Chromium (Cr) is a common element in the Earth’s crust. It may exist in different oxidation states, Cr(0), Cr(III) and Cr(VI), with Cr(III) and Cr(VI) being relatively stable and largely predominant. Chromium’s peculiarity is that its behavior relies on its valence state. Cr(III) is a trace element in humans and plays a major role in glucose and fat metabolism. The beneficial effects of Cr(III) in obesity and types 2 diabetes are known. It has been long considered an essential element, but now it has been reclassified as a nutritional supplement. On the other hand, Cr(VI) is a human carcinogen and exposure to it occurs both in occupational and environmental contexts. It induces also epigenetic effects on DNA, histone tails and microRNA; its toxicity seems to be related to its higher mobility in soil and swifter penetration through cell membranes than Cr(III). The microorganisms Acinetobacter sp. Cr1 and Pseudomonas sp. Cr13 have been suggested as a promising agent for bioremediation of Cr(VI).
- chromium
- essential nutrient
- toxic element
- epigenetics
- remediation
1. Introduction
Heavy metals are pollutants present in the air and in the soil from natural and anthropogenic sources. Among heavy metals, chromium represents a fascinating case. In its prevalent oxidation states, III and VI, it has completely different characteristics in terms of toxicity and essentiality in human health, and behavior in the soil. Cr(III) derives from anthropogenic sources and is an essential nutrient for humans. It has been defined a pharmacologically active element considering its important role in carbohydrate and lipid metabolism and in the maintenance of the structural integrity of nucleic acids [1]. Cr(III) is a constituent of glucose tolerance factor (GTF): this factor is synthesized in vivo from absorbed dietary chromium and it modulates the rate of removal of glucose from blood with an insulin boosting mechanism. Cr(III) improves insulin activity, as it binds to insulin and potentiates its action by about three-fold. Therefore, chromium deficiencies can lead to pathologies associated with carbohydrates and weight loss [2][3][2,3].
Recently, the potential utilization of chromium to fight thermal stress in animals has been reported. Heat stress (HS) may influence nutrient digestion, carcass quality and the immune system. Homeorhetic adaptations, generally caused by increased circulation of insulin, may be also altered. Cr(III) prevents HS-induced lipid peroxidation, increases nutrient metabolism and cortisol hormone activity, stimulates the action of insulin in responsive tissues and thus it may act in fighting the side effects of heat stress in animals [4][5][4,5]. The required daily dose of Cr(III) is 10–40 µg for children up to six months, and 25–35 µg for other ages [6]. Insufficient dietary Cr(III) also induces symptoms equal to diabetes and cardiovascular diseases. The deficiency of this metal can cause blood sugar spikes, elevated cholesterol levels and blood pressure. It may also have other consequences, such as lower resistance to infections, atherosclerosis, hormonal imbalance, nervous disorders, fatigue, etc., [7][8][7,8].
Cr(III) occurs in several foods and supplementation products. It is largely contained in baker’s yeast, red meat, liver, whole grains, red beets; however, it is hardly assimilated (only 3% is retained by body). The most common supplementation products include chromium-picolinate (CrPic), chromium-histidinate (CrHis), chromium-dinicocysteinate (CRDC), and niacin-bound chromium (NBC) [9]. Several studies have been developed to evaluate the safety and efficacy of these supplements as insulin-sensitizing agents useful for prevention and treatment of type 2 diabetes (T2DM) and obesity [10][11][12][13][14][10,11,12,13,14]. However, excessive levels of chromium can determine pathological states. In fact, even if Cr(III) compounds are not able to cross cell membranes, they may accumulate around cells causing alterations in cell functions and damaging the cell-membranes. In fact, long time exposure to Cr(III) may lead to skin allergies and cancer [15]. Levina et al. (2008) reported also that the accumulation of Cr(III)-based dietary supplements, such as CrPic could induce genotoxic effects [16]. In the supplements Cr(III) may undergo a series of chemical transformations in biological media.
Products deriving from partial hydrolysis of nutritional supplements containing Cr(III) may give reactive species while generation of highly reactive Cr(VI/V/IV) species and organic radicals may derive from reactions of Cr(III) with biological oxidants. However, it must be considered that Cr(III) compounds possess low bioavailability [17]. Eastmond et al. (2008) reported that the genotoxic effects in vivo are uncommon but they can occur for elevated physiological intake levels of Cr(III) supplements [18]. While Cr(III) is an essential trace element, found in nature in rocks and soil, readily absorbed by plants, hexavalent chromium is mainly an industrial contaminant and is also produced from anthropogenic activities. Cr(VI) compounds present several applications and are vastly used as pigments for textile tints, paints, inks, plastics, corrosion preventing agents, leather tanning agents and wood preservatives [19]. Hexavalent chromium is mainly used in tanneries or industries dealing with metalworking, stainless steel welding, chromate production and the manufacture of chromium pigments [20]. The release of Cr(VI) into the air is mainly due to these industrial processes. Chromium in the environment may be derived by inhalation of contaminated air and water [9][21][9,21]. High chromium concentration mainly in water bodies may derive from waste from the ferrochrome industry, such as slag, dust and processed water [22]. Chromium discharge in European Union (EU) waters is subjected to nationwide recommendations, which may vary depending on the type of industry and receiving water body [23]. Cr(VI) is classified by IARC (International Agency for Research on Cancer) as a human carcinogen (class I) [24]. The respiratory tract is the major target for the toxic and carcinogenic action of hexavalent chromium. Acute and chronic occupational exposure mainly occurs by inhalation.
2. Chemical Form and Properties of Chromium
Chromium is the 24th element in the periodic table and its symbol is Cr. It was discovered in 1798 by L. N. Vauquelin. It belongs to transition metals and its average atomic weight is 52 g/mol. Its physicochemical properties are summarized in Table 1. Chromium is the first element of the sixth group; it is a steely-grey, shiny, hard and brittle metal. It is the twenty-first most abundant element in the Earth’s crust [25]. While it does not react with water, it may react with acids. Chromium may theoretically occur in oxidation states between −2 and +6, but the most common oxidation states are +3 and +6 [Cr(III) and Cr(VI)]. Cr2+ is unstable and oxidizes to Cr3+ when in contact with air. Elemental chromium (Cr(0)) is not present in nature in the Earth’s crust and is physiologically inert. Cr(0) in its inert metallic form and at a concentration of about 11% is found almost exclusively as a constituent of iron alloys as stainless steel. The addition of 8% nickel to this alloy increases corrosion resistance [26]. Chromium owes its name to its many-colored compounds colors such as black, green, blue, violet, yellow, orange and red; thus, it has been used in paints and pigments. Common uses of chromium are summarized in Table 2. Chromium is both an essential trace element (as Cr3+) ion and an environmental toxicant (as Cr6+). The latter is considered a heavy metal. However, long time exposure to Cr(III) may lead to skin allergy and cancer [15][27][15,27]. In aqueous solutions, Cr(VI) may exist as chromate ion (CrO42−) or as dichromate or bichromate ion (Cr2O72−). Cr3+ in aqueous solutions is green, CrO42− confers a yellow color to water, while Cr2O72− is orange. Cr(VI) solutions are vigorous oxidizing agents in acidic conditions. Chromic acid (H2CrO4) is utilized for cleaning glassware in chemistry laboratories by oxidizing organic residues. In anoxic conditions, a chromium trivalent form prevails, while the hexavalent form prevails in aerobic conditions. Chromate ions, especially potassium chromate (K2CrO4), are used as indicators in the titration of chloride ions with silver nitrate in the Mohr method [28], while the potassium bichromate (K2Cr2O7) titrimetric method is used to determine chemical oxygen demand (COD), that is the amount of oxygen needed to destroy all organic matter contained in water [29].
Table 1. Physical and chemical properties of chromium.
Atomic number | 24 | ||||
Atomic weight | 51.9961 u | ||||
Atomic radius | 130 pm | ||||
Electronic configuration | [Ar] 4s13d5 | ||||
Melting point | 1907 °C | ||||
Boiling point | 2672 °C | ||||
Density at 20 °C | 7.18 g/cm3 | ||||
Heat of fusion | 21 KJ/mol | ||||
Heat of vaporization | 342 KJ/mol | ||||
Pauling electronegativity number | 1.66 | ||||
First ionization energy | 652.4 KJ/mol | ||||
Second ionization energy | 1590.6 KJ/mol | ||||
Third ionization energy | 2987 KJ/mol |
Table 2. Uses of chromium.
| Form | Uses | ||||
|---|---|---|---|---|---|
Cr(O) | Stainless steel production | ||||
Alloy production | |||||
Metal and alloy manufacturing |
), due to Fenton reaction. These ions are very unstable, causing lipid peroxidation, DNA and protein damage [37].
3. Bioavailability, Absorption and Excretion of Cr(III) and Cr(VI)
Brewer’s yeast, sea food, oysters, liver, meat, cheese, fruits, green beans, spinach and broccoli are dietary sources rich in Cr(III) (Table 3). Chromium content in food is influenced by its presence in the soil in which the plants and vegetables grow and by feedstuffs fed to animals and contamination during processing or cooking methods in Cr-containing stainless-steel equipment [48][49][48,49]. The estimated safe and adequate daily dietary intake for Cr(III), established by the National Research Council, is 50–200 μg/day corresponding to 0.71–2.9 μg/kg/day for a 70 kg adult [10]. In 2001, the Food and Nutrition Board at the Institute of Medicine of the National Academies of Sciences (US) specified the daily chromium intake at a value of 25 µg and 35 µg for women and men, respectively [50]. Maximum intake levels are up to 250 µg/day for supplemental intake as suggested by the World Health Organization (WHO); these are in the same order of magnitude as the exposure resulting from normal dietary intake [51]. Cr(III) is a natural constituent of the diet and may be found in a multiplicity of foods and supplements, whereas Cr(VI) mostly derives from industrial processes and may be found in drinking water as a result of anthropogenic contamination [52][53][52,53].
Table 3. Food sources of chromium.
| Food | Cr (µg/kg) | ||||
|---|---|---|---|---|---|
Mussels | 128 | ||||
Brewer’s yeast | 112 | ||||
Brazil nuts | |||||
Cr(III) | Metal and alloy manufacturing | ||||
Brick lining | |||||
Chrome plating and welding | |||||
Leather tanning | |||||
Textiles | |||||
Cr(VI) | Chrome plating and welding | ||||
Copying machine toner | |||||
Chrome plating | |||||
Leather tanning | |||||
Textiles | |||||
Wood preservatives |
Cr(III) is a micronutrient essential in humans for the metabolism of carbohydrates, lipids and proteins. It is considered the main trace mineral involved in the amelioration and prevention of hyperglycemia and hyperlipidemia in T2DM, as a component of GTF. Administration of Cr(III) orally may markedly alleviate the diabetic-like symptoms [30]. Cr(III) enhances insulin signaling in different tissues [31]. Additionally, trivalent chromium inhibits hepatic enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and interferes with cholesterol metabolism [32]. Cr(III) is also involved in the reduction of plasma cholesterol and triglycerides and in the preservation of normal glucose levels in the blood and in the inhibition of oxidative stress [10]. However, recent studies demonstrated that Cr(III) does not induce human low density lipoprotein (LDL) oxidation at pH 4.5 or 7.4 under the experimental conditions used, thus suggesting caution when evaluating LDL oxidation and lipid peroxidation induced by trivalent chromium [33]. Cr(III) present in the soil may undergo natural oxidation processes being converted to Cr(VI); these oxidation processes may also be influenced by manganese levels. High levels of this metal in the soil as well as the high values of pH in the soil may influence oxidation processes [34]. Trivalent chromium may be easily taken up by plants from the soil; in this way, Cr(III) can enter the food chain of the diet of living beings. Cr(III), as well as being in the daily diet, is present in many nutritional supplements in the form of CrPic, CrHis, CRDC and NBC. Among these nutritional supplements, CrPic is the most easily absorbed [35][36][35,36]. Some in vitro studies evidenced that Cr(III) can react with DNA thus causing DNA damage in cell cultures, but in normal conditions, the limited entry of Cr(III) into cells in vivo limits its genotoxic effects in biological systems. Therefore, the genotoxic effects are rare in animals and humans exposed to nutritional doses or moderate supplemental doses of Cr(III), but they are more likely for modestly elevated physiological intake levels [18].
Cr(VI) is seldom found in nature and it mainly derives from industrial and anthropogenic activities. It is used in industry in stainless steel, chrome plating, welding, leather tanning and as corrosion inhibitor. Cr(VI) is toxic and carcinogenic and is a respiratory irritant which determines phospholipid peroxidation, DNA damage, chromosomal aberration, epigenomic instability and cell death. Cr(VI), which structurally resembles phosphate and sulfate anions, may easily cross cell membranes via nonspecific anionic transfer systems, while Cr(III) cannot enter cells [37]. In the environment Cr(VI) may be found as chromate oxyanion (CrO4=), which is structurally similar to sulphate and phosphate anions; for this reason, the anion transport protein, called Band 3, transports sulphate, phosphate and chromate ions, as well [38][39] [38,39] (Figure 1). After food or water ingestion, Cr(VI) may be reduced to Cr(III) by the action of saliva and highly acidic gastric juice and then taken by intestinal bacteria [40]. Reduction of Cr(VI) to Cr(III) into cells generates Cr(V) and Cr(IV) intermediate ions: this process depends on the reducing agent and their concentration. Cysteine acts as a one-electron reducer forming Cr(V); the reducing reaction in the presence of GSH proceeds through either one- or two-electron transfer forming Cr(V) and Cr(IV), while ascorbate is a two-electron donor, giving Cr(IV) [37][41] [37,41] (Figure 1).
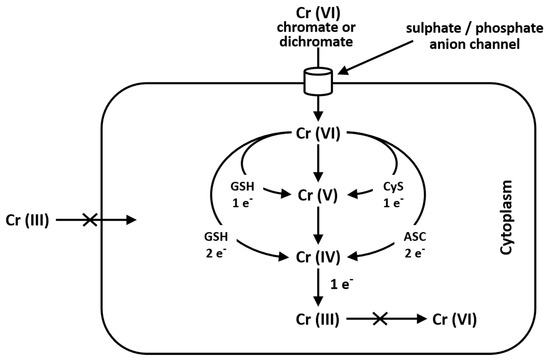
Figure 1.
Pathway of chromium metabolism in the cell. Cr(III) is unable to cross the cellular membrane, while Cr(VI), being structurally similar to phosphate and sulfate, crosses the cellular membrane via a nonspecific anion channel. In the cell, Cr(VI) undergoes metabolic reduction to Cr(III) in the presence of Asc, GSH and Cys [38][41].
Once inside cells, Cr(VI) undergoes metabolic reductions and is converted to Cr(III) in the presence of ascorbate, reduced glutathione (GSH), cysteine (Cys), cytochrome P450 reductase, glutathione reductase, aldehyde oxidase, catalase, glutathione peroxidase, superoxide dismutase, glutathione S-transferase and thioredoxin reductase. Moreover, the mitochondrial electron transport chain (complex I, also called NADH: ubiquinone oxide reductase; complex III, also called ubiquinone: cytochrome C oxide reductase) is a Cr(VI) potent reducing agent [42][43][42,43]. Simultaneously with conversion of Cr(VI) to Cr(III), Fenton reaction produces reactive oxygen species (ROS), like hydrogen peroxide (H2O2), hydroxyl radicals (•OH) and superoxide anion radicals (O2•–) (Figure 2). Several authors demonstrated that chromium is capable of altering the epigenetic profile of cells in DNA methylation, histone and microRNA post-translational modifications [44][45][46][47][44,45,46,47].
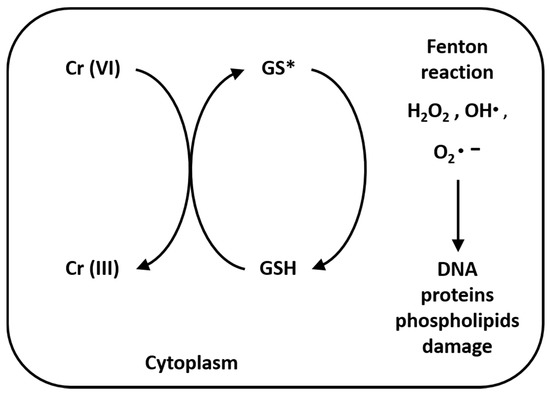
Figure 2. Reduction reaction of Cr(VI) to Cr(III) and Fenton reaction. The reaction process of Cr(VI) to Cr(III), especially in the presence of GSH, produces hydrogen peroxide (H2O2), hydroxyl radicals (•OH) and superoxide anion radicals (O2•
100 | |||||
Oysters | 57 | ||||
Wholemeal bread | 42 | ||||
Rye bread | 30 | ||||
Dried dates | 29 | ||||
Pears | 27 | ||||
Shrimps | 26 | ||||
Broccoli | 25 | ||||
Whole wheat flour | 21 | ||||
Tomatoes | 20 | ||||
Whole meal barley | 13 | ||||
Hazelnuts | 12 | ||||
Whole corn | 9 |
Cr(III) is absorbed in the gut through the unsaturated passive transport. The absorption intake depends on the chromium quantity in the food and the chemical form of this element (i.e., chloride, picolinate, nicotinate, etc.). The absorption of chromium in human beings is much higher in the form of yeast chromium (5–10%) than CrPic (2.8%) or CrCl3 (0.1–0.4%) (European Commission 2003) [54]. Organic chromium (i.e., picolinate, nicotinate, methionine, histidinate) is better absorbed than inorganic [55]. In 2007, Zha and co-authors reported that the highest correlation dose/accumulation in the tissues occurred when chromium nanoparticles were used (average size 40–50 nm) [56]. However, other factors are related to the absorption of this element: ascorbic acid, aspirin, oxalic acid, simple sugar, nicotinic acid and some amino acids may enhance the absorption of chromium, whereas calcium, magnesium, zinc, titanium, iron and phosphate reduce the level of absorption [57]. A chromium deficiency has been noted in athletes after strenuous exercise, pregnant women, and the elderly because of their difficulty in absorbing inorganic chromium in an adequate amount to convert into the active form [58]. Chromium absorbed by passive diffusion represents only about 1% [59].
After being absorbed from the intestine, Cr(III) is released into the bloodstream, and its transportation is mediated by transferrin, a β-globulin, followed by receptor-mediated endocytosis, and then transferred to cells of various tissues, first of all, liver and kidneys [60]. Transferrin is a serum protein, involved in iron metabolism and responsible for transporting Fe(III) in the bloodstream [61]. This protein is also involved in the transport of Cr(III) because of its similarity to the ferric ion both in size and charge [62]. Transferrin holds two binding sites for iron with different affinities depending on pH. Chromium has been shown to bind only to one of these sites. The antagonism between chromium/iron might cause an enhancement in hematological parameters (hemoglobin, hematocrit, erythrocytes and mean erythrocyte volume), described in chromium deficiency [63]. Chromium passes from cells into blood circulation and is eliminated in urine (80%), while the remaining 20% is excreted via feces and sweat [64]. In humans, pregnancy and lactation, exhaustive physical exercise and consumption of large amounts of sugar with the diet lead to increase chromium excretion in the urine. Cr(VI) that is not reduced by gastric fluid may pass into the small intestine [65]. Cr(VI) that has entered the small intestine can be reduced outside cells, excreted in feces, or enter villus enterocytes by anion transporters. Recently, chromium level imbalances have been observed in alcohol-use disorders [66].
4. The Role of Chromium and Its Mechanism of Action in the Body
The first literature study about a physiological role of chromium in the body appeared in 1959 by Schwarz and Mertz [67]. The authors isolated from pig kidney and Brewer’s yeast the GTF, a component capable of rebalancing impaired glucose tolerance in rats; the active component of this factor was discovered to be chromium. About forty years later chromium was considered an element necessary in small quantities for proper functioning of human beings [68]; anyway nowadays, chromium is classified as a nutritional supplementation factor.
In recent years, several studies on the influence of chromium on cholesterol level and lipid profile have been published. Dietary supplementation with high amounts of chromium has been demonstrated that, while decreasing the serum level of total cholesterol, LDL-cholesterol (LDL-c), nonesterified fatty acids and triglycerides, led to an enhanced concentration of high density lipoprotein (HDL)-cholesterol and β-oxidation process [69]. The oral administration in mice of chromium nitrate cancels and counteracts the anticarcinogenic mechanism of selenium effects in the development and growth of mouse mammary tumors caused by mouse mammary tumor virus (MMTV) [70]. Despite the numerous studies published so far, it is still unclear how chromium ions can operate in the metabolism of lipids and carbohydrates. Probably, the active form of Cr(III) is transported through the body via chromodulin, a low-molecular-weight chromium-binding substance (LMWCr), that is thought to influence lipid and carbohydrate metabolism. LMWCr is a small 1500 Da oligopeptide consisting of ten amino acids (2 Gly, 2 Cys, 2 Asp, 4 Glu) [71]. LMWCr has been demonstrated to be within the cytoplasm and nucleus of the cell in an inactive form (i.e., apochromodulin). After insulin binding and activation of the transferrin receptor, there is an internalization in the cell of transferrin-chromium complex. Chromodulin binds four Cr(III) ions, and then is converted into holochromodulin, the biologically active form. This holopeptide may bind the insulin receptor at the level of β-subunit, activating the tyrosine kinase receptor and thus enhancing the insulin signal. All these mechanisms mediate glucose transport into the cell through the cell membrane thanks to the protein glucose transporter 4 (GLUT4) [72]. Other theories are known to justify the importance of chromium in glucose metabolism. For example, according to Pattar and collaborators (2006), the Cr ions act on the membrane fluidity regulating the glucose uptake by the cells [73]. The fluidity of the membrane, also considering the presence of Cr ions, is associated with the reduced content of cholesterol in the cellular membranes. The lower presence of cholesterol, which affects membrane fluidity, is considered to be another factor that diminishes insulin-controlled glucose intake. Furthermore, Raja et al. (2011) demonstrated that Cr(II) ions modify the structure of the lipid bilayer [74]. Adam et al. (2017) studied the mechanism responsible for contact dermatitis by chromium [75]. It seems that Cr(VI) penetrates through the skin and is then reduced to Cr(III), which may react with proteins to form a hapten, thus creating the complex recognized by T cells. Chromium activates T cells leading to inflammation and, lastly, symptoms [76].
