- ice crystal
- aquatic products
1. Introduction
The quality of aquatic products will affect consumers’ preferences and acceptability directly or indirectly [1]. Water is the main component in almost all fresh food, and it is also actively participated and accelerated in spoilage of foods, such as texture, appearance, and protein [2]. In order to minimize quality loss and preserve the safety of products, several techniques including cooling [1][3][4][5], freezing [6][7][8], and freeze-drying [9][10][11], etc. are used in the preservation of aquatic products. Among them, the most commonly used technique in aquatic product processing is freezing [12].
The quality of aquatic products will affect consumers’ preferences and acceptability directly or indirectly [1]. Water is the main component in almost all fresh food, and it is also actively participated and accelerated in spoilage of foods, such as texture, appearance, and protein [2]. In order to minimize quality loss and preserve the safety of products, several techniques including cooling [1,3,4,5], freezing [6,7,8], and freeze-drying [9,10,11], etc. are used in the preservation of aquatic products. Among them, the most commonly used technique in aquatic product processing is freezing [12].
During the freezing process, the water of aquatic products is converted into ice crystals, inhibiting microorganism growth, slowing enzyme activities that degrade proteins or fats, and helping to extend the shelf life [13]. However, the formation of ice crystals may seriously impact the integrity of cells and muscle fibers, resulting in deterioration of quality [14]. It is generally believed that the freezing rate mainly affects the growth of ice crystals and then impacts the size and morphology of the ice crystals and their distribution inside the foods [12]. The formation and uneven distribution of large ice crystals in aquatic products are subjected to irreversible damage of cellular and tissue structure and lead to quality deteriorations of products, such as drip loss increasing after thawing, dehydration, tissue softening, discoloration, protein denaturation, mechanical damage, and so on [12][15][16][17][18]. On the contrary, the ice crystals generated by the high freezing rate have less damage on the quality of aquatic products. During subsequent freezing storage, the size and distribution of ice crystals in food change due to the occurrence of ice crystals recrystallization [19]. In addition, the temperature fluctuation that occurs during freezing storage and transport is often unpredictable and inevitable. Ice crystals melt, as well as recrystallization, which adversely affect cryopreserved foods [20][21].
During the freezing process, the water of aquatic products is converted into ice crystals, inhibiting microorganism growth, slowing enzyme activities that degrade proteins or fats, and helping to extend the shelf life [13]. However, the formation of ice crystals may seriously impact the integrity of cells and muscle fibers, resulting in deterioration of quality [14]. It is generally believed that the freezing rate mainly affects the growth of ice crystals and then impacts the size and morphology of the ice crystals and their distribution inside the foods [12]. The formation and uneven distribution of large ice crystals in aquatic products are subjected to irreversible damage of cellular and tissue structure and lead to quality deteriorations of products, such as drip loss increasing after thawing, dehydration, tissue softening, discoloration, protein denaturation, mechanical damage, and so on [12,15,16,17,18]. On the contrary, the ice crystals generated by the high freezing rate have less damage on the quality of aquatic products. During subsequent freezing storage, the size and distribution of ice crystals in food change due to the occurrence of ice crystals recrystallization [19]. In addition, the temperature fluctuation that occurs during freezing storage and transport is often unpredictable and inevitable. Ice crystals melt, as well as recrystallization, which adversely affect cryopreserved foods [20,21].
Nucleation has a crucial influence on the size of ice crystals and their distribution inside the products [22]. In addition to controlling the freezing rate, another important factors are the supercooling degree of ice crystals during the nucleation process [2][12]. In industrial production, the more traditional freezing methods are used, such as air blast freezing, immersion freezing, plate contact freezing, and so on [23][24]. Although rapid freezing like air blast freezing that requires very low temperatures generates smaller and more numerous ice crystals, it also means more energy and cooling costs [17][25]. Therefore, it is a challenge to reduce ice crystal size without increasing the costs and the energy consumption to freeze aquatic product. In recent decades, several assistance technologies for the freezing method to control ice nucleation and ice crystals growth have been introduced, including high pressure, ultrasounds, magnetic fields, electric fields, and the use of the inhibitors of ice recrystallization [2][20].
Nucleation has a crucial influence on the size of ice crystals and their distribution inside the products [22]. In addition to controlling the freezing rate, another important factors are the supercooling degree of ice crystals during the nucleation process [2,12]. In industrial production, the more traditional freezing methods are used, such as air blast freezing, immersion freezing, plate contact freezing, and so on [23,24]. Although rapid freezing like air blast freezing that requires very low temperatures generates smaller and more numerous ice crystals, it also means more energy and cooling costs [17,25]. Therefore, it is a challenge to reduce ice crystal size without increasing the costs and the energy consumption to freeze aquatic product. In recent decades, several assistance technologies for the freezing method to control ice nucleation and ice crystals growth have been introduced, including high pressure, ultrasounds, magnetic fields, electric fields, and the use of the inhibitors of ice recrystallization [2,20].
2. Effect of Ice Crystals on the Quality of Aquatic Products
2.1. Water
The water in the muscle is composed of three distinct populations: bound water, immobilized water, and free water [26]. The free water of the product becomes ice crystals firstly, followed by the immobilized water, and the bound water is basically unchanged during the freezing process [27]. With the extension of freezing time, the bound water which is tightly bound to proteins migrates to the immobilized water and its content is reduced, especially for the protein denaturation under large ice crystals [28]. Meanwhile, the immobilized water migrates to the free water, and hence freezing at lower temperatures can produce smaller ice crystals and inhibit this migration [29]. Thawing loss is mainly determined by the content of free water [7][30]. Yu et al. [31] found that small ice crystals formed by rapid freezing could better suppress the thaw loss of giant freshwater prawns, compared with the formation of large ice crystals in slow freezing. Generally, the thaw loss is simply attributed to the difference in the mechanical damage of muscle fibers caused by the size and location of ice crystals, and there is not further explanation [32]. Zhang et al. [33] proposed a model to explain thaw loss that water migrated from the inside of the muscle fiber to the outside during growth of ice crystals, and the slow freezing rate increased the mechanical damage caused by transverse shrinkage and deformation of the muscle fiber. Additionally, slow freezing also caused an increase in concentration of protons (lower pH and higher ionic strength) which leads to protein denaturation and the structural protein reabsorbs less water after thawing; nevertheless, some protons are trapped inside small ice crystals formed by rapid freezing. When the aquatic product is exposed to temperature fluctuations, the growth in size of ice crystals leads to an increase of thaw loss and weight loss of the product muscle.
The water transfer during freezing occurs between the product and its surrounding environment, resulting in dehydration which implies a weight loss while the product releases energy to the surrounding environment [34]. Mulot et al. [16] studied the effect of freezing temperature (from room temperature to −100 °C) and gas flow velocity (from 0 to 9 ms
−1) during freezing on food dehydration, and the results indicated that there was a close relationship between freezing temperature and weight loss: lowering the temperature decreases weight loss. For lower freezing temperatures, the increase in flow velocity can reduce weight loss, whereas, for high freezing temperatures, the influence of the gas flow velocity is less pronounced on the weight loss of the product. In order to limit the weight loss of the product, it is essential to reduce the total freezing time (reduction of initial product temperature, for instance). Additionally, Jin et al. [9] found that larger crystal size induced by ice nucleation proteins could facilitate the water vapor flow and thus increase the dehydration rate of the sucrose solution, which was conducive to improve the efficiency of the freeze drying process. The product frozen at a high freezing rate or low freezing temperature is left with small pore sizes resulting in a low void connectivity after freeze drying, which increases the resistance to water vapor flow and has low freeze drying efficiency [35]. However, it is also believed that large ice crystals melt and freeze dry more easily. The dehydration of frozen products inevitably occurs during frozen storage, especially affected by temperature fluctuations. According to Dalvi-Isfahan et al. [36], since the water vapor pressure difference between the surface of frozen products and the water in the air during frozen storage, ice sublimate and dehydrate to equilibrate with the vapor pressure of both sides. The dehydration of frozen products which cause the weight loss irreversibly affects other qualities of products, such as texture, color and protein, etc., resulting in a serious deterioration in the quality and value of frozen products [37][38]. Various techniques can be used to avoid the dehydration of aquatic products during freezing storage, such as glazing [8][39], vacuum packaging [21], and temperature control [36], etc.
2.2. Texture
The texture is directly influenced by the characteristics (shape and position) of ice crystals during the freezing of aquatic products. Under the influence of ice crystals, the myofibrils and the connective tissue around fibers of the sample thawed before or after was significantly fragmented (
Figure 1). It is well documented that ice crystals cause the deterioration of aquatic products, especially the accelerated decrease of hardness caused by large ice crystals, such as common carp [40], horse mackerel [18], and prawns [31]. According to Yang et al. [17], ice crystals played an important role in the microstructure destruction and texture softening of pufferfish, and the even-distributed ice crystals contributed to maintaining the texture characteristics of frozen pufferfish. However, the softening of frozen aquatic products cannot be attributed only to the formation of ice crystals, but also to endogenous proteolytic activity [41] and lipid and protein oxidation [1]. In order to compare the main factors of these three reasons for softening, Yang et al. [15] compared and indicated that fish softening contributed the most was ice crystals during frozen storage, and the fish softening during frozen storage mainly occurred in the initial stage. What is more, the evolution of ice crystals to large ice crystals due to temperature fluctuations aggravate the deterioration of texture, which was likely a result of the decrease in the mechanical strength of connective tissue, water loss, and protein aggregation [42]. Yet, the smaller ice crystals formed through the lower temperature is not necessarily better for the texture of aquatic products. Shi et al. [43] indicated that the perch frozen by liquid nitrogen (−196 °C) had an obvious crack on the muscle surface, which was not suitable for long-term storage of fish. It was suggesting that −85.0 °C should be regarded as the limit temperature for the industrial freezing of fish [44]. Thus, it was not simply considered the effect of freezing temperature on ice crystals for freezing aquatic products but also on the texture and energy consumption to select the appropriate freezing temperature.
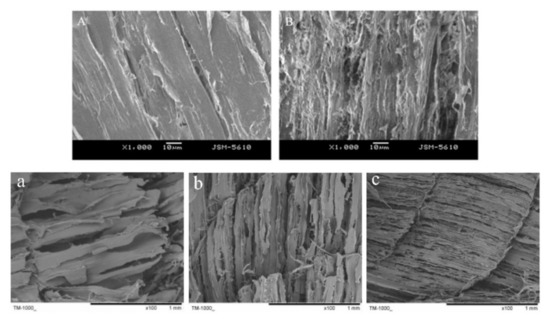
2.3. Protein
2.4. Colour
Colour is the most direct indicator for consumers to evaluate the quality of food and is an important factor in influencing their purchases [13]. Frozen channel catfish have a significant increase in whiteness values after thawing, which is not only associated with the decomposition of myoglobin but also due to the generation of ice crystals during freezing, the increase of free water on their surface after thawing, and the enhanced reflection of light on the surface of the fish resulting in an increase in whiteness values [129]. In addition, as the freezing rate increases, the surface ice crystal size of frozen salmon fillet decreases, L* values (represent the degree of lightness to darkness) increase, a* (represent the degree of redness to greenness) and b* values (range from yellowness to blueness) decrease, and whiteness values increase, which means that less colour change [130]. After a long period of frozen storage, the surface of the common carp fillets gradually tended to be yellow [131]. The L* value of precooked Chinese shrimp decreased from 54.52 to 44.28 at the end of storage (180 d), which was probably due to astaxanthin degradation and lipid oxidation, and the same change trend was observed for b* values [132]. Temperature fluctuations accelerate the myoglobin oxidation in the meat and accelerate the colour change of the product [35]. From Figure 4, it is obvious that the surface color of large yellow croakers became dull and lusterless due to dehydration. However, the mechanisms by which ice crystals affect the colour of aquatic products remain to be explored in depth.
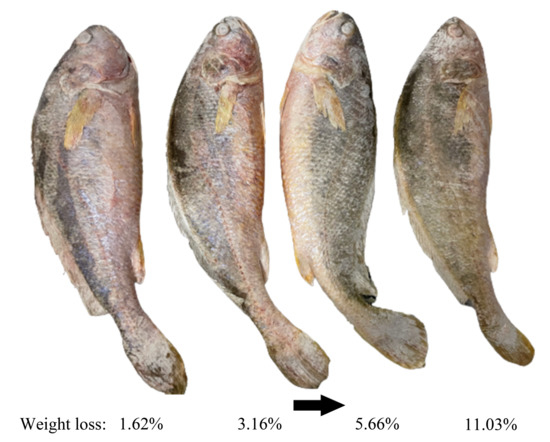
Figure 14. Effects of ice crystalswater sublimation on the structure of aquatic products. The SEM micrographs of peeled shrimp(A: furface color of large yellow croakeresh and B: f dureezing in a freezer at −30 °C) and hairtail (a: cfrozen stonventional air freezing at −20 °C, b: refriagerator. cryogenic freezing at −80 °C, and c: liqThe figuid nitrogen immersion freezing at −196 °C) are adaptede was created from Zhang et al. [45]own experimental imand Luan et al. [46], ges by the authorespectively.
2.3. Protein
Protein molecules are substances that have a spatial conformation (including primary, secondary, tertiary, and quaternary structures) under the combined action of covalent and noncovalent bonds. In general, the mechanism of protein denaturation caused by freezing is due to the increased concentration of solutes in the unfrozen water phase of muscle tissue during ice crystal formation [33][47]. In other words, solutes are gradually concentrated in the unfrozen water phase outside the ice crystals during liquid nitrogen immersion freezing (LIF) at −196 °C freezing, which causes the concentration of protons in the unfrozen water around the protein to increase (lower pH and higher ionic strength) also resulting in protein denaturation, especially for slow freezing. However, several studies over the years have other opinions on this, such as the direct adsorption of proteins onto ice crystals [48], the accumulation of air bubbles at the ice-freeze concentrate interface [49], the mechanical stress associated with ice crystal growth that may result in pressure-induced protein unfolding [50], and the foster of cold denaturation in the presence of ice crystals [51]. Since water molecules that play an important role in maintaining the structure stability of secondary and tertiary of proteins participate in crystallization, led to the changes in the spatial conformation of protein molecules and then protein denaturation [52]. The formation of ice crystals during the freezing of snakehead destroyed the protein secondary structure conformations and compared with small ice crystals, samples under large ice crystals have higher thermal stability [53]. Similarly, the results of Sun et al. [54] showed a decrease in the α-helix proportion of the common carp after freezing and an increase in random coil, indicated that the protein secondary structure changed. In the meantime, the formation of ice crystals leads to the unfolding of the protein tertiary structure and the reduction of thermal stability, but the protein primary structure (including sulfhydryl, carbonyl group, free amino group, dityrosine content, and surface hydrophobicity) of the frozen sample was no significant difference between and the unfrozen sample. The muscle cells are destroyed by ice crystals during freezing, and protein molecules are attacked and oxidized by some preoxidation factors. After thawing, the degree of protein oxidation is increased due to the release of mitochondria, lysosomal enzymes, and other pro-oxidation factors into the sarcoplasm [55][56]. Further, prolonged cryopreservation and temperature fluctuations might cause the growth of ice crystals in the sample, resulting in more serious damage to the protein structure [20].
2.4. Colour
Colour is the most direct indicator for consumers to evaluate the quality of food and is an important factor in influencing their purchases [13]. Frozen channel catfish have a significant increase in whiteness values after thawing, which is not only associated with the decomposition of myoglobin but also due to the generation of ice crystals during freezing, the increase of free water on their surface after thawing, and the enhanced reflection of light on the surface of the fish resulting in an increase in whiteness values [57]. In addition, as the freezing rate increases, the surface ice crystal size of frozen salmon fillet decreases, L* values (represent the degree of lightness to darkness) increase, a* (represent the degree of redness to greenness) and b* values (range from yellowness to blueness) decrease, and whiteness values increase, which means that less colour change [58]. After a long period of frozen storage, the surface of the common carp fillets gradually tended to be yellow [59]. The L* value of precooked Chinese shrimp decreased from 54.52 to 44.28 at the end of storage (180 d), which was probably due to astaxanthin degradation and lipid oxidation, and the same change trend was observed for b* values [60]. Temperature fluctuations accelerate the myoglobin oxidation in the meat and accelerate the colour change of the product [61]. From Figure 2, it is obvious that the surface color of large yellow croakers became dull and lusterless due to dehydration. However, the mechanisms by which ice crystals affect the colour of aquatic products remain to be explored in depth.

Figure 2. Effect of water sublimation on the surface color of large yellow croakers during frozen storage. The figure was created from own experimental images by the authors.
