special case in the field of c.p. Citrus EOs. While these are normally dominated by monoterpene hydrocarbons, and specifically by limonene, which can range from 80% to 95% of the total content [13], BEO has a much lower percentage of limonene and allied monoterpenes, and a much higher percentage of oxygenated terpenes, specifically linalool and linalyl acetate. As for all c.p. EOs, their content can be divided into the volatile fraction and the non-volatile one.
The composition of the volatile fraction is highly variable, and this variability depends on several parameters: phenology, pedoclimatic characteristics of the production area, geographical provenance, genetics (cultivars), long-term climatic changes and changes in agronomical practices, plus the changing technology of expressing apparels [22][48]. This complex of influencing factors, combined with the high market value of the EO, makes BEO a favourite target of fraudulent manipulations and makes the identification of adulteration both critical and difficult, especially when compared to other Citrus EOs. This variability notwithstanding, it can be stated that the main compounds of the EO are limonene, linalyl acetate, linalool, β-pinene, and γ-terpinene, which together comprise more than 90% of the whole EO [52]. In this article, when not explicitly stated, all data on percentage composition should be taken as referring to Calabrian BEOs.
3.1. Volatile Fraction
The volatile fraction amounts to roughly 93–96% of the total content of BEO [53]. The main components of this fraction are limonene, linalyl acetate, and linalool [13], but there are more than 100 compounds detected and described in the scientific literature [54]. Although the content of hydrocarbon compounds of BEO is much lower than that of other Citrus EOs, the volatile fraction is still dominated by monoterpene hydrocarbons, specifically by limonene (25–55%), β-pinene (4–11%), and γ-terpinene (5–11%) [54]. Additionally, in the volatile fraction, there are sesquiterpene hydrocarbons, such as β-bisabolene, (E)-α-bergamotene, and β-caryophyllene [55]. In contrast with other Citrus EOs, however, oxygenated derivatives of mono and sesquiterpene hydrocarbons are present in a much higher percentage and they can be more than 50% of the entire volatile fraction, while in other Citrus EOs they can range from 1% to 6% [56]. Terpenic esters and alcohols (and secondarily aldehydes and oxides) are particularly abundant, although with very wide composition intervals, alongside with small amounts of aliphatic aldehydes, alcohols, and esters [53][54]. From a quantitative point of view, monoterpene esters are the most relevant esters in BEO, and the more abundant ones are linalyl acetate (15–40%), geranyl acetate, linalyl propionate, and neryl acetate. (Z)-Sabinene hydrate acetate and (E)-sabinene hydrate acetate, although not very important in terms of percentage, seem to be exclusive of C. bergamia EO [53][55]. An important non-terpenic ester is the aliphatic ester octyl acetate, which is more abundant in C. bergamia EO than in other Citrus species [55]. In the category of monoterpene alcohols, linalool is the most important and can be present in very high amounts (2–20%), while the sesquiterpene alcohol α-bisabolol, although not present in high amounts, seems to be quite specific to Bergamot [55].
3.2. Non-Volatile Fraction
This fraction amounts to 4–7% of the total content of the EO [53] and, apart from inert substances such as pigments and waxes, is principally made up of oxygen heterocyclic compounds, mainly psoralens and coumarins, plus minor amounts of polymethoxyflavones (sinensetin, tetra-O-methyl scutellarin) (Table 5). In particular, four compounds make up the majority of this fraction, namely the two psoralens bergapten (5-methoxypsoralen, 5-MOP. 0.11–0.32%) and bergamottin (5-geranyloxypsoralen 1.02–2.75%), and the two coumarins geranyloxy-7-methoxycoumarin (0.08–0.22%), and citropten (5-geranyloxy-7-methoxycoumarin. 0.14–0.35%), which are characteristic but not exclusive of C. bergamia [54][55]. The variability ranges of these four components are very large [22] (see Table 5). Other psoralens identified in genuine EOs are bergaptol, byakangelicin, biakangelicol, epoxybergamottin, isoimperatorin, 5-isopentenyloxy-8-methoxypsoralen, oxypeucedanin, and oxypeucedanin hydrate [49].
Table 5. Main heterocyclic compounds in BEO.
| Molecule | Range Years 1961–2006 | Range Years 2007–2011 |
|---|---|---|
| Bergamottin | 10,000–27,500 mg/L | 10,969–23,923 mg/L |
| Bergapten | 1100–4750 mg/L | 1319–3329 mg/L |
| Citropten | 1200–3813 mg/L | 1340–3032 mg/L |
| 5-geranyloxy-7-methoxycoumarin | 400–2700 mg/L | 803–1818 mg/L |
Sawamura and colleagues have analysed the volatile fraction composition of BEO using GC coupled with olfactometry, to identify the most important compounds in the character definition of Bergamot aroma, namely a few molecules present in minute amounts: (Z)-limonene oxide, decanal, linalyl acetate, and geraniol. Another molecule, (Z)-β-ocimene, although not possessing a typical Bergamot character, seems important for the formation of the overall aroma [54]. A graphical representation of BEO composition is displayed in Figure 6, Figure 7 and Figure 8 and chemical description of its most relevant compounds is reported in Table 6.
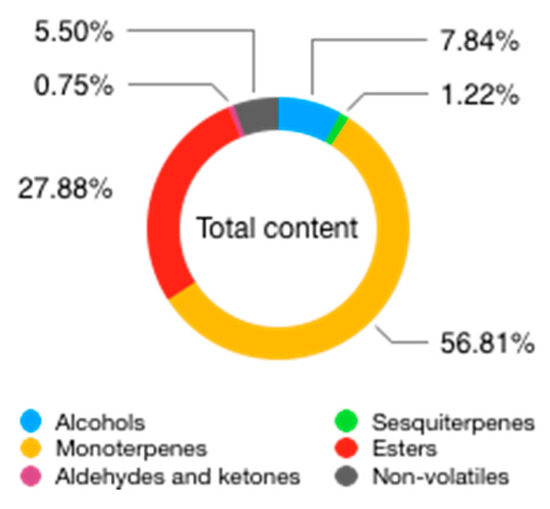
Figure 6. Total contents of BEO.
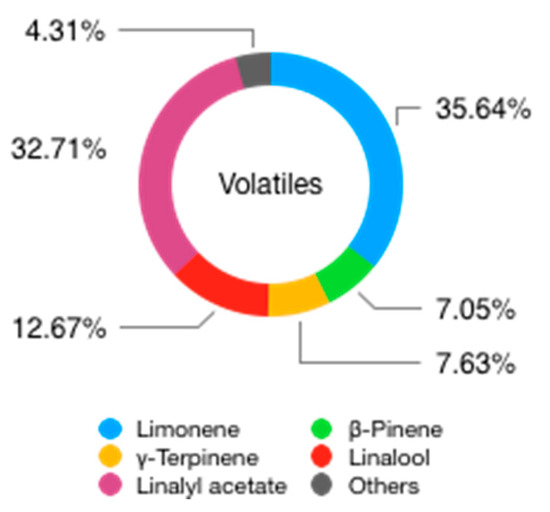
Figure 7. Volatile compounds found in BEO.
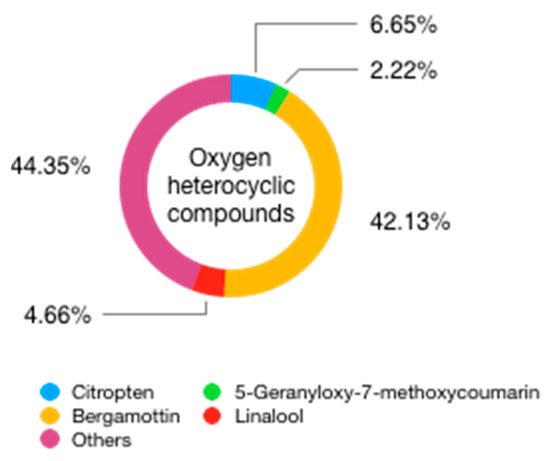
Figure 8. Non-volatile, oxygen heterocyclic compounds found in BEO.
Table 6. Chemical description of the most relevant compounds found in BEO.
.
Table 7. Component ratios of genuine versus adulterated BEO.
| Ratios | Genuine EO | Adulterated EO |
|---|---|---|
| Citronellal/Terpinen-4-ol | 0.167–1.875 | 0.119–0.136 |
| Octyl acetate/α-Terpineol | 8.842–4.742 | 0.5–0.561 |
| γ-Terpinene/Sabinene + β-Pinene | 0.661–12.79 | 0.670–0.733 |
| (E)-Sabinene hydrate acetate/α-Terpineol | 0.704–3.323 | 0.303–0.354 |
In a recent paper on BEO genuine composition, Mangiola and colleagues [57] analyzed six samples of Calabrian BEO, taken from the same lots of EO extracted using the “Speciale” machines in the Reggio Calabria area, during the 2007–2008 season. The physicochemical data on these samples are listed in Table 8.
Table 8. Major physicochemical characteristics of BEO of Calabrian origin.
| Parameters | Values | ||||
|---|---|---|---|---|---|
| Enantiomers | Ratio (%) | Ratio According to Mangiola et al. (2009) (%) [57] | |||
| Relative density at 20 °C | 0.8740–0.8750 | ||||
| Refractive index at 20 °C | |||||
| Linalyl acetate | (3R)-(−)-linalyl acetate/(3S)-(+)-linalyl acetate | 99.7–99.9/0.1–0.3 | 99.6–99.7/0.3–0.4 | 1.4676–1.4690 | |
| Linalool | (3R)-(−)-linalool/(3S)-(+)-linalool | 99.4–99.7/0.3–0.6 | 99.3–99.5/0.5–0.7 | Optical rotation at 20 °C | +28.6–+32.4 |
| α-Pinene | (1S,5S)-(−)-α pinene/(1Esters (%) expressed as linalyl acetate | 33.10–35.96 | |||
| Solubility in ethanol at 90° | Insoluble–1:0.5 | ||||
| Residue on evaporation (%) | 4.7–5.6 | ||||
| CD | 0.71–0.84 | ||||
| UV Max absorption | 1.04–1.20 |
The same authors used chiral analysis to determine the enantiomeric distribution of important compounds in Calabrian BEO. Table 9 lists these results together with data produced by Mosandl and Juchelka [58], Dugo and colleagues [49] and Bonaccorsi and colleagues about genuine EOs but not belonging to the same lots [59].
Table 9. Enantiomeric distribution of important compounds in Calabrian BEO.
| Molecule |
|---|
(3
R)-(−)-linalool/(3S)-(+)-linalool: 99.4–99.5%/0.5–0.6%
Mangiola and colleagues also measured the heterocyclic compounds and found the specific ranges reported in Table 10 [57]. According to Dugo and colleagues, the presence of 5-geranyloxy-8-methoxypsoralen, 5-isopentenyloxy-7-methoxycoumarin, 5-isopentenyl-oxy-8-methoxypsoralen, herniarin and isopimpinellin indicates adulteration with Lime EO [49].
Table 10. Range of heterocyclic compounds found in BEO.
| Molecule | Range |
|---|---|
| R | |
| ,5 | |
| R |
76]. Moreover, the antimicrobial action of BEO has been tested on a wide range of microorganisms, including bacteria commonly involved in gastrointestinal or skin diseases (Helycobacter pylori, Campylobacter jejuni, Escherichia coli 0157, Listeria monocytogenes, Bacillus cereus, and Staphylococcus aureus), and the most relevant mechanism of action appears to be a lytic effect of various EO components on microorganism cell membranes, as observed with electron microscopy [75].
Based on the available evidence, it is possible to list the main pharmacological properties of BEO as follows:
]. While for stress management the level of evidence about BEO efficacy seems quite good, the strength of recommendations for other indications (anxiety and psoriasis) is lower, mostly for the scarcity of specific clinical data. Concerning chronic pain control, evidence derives from in-vivo laboratory models, and clinical investigations are lacking to date. As such, further clinical research on the topic is advised, especially to explore potentially beneficial uses of BEO in chronic pain control and its antimicrobial effects against multi-resistant germs. Some clinical recommendations for the integrative use of BEO along with the strength of available clinical evidence have been reported in Table 11.
Table 11. Clinical recommendations of complementary therapy with BEO [76][77].
| Product | Indications | Dosages for a Complementary Therapy | Evidence * |
|---|
| Bergamottin | 10,969–20,890 mg/L |
| BEO | Stress | Aromatherapy for 15–30 min (inhalation) | B | ||
| Bergapten | 1384–4750 mg/L | ||||
| Anxiety | Aromatherapy for 15–30 min (inhalation) | C | |||
| )-(+)-α pinene | Citropten90.5–93.2/6.8–9.5 | – | |||
| 1340–2124 mg/L | β-Pinene | (1S,5S)-(−)-β pinene/(1R,5R)-(+)-β pinene | 91/9 | 90.5–91.3/8.7–9.5 | |
| 5-Geranyloxy-7-methoxycoumarin | 878–1035 mg/L | Sabinene | (1S,5S)-(−)-sabinene/(1R,5R)-(+)-sabinene | 81.2–85.9/14.1–18.2 | 83.7–86.3/13.7–16.3 |
| Limonene | (4R)-(+)-limonene/(4S)-(−)-limonene | 97.3–98.1/1.9–2.7 | 97.9–98.1/1.9–2.1 | ||
| Terpinen-4-ol | (4R)-(−)-terpinen-4-ol/(4S)-(+)-terpinen-4-ol | 73.7–90.3/9.7–26.3 | 32.3–55.3/44.7–67.7 | ||
| α-Terpineol | (4R)-(+)-α-terpineol/(4S)-(−)-α-terpineol | 30.6–82.5/17.5–69.4 | 31.5–43.7/56.3–68.5 |
| Molecule | Molecular Formula | IUPAC Name | Structure | CAS | Range (%) |
|---|
In terms of comparison, the enantiomeric distribution of linalool and linalyl acetate in genuine BEO from Sicily are the following [60]:
Organoleptic characteristics [61][62]
| Psoriasis | |
| Application on psoriatic plaques 30 min before UVB treatment (topical) | C |
| Chemical class | Monoterpene hydrocarbons | ||||
| Limonene | C10H16 | 1-methyl-4-prop-1-en-2-ylcyclohexene | 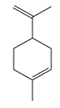 |
138-86-3 | 25–55 |
| α-Pinene | C10H16 | 2,6,6-trimethylbicyclo[3.1.1]hept-2-ene | 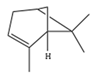 |
80-56-8 | 0.72–1.4 |
| β-Pinene | C10H16 | 6,6-dimethyl-2-methylidenebicyclo[3.1.1]heptane | 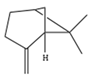 |
127-91-3 | 4–11 |
| γ-Terpinene | C10H16 | 1-methyl-4-propan-2-ylcyclohexa-1,4-diene | 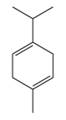 |
99-85-4 | 5–11 |
| cis-β-Ocimene | C10H16 | (3Z)-3,7-dimethylocta-1,3,6-triene | 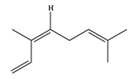 |
338-55-4 | 0.17–0.27 |
| Chemical class | Sesquiterpene hydrocarbons | ||||
| β-Bisabolene | C15H24 | (4S)-1-methyl-4-(6-methylhepta-1,5-dien-2-yl)cyclohexene | 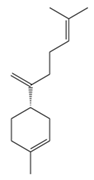 |
495-61-4 | 0.21–0.65 |
| (E)-α-Bergamotene | C15H24 | (1S,5S,6R)-2,6-dimethyl-6-(4-methylpent-3-enyl)bicyclo[3.1.1]hept-2-ene | 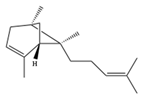 |
13474-59-4 | 0.16–0.50 |
| β-Caryophyllene | C15H24 | (1R,4E,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | 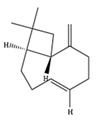 |
87-44-5 | 0.15–0.55 |
| Chemical class | Monoterpene alcohols | ||||
| Linalool | C10H18O | 3,7-dimethylocta-1,6-dien-3-ol | 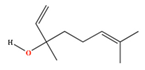 |
78-70-6 | 2–20 |
| Geraniol | C10H18O | (2E)-3,7-dimethylocta-2,6-dien-1-ol | 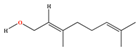 |
106-24-1 | 0–0.01 |
| Chemical class | Sesquiterpene alcohols | ||||
| α-Bisabolol | C15H26O | 6-methyl-2-(4-methylcyclohex-3-en-1-yl)hept-5-en-2-ol | 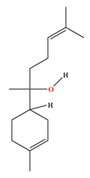 |
72059-10-0 | 0.01–0.03 |
| Chemical class | Monoterpene oxides | ||||
| (Z)-Limonene oxide | C10H16O | Terpene oxide (1S,4S,6R)-1-methyl-4-prop-1-en-2-yl-7-oxabicyclo[4.1.0]heptane | 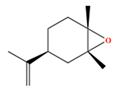 |
32543-51-4 | tr–0.02 |
| Chemical class | Monoterpene esters | ||||
| Linalyl acetate | C12H20O2 | 3,7-dimethylocta-1,6-dien-3-yl acetate | 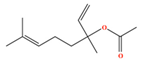 |
115-95-7 | 15–40 |
| Neryl acetate | C12H20O2 | [(2Z)-3,7-dimethylocta-2,6-dienyl] acetate | 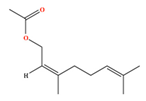 |
141-12-8 | 0.13–0.67 |
| Geranyl acetate | C12H20O2 | [(2E)-3,7-dimethylocta-2,6-dienyl] acetate | 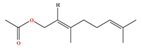 |
105-87-3 | 0.11–0.87 |
| Linalyl propionate | C13H22O2 | 3,7-dimethylocta-1,6-dien-3-yl propanoate | 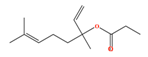 |
144-39-8 | 0.01–0.07 |
| (E)-Sabinene hydrate acetate | C12H20O2 | [(2S,5S)-2-methyl-5-propan-2-yl-2-bicyclo[3.1.0]hexanyl] acetate | 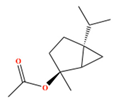 |
Not Available | 0.015–0.13 |
| Chemical class | Aliphatic esters | ||||
| Octyl acetate | C10H20O2 | octyl acetate |  |
112-14-1 | 0.06–0.22 |
| Chemical class | Aliphatic aldehyde | ||||
| Octanal | C8H16O | Octanal |  |
124-13-0 | 0.02–0.09 |
| Decanal | C10H20O | Decanal |  |
112-31-2 | 0.04–0.1 |
| Chemical class | Oxygen heterocyclic compounds (psoralens) | ||||
| Bergaptene | C12H8O4 | 4-methoxyfuro[3,2-g]chromen-7-one | 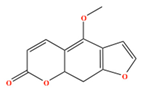 |
484-20-8 | 0.11–0.32 |
| Bergamottin | C21H22O4 | 4-[(2E)-3,7-dimethylocta-2,6-dienoxy]furo[3,2-g]chromen-7-one | 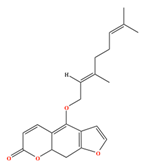 |
7380-40-7 | 1.02–2.75 |
| Chemical class | Oxygen heterocyclic compounds (coumarins) | ||||
| Citropten | C11H10O4 | 5,7-dimethoxychromen-2-one | 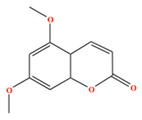 |
487-06-9 | 0.14–0.35 |
| 5-Geranyloxy-7-methoxycoumarin | C20H24O4 | 5-Geranyloxy-7-methoxycoumarin | 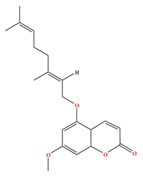 |
7380-39-4 | 0.08–0.22 |
4. Standards and Adulteration
Because of the high prices of BEO, subpar production or downright adulteration of the product are quite common. While the physicochemical standards set up by the ISO, the AFNOR, the Experimental Station for the Citrus Essences and Derivatives Industry (Reggio Calabria, Italy), and the “Disciplinare per la Denominazione di Origine Protetta” (DOP: Protected Designation of Origin, Italy) have been important to establish the genuineness of a product, they are not sufficient anymore when confronted by adulteration with the addition of synthetic compounds or other, more sophisticated techniques [57]. For this reason, more advanced techniques to uncover adulterations have been used, for example, components ratio and enantiomeric distribution using chiral analysis. In 1996, Verzera and colleagues [52] already compared genuine BEO samples with some adulterated commercial EOs and concluded that differentiation was possible through the use of components ratio, as shown in Table 7
Relaxing, calming, and anxiolytic
Antinociceptive and anti-inflammatory
(3
R
)-(−)-linalyl acetate/(3
S
)-(+)-linalyl acetate: 99.7–99.8%/0.2–0.3%
Colour: from green to yellowish-green to yellowish-brown. The colour fades with ageing, particularly when the EO is exposed to daylight.
-
Odour: the top note is extremely rich, fresh, sweet-citrusy, lavandaceous, with a tea-like nuance. The dry-down is oily-herbaceous, balsamic, with a lemony-citrus character, and a slight tobacco/tea note, somewhat reminiscent of clary sage and neryl acetate.
-
The distilled, FCF, EO has a thinner odour, with p-cymene and linalyl acetate notes to the fore, and the dry-down has a pithy, citrusy lemony character, very clean and fresh.
-
The terpeneless EO is much less fresh than the c.p. one, and only a little stronger.
5. Pharmacological Activities of Bergamot Derivatives
In a literature review about the pharmacological properties of various Citrus spp. derivatives, BEO was described to possess melanogenic, antinociceptive, antiproliferative, sedative, anxiolytic, neuroprotective, antioxidant, and antimicrobial activities [63]. Neuropharmacological studies of BEO showed its usefulness in neuroprotection (even in the course of experimental brain ischemia), chronic pain control, and in the management of stress, anxiety and anxiety-related conditions [53][64][65][66][67]. It has been suggested that the pharmacological mechanism of action of BEO on the central nervous system can involve different neuronal pathways, with an integrated effect on both 5-HT and GABA-A receptors and with an anxiety-lowering activity which is not superimposable to that of benzodiazepines [68]. The antinociceptive effect of BEO seems to include possible involvement of peripheral cannabinoid and opioid systems, with the consequent release of endogenous mediators capable of reducing pain perception [65]. This is probably the reason why preclinical evidence indicates that BEO is more effective in the control of neuropathic pain if compared with other types of pain [69]. Interestingly, the effect on pain management can be exerted even when BEO is administered through inhalation [70]. Overall, the most relevant active components of BEO with a potential anxiolytic, relaxing, and antinociceptive effect on the nervous system are believed to be terpenes like pinene, limonene, linalool, and linalyl acetate, whose properties have been described in several studies [71][72][73].
Furthermore, Bergamot derivatives, including the EO, have been hypothesized to have anti-inflammatory [74] and anti-infective properties, especially against bacteria and also against some fungi [75]. In particular, antiphlogistic effects of BEO have been demonstrated in animal models of carrageenan-induced inflammation and are believed to be mostly attributed to the abovementioned terpenes [74][
Antimicrobial
Overall, indications of BEO administration mostly regard psychophysical stress and anxiety (along with anxiety-related conditions) due to its clinically-demonstrated relaxing and calming effects, and psoriasis, for a combination of anti-inflammatory, keratolytic and antimicrobial effects [76][77
6. Toxicology of BEO
According to the European Medicines Agency (EMA), the most relevant concerns about BEO toxicology regards its photosensitive and melanogenic properties, traditionally ascribed to its content in furocoumarins (psoralens) such as bergapten, which may be even photo-mutagenic if applied alone as a pure substance [79]. A study by Zaynoun et al. suggested that psoralen-induced photosensitivity can be influenced by a range of specific factors, including the concentration of psoralens and ethanol in the vehicle, the interval of time between the product application and irradiation, the level of skin hydration, and the degree of natural pigmentation; however, individual factors like sex, age, and the patient’s susceptibility to suntanning seem to play no role in influencing the skin reaction [80]. Despite the larger use of Bergamot-derived products in recent years, there has been no substantial increase in the number of cases describing BEO adverse effects, and this may be due to an under-report of actual cases but also to the spread of safer psoralen-free Bergamot derivatives in the market [79]. Moreover, apart from the psoralen content, the International Fragrance Association (IFRA) recommends that the content of BEO in skin products should never exceed 0.4% to prevent the occurrence of adverse effects [53]. The onset of light-related skin reaction usually follows the application of BEO with psoralens 2 to 72 h after exposure [79][81], and it has the characteristics of bullous dermatitis [79]. According to Tisserand and Young, all of the major constituents (limonene, linalyl acetate, linalool) and most of the minor constituents of BEO are known to be safe in terms of systemic toxicity [82], and acute toxicity of the EO in rats has been reported only for doses above the 10 g/kg threshold [83]. Still, provided that no specific data exists for the safety profile of BEO in lactating and pregnant women, its use has to be avoided in these categories of patients, unless further clinical information becomes available. Additionally, in consideration of limited safety data, caution has to be adopted in more fragile individuals like children and very elderly subjects and even in patients with allergic diathesis. Finally, repeated and excessive use over time may cause problems even in healthy subjects due to in-vitro demonstrated cytotoxic and antiproliferative effects of high doses of BEO [83]. Cases of adverse effects caused by BEO application report that aromatherapy (sauna with vaporized BEO followed by artificial or natural sunbathing) can cause skin photosensitivity [53][84] and the oral intake of large amounts of BEO can be associated with neurological symptoms (muscle cramps, fasciculations, paraesthesias and blurred vision) [85]. Overall, the safety profile of BEO has to be fully outlined yet; however, the most important adverse effects (photosensitivity, neurological symptoms) can be avoided by resorting to psoralen-free formulations and by limiting the intake to short periods.
7. Conclusions and Prospects
BEO can be a valuable food, cosmetic and medicinal resource with a long-lasting tradition in folk medicine and local cuisine. With their remarkable history, production methods of BEO share some technical characteristics and are influenced by the specific chemo-botanical properties of this fruit. Due to its high prices, adulterations are not uncommon and regulatory authorities should monitor market products to protect consumers. Further research is advised to better study any potential therapeutic uses of BEO along with its toxicological profile and to possibly improve its production yield and quality standard assessment methods.
References
- Zhong, G.; Nicolosi, E. Citrus Origin, Diffusion, and Economic Importance. In The Citrus Genome; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 5–21, ISBN 9783030153083.
- Calabrese, F. La Favolosa Storia Degli Agrumi; L’Epos: Palermo, Italy, 1998.
- Inglese, P.; Sortino, G. Citrus History, Taxonomy, Breeding, and Fruit Quality. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: Oxford, UK, 2019; ISBN 9780199389414.
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316, doi:10.1038/nature25447.
- Dugo, G.; Mondello, L. Citrus Oils: Composition, Advanced Analytical Techniques, Contaminants, and Biological Activity; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439800294.
- Distefano, G.; Las Casas, G.; Deng, X.; Chai, L. Citrus Reproductive Biology from Flowering to Fruiting. In The Citrus Genome; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 167–176, ISBN 9783030153083.
- Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; Stevens, P.F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20, doi:10.1111/boj.12385.
- Thorne, R.F. The classification and geography of the flowering plants: Dicotyledons of the class Angiospermae. Bot. Rev. 2000, 66, 441–647, doi:10.1007/BF02869011.
- Swingle, W.T. The botany of Citrus and its wild relations. Citrus Ind. 1967, 1, 190–422.
- Samuel, R.; Ehrendorfer, F.; Chase, M.W.; Greger, H. Phylogenetic analyses of Aurantioideae (Rutaceae) based on non-coding plastid DNA sequences and phytochemical features. Plant Biol. 2001, 3, 77–87.
- Dearaujo, E. What is Citrus? Taxonomic implications from a study of cp-DNA evolution in the tribe Citreae (Rutaceae subfamily Aurantioideae). Org. Divers. Evol. 2003, 3, 55–62.
- Bayer, R.J.; Mabberley, D.J.; Morton, C.; Miller, C.H.; Sharma, I.K.; Pfeil, B.E.; Rich, S.; Hitchcock, R.; Sykes, S. A molecular phylogeny of the orange subfamily (Rutaceae: Aurantioideae) using nine cpDNA sequences. Am. J. Bot. 2009, 96, 668–685, doi:10.3732/ajb.0800341.
- Dugo, G.; Di Giacomo, A. Citrus: The Genus Citrus; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780203216613.
- Goldschmidt, E.E. The Citrus Genome: Past, Present and Future. In The Citrus Genome; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–3, ISBN 9783030153083.
- Velasco, R.; Licciardello, C. A genealogy of the Citrus family. Nat. Biotechnol. 2014, 32, 640–642.
- Carbonell-Caballero, J.; Alonso, R.; Ibañez, V.; Terol, J.; Talon, M.; Dopazo, J. A Phylogenetic Analysis of 34 Chloroplast Genomes Elucidates the Relationships between Wild and Domestic Species within the Genus Citrus. Mol. Biol. Evol. 2015, 32, 2015–2035, doi:10.1093/molbev/msv082.
- Wu, G.A.; Prochnik, S.; Jenkins, J.; Salse, J.; Hellsten, U.; Murat, F.; Perrier, X.; Ruiz, M.; Scalabrin, S.; Terol, J.; et al. Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during Citrus domestication. Nat. Biotechnol. 2014, 32, 656–662.
- Curk, F.; Ollitrault, F.; Garcia-Lor, A.; Luro, F.; Navarro, L.; Ollitrault, P. Phylogenetic origin of limes and lemons revealed by cytoplasmic and nuclear markers. Ann. Bot. 2016, 117, 565–583, doi:10.1093/aob/mcw005.
- Schwartz, T.; Nylinder, S.; Ramadugu, C.; Antonelli, A.; Pfeil, B.E. The Origin of Oranges: A Multi-locus Phylogeny of Rutaceae Subfamily Aurantioideae. Syst. Bot. 2016, 40, 1053–1062, doi:10.1600/036364415X690067.
- Hodgson, R.W. Horticultural varieties of Citrus. In The Citrus Industry; Reuther, W., Webber, H.J., Batchelor, L.D., Eds.; Volume I.; University of California Press: Berkeley, CA, USA, 1967; pp. 431–592.
- Köhler, H.A. Köhler’s Medizinal-Pflanzen. Arch. Pharm. 1887, 225, 276–276.
- Dugo, G.; Bonaccorsi, I. Citrus Bergamia: Bergamot and its Derivatives; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781439862278.
- Uzun, A.; Yesiloglu, T.; Aka-Kacar, Y.; Tuzcu, O.; Gulsen, O. Genetic diversity and relationships within Citrus and related genera based on sequence related amplified polymorphism markers (SRAPs). Sci. Hortic. 2009, 121, 306–312.
- Federici, C.T.; Roose, M.L.; Scora, R.W. RFLP analysis of the origin of Citrus bergamia, Citrus jambhiri, and Citrus limonia. Proc. First Int. Citrus Biotechnol. Symp. 1998, 535, 55–64.
- Nicolosi, E.; Deng, Z.N.; Gentile, A.; La Malfa, S.; Continella, G.; Tribulato, E. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor. Appl. Genet. 2000, 100, 1155–1166, doi:10.1007/s001220051419.
- Li, X.; Xie, R.; Lu, Z.; Zhou, Z. The origin of cultivated Citrus as inferred from internal transcribed spacer and chloroplast DNA sequence and amplified fragment length polymorphism fingerprints. J. Am. Soc. Hortic. Sci. 2010, 135, 341–350.
- Maruca, G.; Laghetti, G.; Mafrica, R.; Turiano, D.; Hammer, K. The Fascinating History of Bergamot (Citrus Bergamia Risso & Poiteau), the Exclusive Essence of Calabria: A Review. JESE-A 2017, 6, doi:10.17265/2162-5298/2017.01.003.
- Guala, G. Integrated Taxonomic Information System (ITIS); National Museum of Natural History, Smithsonian Institution: Washington, DC, USA, 2019.
- Ipni. The International Plant Names Index; Royal Botanic Gardens, Kew: Richmond, UK, 2012.
- Tropicos. Available online: http://www.tropicos.org (accessed on 6 November 2020).
- Govaerts, R.H.A.; Faden, R.B. World Checklist of Selected Plant Families; Royal Botanic Gardens, Kew: Richmond, UK, 2016.
- Search Accessions GRIN-Global. Available online: https://npgsweb.ars-grin.gov/gringlobal/search (accessed on 6 November 2020).
- Mabberley, D.J. Mabberley’s Plant-Book, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008.
- Fatta Del Bosco, S.; Abbate, L.; Mercati, F.; Napoli, E.; Ruberto, G. Essential Oils in Citrus. In The Citrus Genome; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 211–223, ISBN 9783030153083.
- Rapisarda, A.; Caruso, C.; Iauk, L.; Ragusa, S. Applicazione dell’analisi di immagine nello studio delle ghiandole oleifere dei frutti di alcune specie del genere Citrus. Essenze Deriv. Agrum. 1996, 66, 5–12.
- Svoboda, K.P.; Svoboda, T.G.; Syred, A.D. Secretory Structures of Aromatic and Medicinal Plants: A Review and Atlas of Micrographs; Microscopix Publications: Knighton, Wales, 2000.
- Amato, A.; Castellotti, T.; Gaudio, F.; Gaudio, G.; Lovecchio, R.; Pupo D’Andrea, M.R.; Peluso, R. L’agricoltura nella Calabria in cifre 2012; INEA–Istituto Nazionale di Economia Agraria: Roma, Italy, 2013. Available online: http://www.inea.it/pubbl/ (accessed on 6 November 2020).
- Giacomo, A.D. Bergamot in the calabrian economy and the role of consorzio del bergamotto. In Citrus Bergamia; CRC Press: Boca Raton, FL, USA, 2013; pp. 74–85, ISBN 9780429165887.
- Ciani, F.; Huggard, J.; Zervas, T. The Resilience of Bergamot Farmers to Economic Shocks in the Reggio Calabria Province. Reg. Mag. 2016, 302, 23–25, doi:10.1080/13673882.2016.11742705.
- Nesci, F.S.; Sapone, N. Bergamot–A Green and Multifunctional Asset Exclusive from the Province of Reggio Calabria. AEF 2014, 11, 376–379, doi:10.4028/www.scientific.net/AEF.11.376.
- Guenther, E. The Individual Essential Oils of the Plant Families Rutaceae and Labiatae; D. Van Nostrand Company, Inc.: New York, NY, USA, 1950; Volume 3.
- Jin, W.F.; Shen, L.H.; Ren, J.H.; Jin, J.M.; Shen, Y.Y.; Zhu, J.Q.; Liang, Z.S.; Yang, D.F. Research progress on bergamot essential oil and its related product development. Zhong Cao Yao 2016, 47, 857–861.
- Yang, H.; Zhou, A.; Lin, M.; Xie, W.; Chen, S.; Chen, H. Research progress of extraction methods and pharmacological effects of bergamot essential oil. J. Food Saf. Qual. 2013, 4, 1347–1352.
- Dugo, P.; Russo, M. The Oxygen Heterocyclic Components of Citrus Essential Oils. In Citrus Oils: Composition, Advanced Analytical Techniques, Contaminants, and Biological Activity; Dugo, G., Mondello, L., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 405–444, ISBN 9781439800294.
- Hunter, M. Essential Oils: Art, Agriculture, Science, Industry and Entrepreneurship; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010.
- Di Giacomo, A.; Di Giacomo, G. Essential oil production. In Citrus: The Genus Citrus; Dugo, G., di Giacomo, A., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 114–147, ISBN 9780203216613.
- Chen, M.-H.; Huang, T.-C. Volatile and Nonvolatile Constituents and Antioxidant Capacity of Oleoresins in Three Taiwan Citrus Varieties as Determined by Supercritical Fluid Extraction. Molecules 2016, 21, 1735, doi:10.3390/molecules21121735.
- Sawamura, M. Citrus Essential Oils: Flavor and Fragrance; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 9781118074381.
- Dugo, G.; Bartle, K.D.; Stagno D’alcontres, I.; Trozzi, A.; Verzera, A. Advanced analytical techniques for the analysis of Citrus essential oils. Part 3. Oxygen heterocyclic compounds: HPLC, HPLC/MS, OPLC, SFC, Fast HPLC analysis. Essenze Deriv. Agrum. 1999, 69, 251–283.
- Poiana, M.; Rosa Fresa, R.; Mincione, B. Supercritical carbon dioxide extraction of bergamot peels. Extraction kinetics of oil and its components. Flavour Fragr. J. 1999, 14, 358–366.
- Figoli, A.; Donato, L.; Carnevale, R.; Tundis, R.; Statti, G.A.; Menichini, F.; Drioli, E. Bergamot essential oil extraction by pervaporation. Desalination 2006, 193, 160–165.
- Verzera, A.; Lamonica, G.; Mondello, L.; Trozzi, A.; Dugo, G. The composition of bergamot oil. Perfum. Flavor. 1996, 21, 19–34.
- Navarra, M.; Mannucci, C.; Delbò, M.; Calapai, G. Citrus bergamia essential oil: From basic research to clinical application. Front. Pharmacol. 2015, 6, 36, doi:10.3389/fphar.2015.00036.
- Sawamura, M.; Onishi, Y.; Ikemoto, J.; Tu, N.T.M.; Phi, N.T.L. Characteristic odour components of bergamot (Citrus bergamia Risso) essential oil. Flavour Fragr. J. 2006, 21, 609–615, doi:10.1002/ffj.1604.
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12, doi:10.3389/fpls.2019.00012.
- Di Giacomo, A.; Mincione, B. Gli Olii Essenziali Agrumari in Italia. Sottoprogetto 4; Laruffa: Reggio Calabria, Italy, 1994; ISBN 9788872210734.
- Mangiola, C.; Postorino, E.; Gionfriddo, F.; Catalfamo, M.; Manganaro, R.; Calabro, G. Evaluation of the genuineness of cold-pressed bergamot oil. Perfum. Flavorist 2009, 34, 26–32.
- Mosandl, A.; Juchelka, D. Advances in the Authenticity Assessment of Citrus Oils. J. Essent. Oil Res. 1997, 9, 5–12.
- Bonaccorsi, I.; Sciarrone, D.; Cotroneo, A.; Mondello, L.; Dugo, P.; Dugo, G. Enantiomeric distribution of key volatile components in Citrus essential oils. Rev. Bras. Farmacogn. 2011, 21, 841–849.
- Verzera, A.; La Rosa, G.; Zappala, M.; Cotroneo, A. Essential oil composition of different cultivars of bergamot grown in Sicily. Ital. J. Food Sci. 2000, 12, 493–502.
- Burfield, T. Natural aromatic materials–Odours & origins. Int. J. Aromather. 2001, 11, 47–48, doi:10.1016/S0962-4562(01)80070-9.
- Arctander, S. Perfume and Flavor Materials of Natural Origin: A Perfumer’s and Flavorist’s Practical Description of Available Materials, Their Origin, Production and Processing, Appearance, Odor and Flavor Type, Evaluation, Application and Availability with Brief Notes on Their Main Constituents, Replacements and Most Common Adulterants; Allured Publishing: Carol Stream, IL, USA, 1994.
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966, doi:10.3390/ijms19071966.
- Scuteri, D.; Rombolá, L.; Tridico, L.; Mizoguchi, H.; Watanabe, C.; Sakurada, T.; Sakurada, S.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Neuropharmacological Properties of the Essential Oil of Bergamot for the Clinical Management of Pain-Related BPSDs. Curr. Med. Chem. 2019, 26, 3764–3774, doi:10.2174/0929867325666180307115546.
- Rombolà, L.; Amantea, D.; Russo, R.; Adornetto, A.; Berliocchi, L.; Tridico, L.; Corasaniti, M.T.; Sakurada, S.; Sakurada, T.; Bagetta, G.; et al. Rational Basis for the Use of Bergamot Essential Oil in Complementary Medicine to Treat Chronic Pain. Mini Rev. Med. Chem. 2016, 16, 721–728, doi:10.2174/1389557516666160321113913.
- Scuteri, D.; Rombolà, L.; Morrone, L.A.; Bagetta, G.; Sakurada, S.; Sakurada, T.; Tonin, P.; Corasaniti, M.T. Neuropharmacology of the Neuropsychiatric Symptoms of Dementia and Role of Pain: Essential Oil of Bergamot as a Novel Therapeutic Approach. Int. J. Mol. Sci. 2019, 20, 3327, doi:10.3390/ijms20133327.
- Bagetta, G.; Morrone, L.A.; Rombolà, L.; Amantea, D.; Russo, R.; Berliocchi, L.; Sakurada, S.; Sakurada, T.; Rotiroti, D.; Corasaniti, M.T. Neuropharmacology of the essential oil of bergamot. Fitoterapia 2010, 81, 453–461, doi:10.1016/j.fitote.2010.01.013.
- Rombolà, L.; Scuteri, D.; Adornetto, A.; Straface, M.; Sakurada, T.; Sakurada, S.; Mizoguchi, H.; Corasaniti, M.T.; Bagetta, G.; Tonin, P.; et al. Anxiolytic-Like Effects of Bergamot Essential Oil Are Insensitive to Flumazenil in Rats. Evid. Based. Complement. Alternat. Med. 2019, 2019, 2156873, doi:10.1155/2019/2156873.
- Bagetta, G.; Berliocchi, L.; Rombolà, L.; Morrone, L.A.; Sakurada, T.; Corasaniti, M.T.; Sakurada, S. Preclinical evidence for rational use of bergamot essential oil in pain trials. Planta Med. 2015, 81, doi:10.1055/s-0035-1565356.
- Scuteri, D.; Crudo, M.; Rombolà, L.; Watanabe, C.; Mizoguchi, H.; Sakurada, S.; Sakurada, T.; Greco, R.; Corasaniti, M.T.; Morrone, L.A.; et al. Antinociceptive effect of inhalation of the essential oil of bergamot in mice. Fitoterapia 2018, 129, 20–24, doi:10.1016/j.fitote.2018.06.007.
- Rombolà, L.; Tridico, L.; Scuteri, D.; Sakurada, T.; Sakurada, S.; Mizoguchi, H.; Avato, P.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Bergamot Essential Oil Attenuates Anxiety-Like Behaviour in Rats. Molecules 2017, 22, 614, doi:10.3390/molecules22040614.
- Donelli, D.; Antonelli, M.; Bellinazzi, C.; Gensini, G.F.; Firenzuoli, F. Effects of lavender on anxiety: A systematic review and meta-analysis. Phytomedicine 2019, 65, 153099, doi:10.1016/j.phymed.2019.153099.
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2020, 17, 6506, doi:10.3390/ijerph17186506.
- Ferlazzo, N.; Cirmi, S.; Calapai, G.; Ventura-Spagnolo, E.; Gangemi, S.; Navarra, M. Anti-Inflammatory Activity of Citrus bergamia Derivatives: Where Do We Stand? Molecules 2016, 21, 1273, doi:10.3390/molecules21101273.
- Cirmi, S.; Bisignano, C.; Mandalari, G.; Navarra, M. Anti-infective potential of Citrus bergamia Risso et Poiteau (bergamot) derivatives: A systematic review. Phytother. Res. 2016, 30, 1404–1411, doi:10.1002/ptr.5646.
- Perna, S.; Spadaccini, D.; Botteri, L.; Girometta, C.; Riva, A.; Allegrini, P.; Petrangolini, G.; Infantino, V.; Rondanelli, M. Efficacy of bergamot: From anti-inflammatory and anti-oxidative mechanisms to clinical applications as preventive agent for cardiovascular morbidity, skin diseases, and mood alterations. Food Sci. Nutr. 2019, 7, 369–384, doi:10.1002/fsn3.903.
- Mannucci, C.; Navarra, M.; Calapai, F.; Squeri, R.; Gangemi, S.; Calapai, G. Clinical Pharmacology of Citrus bergamia: A Systematic Review. Phytother. Res. 2017, 31, 27–39, doi:10.1002/ptr.5734.
- Kavanagh, B.P. The GRADE system for rating clinical guidelines. PLoS Med. 2009, 6, e1000094, doi:10.1371/journal.pmed.1000094.
- European Medicines Agency Assessment Report on Citrus Bergamia Risso et Poiteau, Aetheroleum. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-citrus-bergamia-risso-et-poiteau-aetheroleum_en.pdf (accessed on 24 November 2020).
- Zaynoun, S.T.; Johnson, B.E.; Frain-Bell, W. A study of oil of bergamot and its importance as a phototoxic agent. II. Factors which affect the phototoxic reaction induced by bergamot oil and psoralen derivatives. Contact Dermat. 1977, 3, 225–239, doi:10.1111/j.1600-0536.1977.tb03667.x.
- Dubertret, L.; Serraf-Tircazes, D.; Jeanmougin, M.; Morlière, P.; Averbeck, D.; Young, A.R. Phototoxic properties of perfumes containing bergamot oil on human skin: Photoprotective effect of UVA and UVB sunscreens. J. Photochem. Photobiol. B Biol. 1990, 7, 251–259, doi:10.1016/1011-1344(90)85160-X.
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Churchill Livingstone: London, UK; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780443062414.
- Berliocchi, L.; Ciociaro, A.; Russo, R.; Cassiano, M.G.V.; Blandini, F.; Rotiroti, D.; Morrone, L.A.; Corasaniti, M.T. Toxic profile of bergamot essential oil on survival and proliferation of SH-SY5Y neuroblastoma cells. Food Chem. Toxicol. 2011, 49, 2780–2792, doi:10.1016/j.fct.2011.08.017.
- Kaddu, S.; Kerl, H.; Wolf, P. Accidental bullous phototoxic reactions to bergamot aromatherapy oil. J. Am. Acad. Dermatol. 2001, 45, 458–461, doi:10.1067/mjd.2001.116226.
- Finsterer, J. Earl Grey tea intoxication. Lancet 2002, 359, 1484, doi:10.1016/S0140-6736(02)08436-2.
