Endocytosis is a fundamental process involved in trafficking of various extracellular and transmembrane molecules from the cell surface to its interior. This enables cells to communicate and respond to external environments, maintain cellular homeostasis, and transduce signals. G protein-coupled receptors (GPCRs) constitute a family of receptors with seven transmembrane alpha-helical domains (7TM receptors) expressed at the cell surface, where they regulate physiological and pathological cellular processes. Several herpesviruses encode receptors (vGPCRs) which benefits the virus by avoiding host immune surveillance, supporting viral dissemination, and thereby establishing widespread and lifelong infection, processes where receptor signaling and/or endocytosis seem central. vGPCRs are rising as potential drug targets as exemplified by the cytomegalovirus-encoded receptor US28, where its constitutive internalization has been exploited for selective drug delivery in virus infected cells. Therefore, studying GPCR trafficking is of great importance.
- endocytosis
- G-protein coupled receptors
- herpesvirus
1. Introduction
Endocytosis encompasses different routes by which a cell uptakes extracellular material from the surface and transports it into the cell thereby maintaining homeostasis between the extracellular and intracellular environment [1]. Nutrients, receptor-ligand complexes, extracellular matrix, cell debris, bacteria, and viruses can enter the cell by different endocytic mechanisms [2][3]. Importantly, endocytosis is also linked with cellular signaling at the plasma membrane (PM) where receptors bind specific signaling molecules and initiate internalization [4].
Receptors located at the cell surface coordinate many different physiological processes in the cell. Among them, G protein coupled receptors (GPCRs) with seven transmembrane domains (7TM) are a major family of receptors able to stimulate important intracellular signaling pathways in response to various extracellular stimuli [5]. It has been recognized that after initial activation and desensitization on the cell membrane, GPCRs subsequently enter the cell via endocytosis. Endocytosis can occur either constitutively (without ligand stimulus) or in response to certain stimuli, including growth factors, viruses (Ebola, SARS and other coronaviruses), and different ligands [6]. In the cell, further sorting of internalized GPCRs between degradation and recycling pathways occurs. Therefore, cells can tightly regulate GPCR surface availability for further signaling events [7].
Endocytosis is also a mechanism used by several herpesviruses (for example Epstein-Barr virus, Kaposi's sarcoma associated herpesvirus, cytomegalovirus, and varicella zoster virus) for initial entry into the cell [8]. Herpesviruses are widespread DNA viruses employing a special bipartite life cycle where latent and lytic phases interchange in order to persist in the infected host for their whole life [9]. The herpesvirus virion consists of three main parts: a nucleocapsid containing linear double-stranded DNA, an envelope, and tegument. In the envelope, different glycoproteins are involved in the initial binding and endocytosis events upon infection of susceptible cells. Herpesvirus genomes encode between 100 to 200 proteins. These proteins are involved in DNA replication (e.g., DNA polymerase), viral entry, cell-to-cell spread, immunevasion, and pathogenesis. Among these regulatory proteins, herpesviruses encode GPCRs (vGPCRs). It is believed that during evolutionary processes, viruses took over genes for these receptors from their hosts and rearranged them to function in the benefit of the virus [10][11][12]. They imitate the function of endogenous human receptors and therefore use them to subvert cellular signaling, avoid cell immune responses, induce cell transformation, and support viral dissemination and replication [12][13].
Many vGPCRs resemble endogenous chemokine receptors structurally, and bind a broad spectrum of both endogenous and virally encoded chemokines, leading to activation of downstream signaling pathways [12][14]. Others are described as "orphan" vGPCRs for which no ligand has yet been identified. Additionally, BILF1 receptors, encoded by gammaherpesviruses, have recently been recognized as the first immune evasive vGPCR able to downregulate surface MHC class I molecules at the cell surface [15].
2. Different Endocytic Pathways
In general, endocytosis is divided into two processes: pinocytosis, by which cells take in fluid and small particles, and phagocytosis, which is performed by specialised cells that have the capacity to uptake larger particles (>500 nm) such as microorganisms and cell debris (Figure 1). Pinocytosis is further divided into macro- and micropinocytosis. By macropinocytosis, cells take in extracellular fluid via large endocytic vesicles which are heterogeneously sized (200–500 nm) termed macropinosomes. In micropinocytosis, specific molecules enter the cell through smaller vesicles and can be further divided to clathrin-mediated endocytosis (70–150 nm) (CME), caveolae-mediated endocytosis (60–80 nm) or non-coated vesicles (Figure 1) [3][16].
The most extensively studied form of endocytosis is the clathrin-mediated pathway, which occurs in all mammalian cells. This pathway has traditionally been described as the most commonly used endocytic pathway for the majority of GPCRs [17]. Among vGPCRs, US28 and ORF74 use the clathrin-mediated pathway as one of the mechanisms for internalization, however, their mechanism for cell entry is promiscuous, as described later.
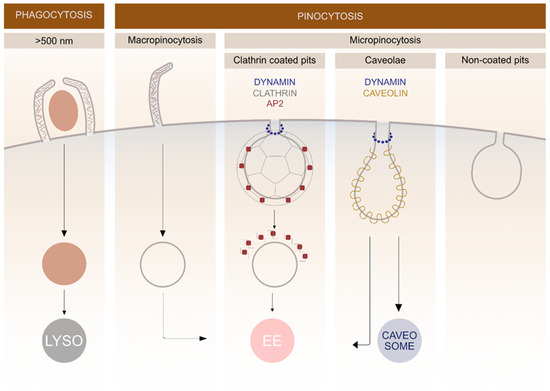
Figure 1. Schematic representation of different endocytic pathways in mammalian cells. The endocytosis is divided into various subgroups based on the size of the cargo entering the cell. Different membrane proteins are involved in clathrin and caveolin-mediated pathways and the fate of cargo molecules depends on specific endocytic mechanisms. Lysosomes (LYSO), adaptor protein 2 (AP2), early endosomes (EE).
After pinching off the PM, the majority of endocytic vesicles are fused with early endosomes where the cargo is sorted. Later, it can either recycle back to the PM or it can be directed into degradation pathways. Late endosomes are formed via fusion of early endosomes in an endosome maturation process, where the structures of membrane proteins change. After endosome maturation, the recycling to the PM stops and non-recycled material is further directed into degradation pathways [18]. Degradation takes place in lysosomes, which are formed by fusion of late endosomes and pre-existing lysosomes. During maturation, transport from the trans-Golgi network to endosomes occurs, providing newly synthesized lysosomal enzymes for the endosomes [19].
3. Endocytic Properties of The Most Commonly Studied vGPCRs
3.1. Cytomegalovirus (CMV)
Cytomegalovirus is a severe virus which causes deadly infections among immunosuppressed patients [20]. Human cytomegalovirus (HCMV) open reading frames (ORF) encodes four vGPCRs: US28, US27, UL33, and UL78; the first three mimic chemokine receptor structure. Mouse and rat CMV encodes homologs of UL33 and UL78, but not US28 and US27 [12].
US28 is an extensively studied receptor that has been shown to be functionally important in various aspects of HCMV infection. It displays high constitutive (ligand independent) activity and binds a broad range of chemokines [21][22][23]. It is the first vGPCR used as an antiviral drug target to selectively kill HCMV infected cells using fusion toxin protein [24][25][26]. This strategy relies on the ability of this receptor to internalize constitutively and thereby deliver the immunotoxin intracellularly [25][27]. The majority of US28 receptors is located intracellularly in endosomes in the perinuclear region with only a small amount located in the PM (Figure 2). It was revealed that constitutive endocytosis is β-arrestin independent; nevertheless, it still employs a pathway through clathrin-coated vesicles as shown in embryonic fibroblasts acquired from β-arrestin-1 and -2 knockout mice using siRNA against the μ2 subunit of AP-2 adaptor protein [27]. When comparing the endocytosis of US28 in the control and β-arrestin knockout cells, no differences were observed [27].
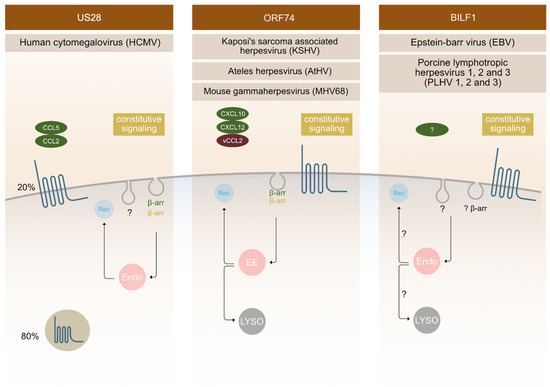
Figure 2. Endocytic mechanisms employed by vGPCRs. Different vGPCRs use different mechanisms to enter the cell. Besides ligand dependent endocytosis (as shown on the Figure for US28 with ligands CCL5 and CCL2 and for ORF74 with ligands CXCL10, CXCL 12 and VCCL2), constitutive (ligand independent) endocytosis is a common feature observed for these receptors. The fate of receptors inside the cell is tightly regulated and has an important impact on receptor function outcome. Localization of vGPCRs differs, with ORF74 and BILF1 receptors predominantly localizing at the surface and US28 localizing intracellularly in 80% and at the surface at 20%. Endosomes (endo), recycling (Rec), β-arrestin (β-arr), early endosomes (EE), lysosomes (LYSO).
It is assumed that US28 internalization could partially use caveolae or lipid rafts, since palmitoylation of US28 has been observed. Palmitoylation is a step in the receptor activation process and serves as a targeting signal for receptors to caveolae [28]. Typically, after ligand-mediated receptor activation, the C-terminal tail is phosphorylated, which eventually leads to β-arrestin binding and endocytosis. The C-terminal tail of US28 receptor contains many serine and threonine residues which represent potential phosphorylation sites. Research shows that the C-terminal end is constitutively phosphorylated, therefore it enables the constitutive activity of receptors [27].
Similar to US28, US27 and UL33 (HCMV) are located intracellularly. The intracellular localization of UL33 was however only observed in HeLa and COS-7 cells, but not in HEK-293, where the receptor was mostly located at the PM. This suggests that the receptor localization is cell type specific. Co-localization of US27-YFP/UL33-GFP with LAMP1, the marker of late endosomes/lysosomes, was shown in Hela cells, which indicates that these receptors are located in late endocytic compartments [29]. It is believed that localization within these compartments enables US28, US27, and UL33 to bud into a viral membrane when the virus is exiting the cell [29]. Immunohistochemistry on cryosections showed that UL33 is located at multivesicular bodies (MVB), but it is still unknown whether UL33 can recycle back to the PM or it enters a degradation pathway through lysosomes [29].
When co-expressing US27 and US28, colocalization was observed, suggesting that both proteins are located in late endosomes/lysosomes. Based on this overlap, it was examined whether US27 is similarly internalized and revealed that a large amount of this receptor is found in intracellular vesicles, indicating receptor internalization in a constitutive manner, like for US28 [29].
Little is known about UL78. It is believed, that this receptor is not necessary for viral replication in cell cultures, but its function in vivo remains to be studied [29]. This receptor is mainly localized within the cytoplasm of the endoplasmatic reticulum, although surface expression has also been proposed. Furthermore, it has been shown that UL78 undergoes constitutive endocytosis and recycling back to PM [30].
3.2. Kaposi's Sarcoma Associated Herpesvirus (KSHV)
The ORF74 receptor family is encoded by γ2-herpesviruses, such as: KSHV, MHV68, HVS, AtHV, and EHV2. They bind CXC chemokines, and most often also display constitutive activity. ORF74 encoded by KSHV is the most thoroughly characterized receptor in this group, followed by ECRF3 from HVS. Both bind CXC-chemokines; KSHV-ORF74- with a broader spectrum compared to ECRF3-HVS [31][32][33][34][35]. The constitutive signalling of KSHV-ORF74-has been directly linked to tumorigenesis in several mouse models [36][37][38]. In terms of cellular localization, KSHV-ORF74- has been described to be mostly located at the PM (Figure 2), but also to undergo internalization.
CXCL1-, and CXCL8-mediated endocytosis was shown to depend on β-arrestin 1 and 2, whereas the constitutive endocytosis occurs independently of β-arrestin [39]. The AP-2 complex plays an important role in constitutive internalization of ORF74 (Figure 2). This adaptor complex is also important for clathrin-coated vesicles mediated endocytosis [40]. After internalization, the receptor is found in early endosomes, and from there it recycles back at the PM or it fuses with lysosomes and enters the degradation pathway [41].
3.3. Epstein-Barr Virus (EBV) and Its Closely Related Lymphocryptoviruses
The first BILF1 receptors were recognized as GPCR's in 2005 [42]. They are encoded by different γ-1-herpesviruses (EBV, other primate lymphocryptoviruses, porcine lymphotropic herpesviruses (PLHV1, 2, 3) and other ungulate gammaherpesviruses) [43][44][45]. The sequence identity of BILF1 receptors varies, with ape γ-1 herpesvirus BILFs being highly conserved and ungulate γ-1 herpesvirus BILFs being more distantly related [45]. However, ORF encoding BILF1 receptors was described for all above mentioned γ-1 herpesviruses at a similar genomic position [46][47][48]. Functionally, EBV-BILF1 remains the most studied among BILF1 receptors until this day. It is an orphan receptor with well-established constitutive signalling and internalization properties. Confocal microscopy has shown that EBV-BILF1, similar to KSHV-ORF74-, predominantly locates at the cell surface, but there is a difference among BILF1 orthologs of this family [42][45]. Studies of BILF1 receptors from different primate lymphocryptoviruses showed that BILF1 receptor encoded in PtroLCV1 (lymphocryptovirus from chimpanzee) and PpygLCV1 (Lymphocryptovirus from orangutan) predominantly locates intracellularly and not at the PM. It was therefore shown that localization is not preserved among the family [45]. However, EBV-BILF1 has been shown to internalize in a constitutive manner [45], a cellular phenomenon that seems linked to the main function of BILF1 as a MHC-1 downregulating molecule [15]. This immunevasive property has also been confirmed for RhLCV-BILF1, but not for CalHV3-BILF1 [15][49]. Further investigation on BILF1 mediated endocytic routes is therefore needed in order to fully understand its functional properties.
34. Conclusions
vGPCRs play important roles in virus life cycle from the primary infection, replication, and latency establishment to various pathological outcomes. In this respect, understanding molecular and cellular behavior is of huge importance. Despite their distant genetic relationships, US28, ORF74, and BILF1 receptors have been linked to the development of cancer. ORF74 was described as the driver of KSHV-related malignancies inducing angiogenesis, cellular transformation, and inflammation [36][38][50]. HCMV-US28 has been detected in glioblastoma and colorectal cancers and is described as an onco-modulator constitutively upregulating angiogenesis and proliferation [51][52]. For BILF1, constitutive Gαi dependent signaling has been linked to EBV-mediated cell transformation ability both in vitro and in vivo [53]. The involvement of all three receptor classes in tumor development makes them attractive drug targets for the treatment of virus-related cancers. As shown for US28, one potential strategy to target infected cells is the use of fusion toxin protein (FTP) to specifically target and kill HCMV infected cells [24][25][26]. For US28, a predominantly intracellular localization pattern was described as a consequence of a rapid constitutive endocytosis [29][54]. This ability was used for the delivery of the toxin intracellularly. For US28, β-arrestin independent, constitutive CME has been shown. This is additionally supported by observations that US28 expresses a YXXΦ motif known to interact with AP-2, a protein important for clathrin coat assembly. BILF1 has recently been described as an immunevasive protein playing a role in downregulating surface expressed MHC class I molecules [15]. With its cell surface localization, BILF1 has been proposed to bind MHC class I molecules and internalize in complex leading to its degradation in lysosomes.
Given the close link between signaling and internalization, and for both of these phenomenon a close link to the pathophysiology of the viruses, future studies of the internalization properties of these receptors are needed to finally establish how, when, and where to target them from a therapeutic point of view.
References
- Hanyaloglu, A.C.; von Zastrow, M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharm. Toxicol. 2008, 48, 537–568, doi:10.1146/annurev.pharmtox.48.113006.094830.
- Joseph, J.G.; Liu, A.P. Mechanical Regulation of Endocytosis: New Insights and Recent Advances. Adv Biosyst. 2020, 4, e1900278, doi:10.1002/adbi.201900278.
- Sigismund, S.; Confalonieri, S.; Ciliberto, A.; Polo, S.; Scita, G.; Di Fiore, P.P. Endocytosis and signaling: Cell logistics shape the eukaryotic cell plan. Physiol. Rev. 2012, 92, 273–366, doi:10.1152/physrev.00005.2011.
- Barbieri, E.; Di Fiore, P.P.; Sigismund, S. Endocytic control of signaling at the plasma membrane. Curr. Opin. Cell Biol. 2016, 39, 21–27, doi:10.1016/j.ceb.2016.01.012.
- Lobingier, B.T.; von Zastrow, M. When trafficking and signaling mix: How subcellular location shapes G protein-coupled receptor activation of heterotrimeric G proteins. Traffic 2019, 20, 130–136, doi:10.1111/tra.12634.
- Kumari, S.; Mg, S.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256, doi:10.1038/cr.2010.19.
- Hanyaloglu, A.C. Advances in Membrane Trafficking and Endosomal Signaling of G Protein-Coupled Receptors. Int. Rev. Cell Mol. Biol. 2018, 339, 93–131, doi:10.1016/bs.ircmb.2018.03.001.
- Sobhy, H. A comparative review of viral entry and attachment during large and giant dsDNA virus infections. Arch. Virol. 2017, 162, 3567–3585, doi:10.1007/s00705-017-3497-8.
- Traylen, C.M.; Patel, H.R.; Fondaw, W.; Mahatme, S.; Williams, J.F.; Walker, L.R.; Dyson, O.F.; Arce, S.; Akula, S.M. Virus reactivation: A panoramic view in human infections. Future Virol. 2011, 6, 451–463, doi:10.2217/fvl.11.21.
- Alcami, A.; Lira, S.A. Modulation of chemokine activity by viruses. Curr. Opin. Immunol. 2010, 22, 482–487, doi:10.1016/j.coi.2010.06.004.
- Raftery, M.; Muller, A.; Schonrich, G. Herpesvirus homologues of cellular genes. Virus Genes 2000, 21, 65–75.
- Rosenkilde, M.M.; Smit, M.J.; Waldhoer, M. Structure, function and physiological consequences of virally encoded chemokine seven transmembrane receptors. Br. J. Pharm. 2008, 153 Suppl 1, S154-166, doi:10.1038/sj.bjp.0707660.
- van Senten, J.R.; Fan, T.S.; Siderius, M.; Smit, M.J. Viral G Protein-Coupled Receptors as Modulators of Cancer Hallmarks. Pharmacol. Res. 2020, doi:10.1016/j.phrs.2020.104804.
- Vischer, H.F.; Siderius, M.; Leurs, R.; Smit, M.J. Herpesvirus-encoded GPCRs: Neglected players in inflammatory and proliferative diseases? Nat. Rev. Drug Discov. 2014, 13, 123–139, doi:10.1038/nrd4189.
- Zuo, J.; Currin, A.; Griffin, B.D.; Shannon-Lowe, C.; Thomas, W.A.; Ressing, M.E.; Wiertz, E.J.; Rowe, M. The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. Plos Pathog. 2009, 5, e1000255, doi:10.1371/journal.ppat.1000255.
- Weeratunga, S.; Paul, B.; Collins, B.M. Recognising the signals for endosomal trafficking. Curr. Opin. Cell Biol. 2020, 65, 17–27, doi:10.1016/j.ceb.2020.02.005.
- Wolfe, B.L.; Trejo, J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 2007, 8, 462–470, doi:10.1111/j.1600-0854.2007.00551.x.
- McNally, K.E.; Cullen, P.J. Endosomal Retrieval of Cargo: Retromer Is Not Alone. Trends. Cell Biol. 2018, 28, 807–822, doi:10.1016/j.tcb.2018.06.005.
- Huotari, J.; Helenius, A. Endosome maturation. Embo. J. 2011, 30, 3481–3500, doi:10.1038/emboj.2011.286.
- Limaye, A.P.; Babu, T.M.; Boeckh, M. Progress and Challenges in the Prevention, Diagnosis, and Management of Cytomegalovirus Infection in Transplantation. Clin. Microbiol. Rev. 2020, 34, doi:10.1128/cmr.00043-19.
- Kledal, T.N.; Rosenkilde, M.M.; Schwartz, T.W. Selective recognition of the membrane-bound CX3C chemokine, fractalkine, by the human cytomegalovirus-encoded broad-spectrum receptor US28. Febs. Lett. 1998, 441, 209–214, doi:10.1016/s0014-5793(98)01551-8.
- Neote, K.; DiGregorio, D.; Mak, J.Y.; Horuk, R.; Schall, T.J. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell 1993, 72, 415–425, doi:10.1016/0092-8674(93)90118-a.
- McLean, K.A.; Holst, P.J.; Martini, L.; Schwartz, T.W.; Rosenkilde, M.M. Similar activation of signal transduction pathways by the herpesvirus-encoded chemokine receptors US28 and ORF74. Virology 2004, 325, 241–251, doi:10.1016/j.virol.2004.04.027.
- Krishna, B.A.; Spiess, K.; Poole, E.L.; Lau, B.; Voigt, S.; Kledal, T.N.; Rosenkilde, M.M.; Sinclair, J.H. Targeting the latent cytomegalovirus reservoir with an antiviral fusion toxin protein. Nat. Commun. 2017, 8, 14321, doi:10.1038/ncomms14321.
- Spiess, K.; Jeppesen, M.G.; Malmgaard-Clausen, M.; Krzywkowski, K.; Dulal, K.; Cheng, T.; Hjorto, G.M.; Larsen, O.; Burg, J.S.; Jarvis, M.A., et al. Rationally designed chemokine-based toxin targeting the viral G protein-coupled receptor US28 potently inhibits cytomegalovirus infection in vivo. Proc. Natl. Acad. Sci. U S A 2015, 112, 8427–8432, doi:10.1073/pnas.1509392112.
- Spiess, K.; Jeppesen, M.G.; Malmgaard-Clausen, M.; Krzywkowski, K.; Kledal, T.N.; Rosenkilde, M.M. Novel Chemokine-Based Immunotoxins for Potent and Selective Targeting of Cytomegalovirus Infected Cells. J. Immunol. Res. 2017, 2017, 4069260, doi:10.1155/2017/4069260.
- Fraile-Ramos, A.; Kohout, T.A.; Waldhoer, M.; Marsh, M. Endocytosis of the viral chemokine receptor US28 does not require beta-arrestins but is dependent on the clathrin-mediated pathway. Traffic 2003, 4, 243–253, doi:10.1034/j.1600-0854.2003.00079.x.
- Droese, J.; Mokros, T.; Hermosilla, R.; Schülein, R.; Lipp, M.; Höpken, U.E.; Rehm, A. HCMV-encoded chemokine receptor US28 employs multiple routes for internalization. Biochem. Biophys. Res. Commun. 2004, 322, 42–49, doi:10.1016/j.bbrc.2004.07.076.
- Fraile-Ramos, A.; Pelchen-Matthews, A.; Kledal, T.N.; Browne, H.; Schwartz, T.W.; Marsh, M. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic 2002, 3, 218–232, doi:10.1034/j.1600-0854.2002.030307.x.
- Wagner, S.; Arnold, F.; Wu, Z.; Schubert, A.; Walliser, C.; Tadagaki, K.; Jockers, R.; Mertens, T.; Michel, D. The 7-transmembrane protein homologue UL78 of the human cytomegalovirus forms oligomers and traffics between the plasma membrane and different intracellular compartments. Arch. Virol. 2012, 157, 935–949, doi:10.1007/s00705-012-1246-6.
- Ahuja, S.K.; Murphy, P.M. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J. Biol. Chem. 1993, 268, 20691–20694.
- Rosenkilde, M.M.; Kledal, T.N.; Brauner-Osborne, H.; Schwartz, T.W. Agonists and inverse agonists for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogene product, ORF-74. J. Biol. Chem. 1999, 274, 956–961, doi:10.1074/jbc.274.2.956.
- Rosenkilde, M.M.; Kledal, T.N.; Holst, P.J.; Schwartz, T.W. Selective elimination of high constitutive activity or chemokine binding in the human herpesvirus 8 encoded seven transmembrane oncogene ORF74. J. Biol. Chem. 2000, 275, 26309–26315, doi:10.1074/jbc.M003800200.
- Rosenkilde, M.M.; Schwartz, T.W. Potency of ligands correlates with affinity measured against agonist and inverse agonists but not against neutral ligand in constitutively active chemokine receptor. Mol. Pharm. 2000, 57, 602–609, doi:10.1124/mol.57.3.602.
- Rosenkilde, M.M.; McLean, K.A.; Holst, P.J.; Schwartz, T.W. The CXC chemokine receptor encoded by herpesvirus saimiri, ECRF3, shows ligand-regulated signaling through Gi, Gq, and G12/13 proteins but constitutive signaling only through Gi and G12/13 proteins. J. Biol. Chem. 2004, 279, 32524–32533, doi:10.1074/jbc.M313392200.
- Yang, T.Y.; Chen, S.C.; Leach, M.W.; Manfra, D.; Homey, B.; Wiekowski, M.; Sullivan, L.; Jenh, C.H.; Narula, S.K.; Chensue, S.W., et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J. Exp. Med. 2000, 191, 445–454, doi:10.1084/jem.191.3.445.
- Holst, P.J.; Rosenkilde, M.M.; Manfra, D.; Chen, S.C.; Wiekowski, M.T.; Holst, B.; Cifire, F.; Lipp, M.; Schwartz, T.W.; Lira, S.A. Tumorigenesis induced by the HHV8-encoded chemokine receptor requires ligand modulation of high constitutive activity. J. Clin. Invest. 2001, 108, 1789–1796, doi:10.1172/jci13622.
- Grisotto, M.G.; Garin, A.; Martin, A.P.; Jensen, K.K.; Chan, P.; Sealfon, S.C.; Lira, S.A. The Human Herpesvirus 8 Chemokine Receptor vGPCR Triggers Autonomous Proliferation of Endothelial Cells. J. Clin. Investig. 2006, 116, doi:10.1172/JCI26666.
- de Munnik, S.M.; Kooistra, A.J.; van Offenbeek, J.; Nijmeijer, S.; de Graaf, C.; Smit, M.J.; Leurs, R.; Vischer, H.F. The Viral G Protein-Coupled Receptor ORF74 Hijacks β-Arrestins for Endocytic Trafficking in Response to Human Chemokines. Plos One 2015, 10, e0124486, doi:10.1371/journal.pone.0124486.
- Azzi, S.; Smith, S.S.; Dwyer, J.; Leclair, H.M.; Alexia, C.; Hebda, J.K.; Dupin, N.; Bidère, N.; Gavard, J. YGLF motif in the Kaposi sarcoma herpes virus G-protein-coupled receptor adjusts NF-κB activation and paracrine actions. Oncogene 2014, 33, 5609–5618, doi:10.1038/onc.2013.503.
- de Munnik, S.M.; Smit, M.J.; Leurs, R.; Vischer, H.F. Modulation of cellular signaling by herpesvirus-encoded G protein-coupled receptors. Front. Pharm. 2015, 6, doi:10.3389/fphar.2015.00040.
- Paulsen, S.J.; Rosenkilde, M.M.; Eugen-Olsen, J.; Kledal, T.N. Epstein-Barr virus-encoded BILF1 is a constitutively active G protein-coupled receptor. J Virol. 2005, 79, 536–546, doi:10.1128/jvi.79.1.536-546.2005.
- Chmielewicz, B.; Goltz, M.; Franz, T.; Bauer, C.; Brema, S.; Ellerbrok, H.; Beckmann, S.; Rziha, H.-J.; Lahrmann, K.-H.; Romero, C., et al. A novel porcine gammaherpesvirus. Virology 2003, 308, 317–329, doi:10.1016/S0042-6822(03)00006-0.
- Lindner, I.; Ehlers, B.; Noack, S.; Dural, G.; Yasmum, N.; Bauer, C.; Goltz, M. The porcine lymphotropic herpesvirus 1 encodes functional regulators of gene expression. Virology 2007, 357, 134–148, doi:10.1016/j.virol.2006.08.008.
- Spiess, K.; Fares, S.; Sparre-Ulrich, A.H.; Hilgenberg, E.; Jarvis, M.A.; Ehlers, B.; Rosenkilde, M.M. Identification and functional comparison of seven-transmembrane G-protein-coupled BILF1 receptors in recently discovered nonhuman primate lymphocryptoviruses. J. Virol. 2015, 89, 2253–2267, doi:10.1128/jvi.02716-14.
- Goltz, M.; Ericsson, T.; Patience, C.; Huang, C.A.; Noack, S.; Sachs, D.H.; Ehlers, B. Sequence analysis of the genome of porcine lymphotropic herpesvirus 1 and gene expression during posttransplant lymphoproliferative disease of pigs. Virology 2002, 294, 383–393, doi:10.1006/viro.2002.1390.
- Hart, J.; Ackermann, M.; Jayawardane, G.; Russell, G.; Haig, D.M.; Reid, H.; Stewart, J.P. Complete sequence and analysis of the ovine herpesvirus 2 genome. J. Gen. Virol. 2007, 88, 28–39, doi:10.1099/vir.0.82284-0.
- Boudry, C.; Markine-Goriaynoff, N.; Delforge, C.; Springael, J.Y.; de Leval, L.; Drion, P.; Russell, G.; Haig, D.M.; Vanderplasschen, A.F.; Dewals, B. The A5 gene of alcelaphine herpesvirus 1 encodes a constitutively active G-protein-coupled receptor that is non-essential for the induction of malignant catarrhal fever in rabbits. J. Gen. Virol. 2007, 88, 3224–3233, doi:10.1099/vir.0.83153-0.
- Griffin, B.D.; Gram, A.M.; Mulder, A.; Van Leeuwen, D.; Claas, F.H.; Wang, F.; Ressing, M.E.; Wiertz, E. EBV BILF1 evolved to downregulate cell surface display of a wide range of HLA class I molecules through their cytoplasmic tail. J. Immunol. 2013, 190, 1672–1684, doi:10.4049/jimmunol.1102462.
- Moore, P.S.; Boshoff, C.; Weiss, R.A.; Chang, Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 1996, 274, 1739–1744, doi:10.1126/science.274.5293.1739.
- Maussang, D.; Verzijl, D.; van Walsum, M.; Leurs, R.; Holl, J.; Pleskoff, O.; Michel, D.; van Dongen, G.A.; Smit, M.J. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc. Natl. Acad. Sci. U S A 2006, 103, 13068–13073, doi:10.1073/pnas.0604433103.
- Miller, W.E.; Zagorski, W.A.; Brenneman, J.D.; Avery, D.; Miller, J.L.; O’Connor, C.M. US28 is a potent activator of phospholipase C during HCMV infection of clinically relevant target cells. Plos One 2012, 7, e50524, doi:10.1371/journal.pone.0050524.
- Lyngaa, R.; Norregaard, K.; Kristensen, M.; Kubale, V.; Rosenkilde, M.M.; Kledal, T.N. Cell transformation mediated by the Epstein-Barr virus G protein-coupled receptor BILF1 is dependent on constitutive signaling. Oncogene 2010, 29, 4388–4398, doi:10.1038/onc.2010.173.
- Miller, W.E.; Houtz, D.A.; Nelson, C.D.; Kolattukudy, P.E.; Lefkowitz, R.J. G-protein-coupled receptor (GPCR) kinase phosphorylation and beta-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J. Biol. Chem. 2003, 278, 21663–21671, doi:10.1074/jbc.M303219200.
