Proper nutrition is crucial for normal brain and neurocognitive development. Failure to optimise neurodevelopment early in life can have profound long-term implications for both mental health and quality of life. Although the first 1000 days of life represent the most critical period of neurodevelopment, the central and peripheral nervous systems continue to develop and change throughout life. Besides their individual contributions, the interaction of nutrients with other micro- and macronutrients and the way in which they are organised in the food matrix are all of crucial importance for normal neurocognitive development. Also the gut-brain axis, including the gut microbiota, is an important modifier in this respect.
- brain
- neurodevelopment
- childhood
- protein quality
- tyrosine
- tryptophan
- poly-unsaturated fatty acids
- polar lipids
- minerals
- vitamins
- kynurenine
- gut-brain axis
- prebiotics
- probiotics
1. Introduction
Nutrition is critical in supporting healthy brain development early in life, with long-lasting, and often, irreversible effects on an individual’s cognitive development and life-long mental health. In this review, we present recent human and pre-clinical evidence on the role of nutrition, with particular focus on more emerging nutrients, in neurocognitive development in healthy infants and children aged 0–59 months.
Development of the human brain starts with the closure of the neural tube by the fourth week of pregnancy [1] and the proliferation of neurons in the germinal layers near the ventricles during the early phases of gestation (from week six of pregnancy) [2]. This is followed by the migration of neurons to their final destination and simultaneous initiation of neuronal differentiation (Figure 1), adapted from [3]). Neuronal differentiation includes the formation of dendrites and axons, the production of neurotransmitters, the development of synapses and intracellular signaling systems, and establishment of complex neural membranes starting from late pregnancy until the first few months postnatally. The formation of synapses continues throughout life [2][4][2,4], whereas the production of various neurotransmitters starts prenatally and reaches mature levels around the age of three years [5]. In parallel, glial cell production begins during the second trimester (32 weeks of gestation) [2][5][2,5]; glial cells insulate the axons by surrounding them with a membranous myelin sheath (axonal myelination), a process that predominantly takes place between the second trimester of gestation and the first year of life. Myelination continues, but starts to decline in early adulthood after which it stops at around the age of 40 years [2].
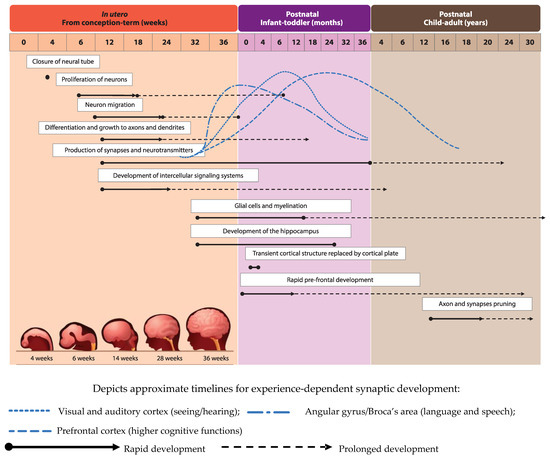
Development of brain structures occurs in several phases. The major transition takes place when the transient cortical structure, mediating fetal and neonatal behavior, is replaced by the cortical plate at three to four months of age. As a result, motor behavior changes from non-directed general movements to more goal-directed movements, such as reaching. The hippocampus, which is important for facial and scene-recognition, as well as spatial memory, develops at approximately 32 weeks of gestation until at least 18 months postnatally [4][5][4,5]. The prefrontal cortex, responsible for complex processing tasks such as attention and multi-tasking, exhibits initial rapid development during the first 6 months of life [5]. Note, that prefrontal development continues well into the third decade of life [6]. The pruning of axons and synapses to further optimize the brain’s functioning usually starts between the onset of puberty and early adulthood [2].
2. Nutrients that Play a Role in Neurodevelopment
2.1. Lipids
2.1.1. Long-Chain Polyunsaturated Fatty Acids
Neurodevelopment is influenced by a number of factors ranging from gestational age at birth and social environment to nutrition. Dietary fat in particular is an important modifiable nutritional factor illustrated by the crucial role of the polyunsaturated fatty acids (PUFAs) linoleic acid (LA; 18:2 n-6), alpha-linoleic acid (ALA, 18:3 n-3), docosahexanoic acid (DHA; 22:6 n-3) and arachidonic acid (AA, 20:4 n-6) in normal brain formation and neuronal myelination during infant neurodevelopment [2][7][2,7]. Furthermore, long-chain PUFAs (LCPUFAs) have been shown to affect the production of various neurotransmitters [5], with profound effects on monoaminergic, cholinergic, and gamma-aminobutyric acid (GABA) ergic systems. DHA is especially important for visual and prefrontal cortex development, the latter of which mediates attention, inhibition and impulsivity actions [5].
In addition to sufficient intake via diet or supplementation, a balanced ratio between LA and ALA is important as well [7][8]. In the prospective Mothers and Children’s Environmental Health (MOCEH) cohort of 960 pregnant women in Korea, an inverse association between LA/ALA ratio during pregnancy and Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) scores in the offspring at six months of age was reported [8][7]. The average LA/ALA ratio in this population amounted to 11.12 ± 6.9.
LA is abundantly present in daily food and high intake can have negative health consequences [7][8]. High LA in colostrum and breastmilk has been associated with poorer motor and cognitive scores at two and three years of age [9] and a lower verbal intelligence quotient (IQ) at five-to-six years [10] in the observational EDEN (Etude de cohorte généraliste, menée en France sur les Déterminants pré et post natals précoces du développement psychomoteur et de la santé de l’Enfant) cohort in France. This negative impact was postulated to take effect through several mechanisms, namely the suppression of biosynthesis of n-3 PUFAs (due to enzymatic competition to convert n-6 and n-3 PUFA to LCPUFA), which supplies necessary DHA for brain development, as well as by decreased uptake of circulating DHA by the brain and thus impaired accretion of DHA in the brain. Lastly, n-6 PUFAs are precursors for several pro-inflammatory eicosanoids that can be produced in early life and may have a negative impact on cognitive function [10].
Several observational studies reported that high DHA levels in pre- and postnatal periods seem to improve specific cognitive skills ranging from processing ability and attention to overall IQ in the offspring, even up to 12 years of life [11][12][13][11,12,13]. Nonetheless, the impact of DHA supplementation during pregnancy remains controversial. Daily 400 mg DHA supplementation for 20 weeks in pregnant mothers showed positive effects on the infant’s attention ability at 5 years of age [14], while an earlier study among 2499 pregnant women in Australia found that daily 800 mg DHA supplementation did not affect cognitive and language development of the offspring at 12 and 18 months [15]. The latter is further supported by the results of a Cochrane systematic review (2015) stating that there was no effect of DHA supplementation in breastfeeding mothers on language development, problem-solving abilities, psychomotor development, and general movement ability of their offspring [16]. Another Cochrane review (2017) reported that, although there was no concern over the safety of infant formulas supplemented with DHA and AA, the majority of evaluated randomized controlled trials (RCTs) did not show any beneficial effects on neurodevelopmental outcomes in term infants [17]. In addition, the authors concluded that the positive effect on visual acuity had not been consistently demonstrated. Although the review included the study by Colombo et al. [18], reporting a beneficial effect of DHA/AA supplementation (up to 0.64% DHA/0.64% AA) in infant formula on problem-solving skills at nine months [18], it did not include earlier studies on the topic [19][20][19,20]. These studies indicated that routine supplementation of term infant milk formula with DHA (at the level of 0.3% PUFA), from birth to four months of age, was associated with improved neurodevelopmental outcome at four months [20] and higher MDI scores at 18 months [19]. In addition, better inhibitory control measured by behavioral and brain electrophysiology responses among those supplemented with the above-mentioned dose at 5.5 years of age has been described as well [21]. Importantly, dosing LCPUFAs at a level higher than 0.64% in early life may have negative effects on cognitive development at a later stage [18].
The impact of essential fatty acids (EFA) and LCPUFAs on cognition and brain development appears to be particularly evident in older children. Interestingly, DHA and AA supplementation of 200 mg daily in Growing Up Milk (GUM) among toddlers aged 13 months for the duration of one year increased the Bayley III language composite score at 24 months as compared to those receiving standard GUM without LCPUFA. The same study reported fewer inattention episodes among boys receiving LCPUFA-supplemented GUM as compared to their unsupplemented counterparts [22].
Two separate studies in two-to-six years old children in Ghana and Tanzania revealed that children with the highest levels of blood EFA and DHA had at least a three times higher chance of successfully passing an executive function test [23][24][23,24]. In older pre-school children, the consumption of 978 g of fish over one week influenced cognitive function compared to those consuming 850 mg of meat, after adjusting for dietary compliance; information on EFA and DHA blood levels was not included [25].
2.1.2. Polar Lipids
Polar lipids are amphiphilic in nature and contain a hydrophobic tail and a hydrophilic head. Phospholipids (glycerophospholipids and sphingomyelin) and sphingolipids (ceramides, cerebrosides and gangliosides) are the main representatives of this group. Polar lipids make up biological membranes but are also found in circulating fluids. In mammalian milk, the milk fat globule membrane (MFGM), the trilayer membrane structure surrounding each fat globule, is an important source of polar lipids [26][27][26,27], as are nanovesicles (exosomes). Nanovesicles are secreted into milk by mammary gland cells and are implicated in cell-to-cell communication by virtue of their functionally active cargo (e.g., messenger-RNA (mRNA), micro-RNA (miRNA), and different proteins; [28]). Human as well as bovine milk contains approximately 4% fat in the form of fat globules [29]. These globules are filled with triglycerides, constituting 98% of total fat. The lipids within the MFGM primarily include polar lipids but also comprise neutral lipids (like cholesterol). The main polar lipids present in human and bovine MFGMs are phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), and sphingomyelin (SM) [30][31][30,31]; human milk contains higher levels of SM and PS, whereas relatively more PE is present in bovine milk fat [32].
To date, no intake recommendations or guidelines for polar lipids have been proposed or implemented by health authorities. However, adequate intakes have been defined for two nutrients that serve as structural parts of polar lipids, choline and DHA [33][34][33,34]. Presently, only limited scientific evidence exists on the brain bioavailability of polar lipids via placental transfer or transport over the blood brain barrier (BBB) [35][36][37][35,36,37]. Nevertheless, supplementing polar lipids in wild-type animal models and healthy infants does suggest benefits for cognitive performance.
The putative role of MFGM polar lipids in brain and neurocognitive development has received significant attention. In Sprague-Dawley rats, oral gavage supplementation with MFGM led to neurocognitive benefits by early upregulation of genes involved in brain function, such as brain-derived neurotrophic factor (BDNF) and St8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 4 [38]. Somewhat unexpectedly, a human RCT evaluating the effects of maternal dietary supplementation of complex milk lipids (CML; gangliosides and phospholipids) from the MFGM during pregnancy on fetal growth showed no effects on any of the fetal biometric dimensions measured [39]. The lack of effect could be due to the application of an inadequate dose of polar lipids. In a Belgian study, a phospholipid-rich MFGM concentrate given daily to preschool children aged 2.5–6 years for a period of four months decreased behavioral problems and reduced days with fever during the intervention period [40]. Notwithstanding this encouraging result, Timby and colleagues (2017) concluded that while MFGM interventions seem safe, it is still unclear which MFGM fractions are most suitable for supplementation and at what concentration at which age. Furthermore, it was pointed out that the evidence base for the effects of MFGM polar lipids on brain and neurocognitive development is still limited [41]. Since then, several studies have provided additional evidence for the importance of the MFGM in early life with mixed outcomes. A study in 451 healthy term infants showed that receiving formula with added bovine MFGM and bovine lactoferrin (LF) resulted in accelerated neurodevelopment at day 365 as evidenced by higher mean cognitive (+8.7), language (+12.3), and motor (+12.6) Bayley-III scores, and improved global development scores from day 120 to day 275 and attention at day 365 in the MFGM + LF group [42]. In addition, enhanced language skills at day 545 were observed (some subcategories of the MacArthur-Bates Communicative Development Inventories were higher in the MFGM + LF group).
There has been growing interest in the use of gangliosides as part of a supplement for either the infant or the mother because these polar lipids serve a crucial role in pre- and postnatal development of the brain, which coincides with the critical window of rapid brain growth around birth [43]. Ceramides are essential for neural development contributing to ganglioside synthesis in utero. Interestingly, the phlorizin domain of the lactase enzyme splits ceramides from glucosyl, galactosyl, and lactosyl-cerebrosides [44]. In addition, lactase splits lactose into glucose and galactose. As nearly all healthy infants are lactase persistent, lactase activity in their small intestine yields ceramide, glucose, and galactose moieties from dietary lactose and glycosphingolipid intake, all important building blocks for the developing nervous system. Gangliosides are glycolipids that contain sialic acid, which is an essential nutrient for optimal brain development and cognition. Endogenous production of sialic acid is possible but limited. Rather, it is available in human milk oligosaccharides in relatively large quantities, predominantly in the form of Neu5Ac (N-acetylneuraminic acid), which is the precursor of various neural brain glycoproteins, including polysialic acid, gangliosides, glycosaminoglycans, and mucins. The major protein carrier of polysialic acids is NCAM (Neural Cell Adhesion Molecule); polysialylated-NCAM is a key neuroplastic molecule involved in neuronal plasticity and of crucial importance for memory formation. Other examples of sialylated proteins are synaptic cell adhesion molecule 1 and scavenger receptor CD36 [45]. Sialic acid is particularly abundant in neuronal cell membranes. Notably, the location and amount of sialic acid in different regions of the brain change dramatically during development.
The high sialic acid content of human breast milk in addition to its role in brain development suggest that sialic acid in breast milk has an impact on infant cognitive development. This could also imply that brain growth creates a greater need for sialic acid than can be provided by endogenous biosynthesis in the infant. This is supported by findings from a study in which sialic acid was measured in brain samples from infants (1–38 weeks) that died of sudden infant death showing that the sialic acid content was higher in the brains of breast-fed infants than in those of formula-fed infants [46].
2.2. Minerals
2.2.1. Iron
In addition to lipids, micronutrients are critical for normal neurodevelopment as well. Iron, for instance, is vital for energy production, oxygen transportation, and DNA synthesis. It plays a crucial role in hippocampal development, myelination and production of neurotransmitters, such as dopamine, serotonin, and norepinephrine as shown in pre-clinical studies [5][47][48][5,47,48]. Iron deficiency results in reduced 6-desaturase enzyme activity that is required for the synthesis of essential fatty acids and can therefore impair the synthesis of α
-linoleic acid (ALA, 18:3 n-3) into DHA [49]. Currently, no literature is available on the impact of combined iron and LCPUFA deficiencies on cognitive neurodevelopment.
Limited evidence from recent studies suggest that iron supplementation during pregnancy and infancy may positively influence the psychomotor development of children [47][48][50][47,48,50]. A positive effect of prenatal iron supplementation during pregnancy on overall cognitive development of the child has been described only for anemic pregnant women. In this group, a favorable effect on cognitive performance in children under two years of age, toddlers and primary school children was observed [48][50][51][48,50,51].
Based on a systematic review and a follow-up study, the effects of iron supplementation during early life on cognitive function are unclear at 12 months of age. In addition, no benefit on cognitive function at 18 months could be detected [47][50][47,50]. Notably, the provision of iron to iron-replete infants could have a negative effect on long-term cognitive development as shown in a cohort study among infants in Chile [47][52][47,52]. Positive impact of iron supplementation on cognitive function seems to be observed only in anemic primary school children [47][51][47,51]. Based on the available evidence, adequate dietary iron intake should be encouraged during pregnancy and post-natal life up to adulthood. With regard to iron supplementation, a different picture emerges. Given the uncertainty of the efficacy of iron supplementation due to significant supplementation heterogeneity across the various studies (i.e., type and format of iron supplementation, dosage, length of supplementation, presence of other nutrients) [47][50][47,50], combined with the potential negative impact of providing high iron dosages to iron-replete infants [52], it seems advisable to restrict the provision of iron supplements, in the right form and dosage, to anemic individuals.
2.2.2. Zinc
Zinc is necessary for central nervous system (CNS) development and is one of the most ubiquitous metals found in the brain; it is present in many enzymes involved in brain growth and is important for neurotransmission. In animal models, zinc has been shown to be involved in neurogenesis, neural migration, synaptogenesis, and regulation of GABA-Ergic neurotransmitter release [5][53][5,53].
Zinc deficiency during pregnancy and early infancy has long been associated with developmental deficits, such as poorer learning, attention, memory, and mood [5]. However, there is currently no convincing evidence that maternal zinc supplementation improves cognitive development in the offspring [54][55][54,55].
Evidence on the association between zinc intake and cognitive development is quite limited for infants. Six-month old infants receiving either a combination of micronutrients (10 mg/d zinc, 10 mg/d iron and 0.5 mg/d copper) or iron and copper alone for the duration of 6 months presented with different outcomes. In the group receiving zinc, there was an improvement in normative information processing and active attentional profiles at two years of age. However, no differences were reported regarding other parameters, which included Bayley Scales of Infant Development (BSID) at six, 12, and 18 months. Infants receiving zinc supplementation were also able to maintain a better zinc status [55].
A Cochrane systematic review (2012) concluded that there is no significant effect of zinc intake on mental and motor development in children [56]. This was nuanced by another systematic review, published later that year, stating that the effect of zinc supplementation on cognitive function might be dependent on the dose of supplementation and the duration of the intervention [57].
2.2.3. Iodine
Iodine plays an important role in brain development in the form of thyroxine and triiodothyronine. It affects the timing of differentiation of neural tissue in the brain prenatally and determines the number of glial cells for myelin sheath production postnatally. Maternal thyroid hormone can be found in the embryonic cavities at the end of week 4 post-conception when the formation of the brain cortex and the anterior part of the neural tube takes place [58].
The impact of pre-conception iodine levels has been recently investigated in the Southampton Women Cohort [59]. The results revealed that a low maternal urinary iodine concentration, measured by iodine/creatinine (I/Cr) ratio, at 3.3 years before conception was associated with low overall childhood cognitive function at 6–7 years as assessed by the Wechsler Abbreviated Scale of Intelligence (WASI) [59]. Around 8.9% of the women in this cohort presented with a low I/Cr ratio. Unexpectedly, the same study reported no influence of pre-conception iodine levels on specific measures of executive function at the age of six-to-seven years [59].
The Generation R cohort study showed that mild-to-moderate iodine deficiency in early pregnancy affects the offspring’s behavior and risk for development of ADHD at eight years of age [60]. Severe iodine deficiency during pregnancy is well-known to result in maternal and fetal hypothyroidism and has been shown to be associated with serious adverse health effects in the offspring, including congenital hypothyroidism, growth retardation and impaired cognition encompassing deficits in hearing, speech, gait and IQ [5][61][5,61]. Still, several iodine supplementation studies during pregnancy on offspring cognitive function reported inconclusive findings. This, in part, may be explained by the application of age-inappropriate global development assessments that may have caused misclassification and lack of correlation with cognitive function at that particular time [58][62][63][58,62,63].
Post-natally, iodine continues to play a role in neurocognitive development. The level of iodine in colostrum predicts the motor development capability of infants at 18 months, but does not relate to other abilities, such as language development or overall cognition [64]. Interestingly, a study on iodine supplementation using iodized salts for children in areas where the incidence of iodine deficiency is high, reported no benefits on cognitive function in children older than three years of age despite the improvement in iodine status [65].
2.3. Vitamins
Despite extensive research conducted on vitamin supplementation, only limited recent evidence exists to suggest that vitamin supplementation during pregnancy and early intake by infants positively influences cognitive development of children. Nevertheless, vitamin A, vitamin B12 (cobalamin), folate, and vitamin D are well-recognized for their capacity to critically influence early cognitive development [5][49][5,49], and micronutrient deficiencies early in life can lead to impairments of the CNS.
2.3.1. Vitamin A
2.3.2. Vitamin B12
A study on maternal intake of methyl-donor nutrients and child cognition at three years of age revealed a weak inverse association for vitamin B12 intake and a linear association for folate intake during the first and second trimester with the Peabody Picture Vocabulary Test III (PPVT-III scores) [68]. Each 600 mcg/day increment in total folate intake during the first trimester was associated with an increase of 1.6 points in PPVT-III scores. No correlations were found between choline, betaine, or methionine and cognitive function [68]. Recent findings from the GUSTO (Growing Up in Singapore Towards healthy Outcomes) cohort showed that maternal B12 deficiency was associated with lower cognitive scores of infants at 24 months when compared to infants from vitamin B12-replete mothers [69]. The level of vitamin B12 at 2–12 months correlated with development and performance in social perception tasks and visuo-spatial abilities at 5 years of age among 330 children in Nepal [70]; an increase of one unit in vitamin B12 status was associated with an increase of 4.88 in the Ages and Stages Questionnaires (ASQ-3), 0.82 in recognition score, 0.59 in geometric puzzle score, and 0.24 in block construction scores. In another study (650 children, India), vitamin B12 status at four-months was associated with increased BSID-II score at 12–14 months [71]. Vitamin B12 plays an important mechanistic role in neural myelination, synaptogenesis, and neurotransmitter synthesis in pre- and postnatal periods. In addition, it promotes development of the hippocampus and is therefore relevant to memory, language, and visual processing [69]. Vitamin B12 and folate are required for cell division and generation of methionine, which is needed to produce neurotransmitters and myelin [70]. A relationship between folate and vitamin B12 was also reported by Strand and colleagues, demonstrating that the plasma folate concentration was independently associated with mental development scores when children with poor vitamin B12 status were excluded from the analysis [72]. Timing of folic acid intake (and the resultant endogenous folate level) is likely critical, as it is well-known that severe folate deficiency during pre-conception and early pregnancy is associated with inadequate closure of the neural tube resulting in severe brain defects, including spina bifida [1]. However, in older children, supplementation with folic acid, vitamin B2, B5, and calcium did not affect verbal IQ, short term memory, or processing speed [73].2.3.3. Vitamin D
In a rodent model, low maternal vitamin D (1,25-dihydroxycholecalciferol) status during pregnancy was associated with structural changes in the brain, such as enlarged lateral ventricles, a thinner cortex, and increased cell proliferation [49][74][49,74]. Low prenatal vitamin D status was also linked to the severity of schizophrenia and autism symptoms in epidemiological studies [75][76][75,76]. In addition, vitamin D status during pregnancy was shown to be related to cognitive development, and maternal 25(OH)D levels <50 nmol/L were independently associated with low MDI and PDI scores at six months [77]. This prospective cohort of 363 mother-infant pairs in China also reported an inverted U–shaped relation between vitamin D levels in cord blood and neurocognitive score at 16–18 months [77]. Interestingly, vitamin D has been shown to be able to upregulate serotonin expression [78].2.4. Dietary Protein and Amino Acids
A classic example of the importance of proteins to behavioral and neurocognitive development in infants is the longitudinal study by Chavez et al. [79] which evaluated the effects of nutritional supplementation on infants’ physical, mental, and social development in two groups of 17 mother-child pairs in a poor rural Mexican community. In this study, one group of mothers was supplemented daily with 205 calories and 15 g of protein during pregnancy and 305 calories and 15 g of protein during lactation, whilst the other group followed the usual feeding habits of the community. Between the 12th and 16th week of life, the supplemented infants began to receive whole cow’s milk ad libitum and prepared baby food in quantities sufficient to maintain adequate rates of growth. At 18 months of intervention, the mothers of supplemented children displayed more complex interactions with their children, who were more restless, playful, demanding and disobedient than those non-supplemented. These results suggest a beneficial effect of protein (and energy) supplementation on the behavioral patterns within the family, with the more active children eliciting greater stimulation from their parents. Another historic study [80] demonstrated that protein supplementation, rather than energy, during early childhood improved psycho-educational performance. Therefore, Guatemalan children exposed to protein supplement scored significantly higher on tests of knowledge, numerical aptitude, reading and vocabulary as compared to those that only received energy supplementation. Two decades later, 130 female subjects were re-evaluated and, interestingly, women exposed to protein supplementation during early childhood had better educational achievements than those from the energy group [81].
2.4.1. The Importance of Protein Quality
2.4.2. mTORC1: Linking Protein (in)Adequacy to Brain Development
One plausible mechanistic pathway that may explain the link between protein (in)adequacy and brain development is the activity of the serine/threonine kinase mammalian target of rapamycin complex 1 (mTORC1), which is a master regulator of all cell growth and metabolism. This kinase integrates signals triggered by different stimuli, such as variations in amino acid supply, changes in the cellular energy state, growth factors (e.g., BDNF, insulin, and IGF1 (insulin-like growth factor 1)), and (within the brain) by transduction of neurotransmitters and neurotrophin signals [88][89][90][88,89,90]. After influx through L-amino acid transporters, leucine activates mTORC1 in neurons [91]. In addition, uptake of arginine by the cationic amino acid transporters CAT1 and CAT3 has also been demonstrated to activate mTORC1 in neurons [92]. Amongst growth factors, insulin and IGF1 enhance mRNA translation in neurons possibly through mTORC1 [93][94][95][96][93,94,95,96]. BDNF, the most prominent neurotrophic factor in the CNS [97][98][97,98], has been shown to activate mTORC1 signaling and enhance de novo protein synthesis in cortical neurons [99][100][99,100]. Several studies suggest that amino acid sufficiency is essential for the insulin-induced activation of mTORC1 in several cell lines [101][102][101,102], but not for BDNF-induced mTORC1 activation in neurons [103]. Neurotransmitters such as serotonin (5-HT) have also been reported to possibly activate mTORC1 [104][105][106][104,105,106]. During early CNS development, mTORC1 is involved in neural stem cell proliferation, migration, and differentiation, axonal and dendrite development, gliogenesis, synaptic plasticity, and learning and memory storage [107][108][107,108]. Aberrant mTORC1 signaling alters neural development and can result in a wide spectrum of neurological developmental disorders, including learning disabilities and mental retardation.3. Nutrient Interactions through the Gut-Brain Axis (GBA)
3.1. What Is the Microbiome Gut-Brain Axis?
3.3. The Impact of Pro- and Prebiotics through the GBA
One way of influencing microbial diversity and reversing dysbiosis is via nutrition and drastic changes in diet have been found to alter microbial diversity in mere days [135][192]. Recent research suggests that microbial ecology can be therapeutically modified via the intake of so-called ‘psychobiotics’ [136][193], referring to active compounds that are capable of acting on the nervous system, consequentially shaping psychological processes and behavior, ultimately exerting health benefits in persons with psychiatric conditions. Indeed, psychobiotics have anxiolytic and antidepressant effects marked by changes in emotional, cognitive, systemic and neural indices [136][193]. Initially, only probiotics were considered as psychobiotics since they have the ability to release neuroactive substances (depending on the strain of belonging) [137][194]; this includes, for example, the production of dopamine and noradrenaline by members of the Bacillus genus, GABA by the Bifidobacteria genus, serotonin by the Enterococcusand Streptococcus genera, noradrenaline and serotonin by the Escherichia genus, and GABA and acetylcholine by the Lactobacilli genus [136][193]. Researchers have consistently outlined the psychotropic effects of probiotics: in an animal study, Barrett and colleagues demonstrated that Lactobacillus (L.) brevis and Bifidobacterium (B.) dentiumincreased GABA concentrations in vitro [138][195]. These findings were supported by a study using an in vivo mouse model showing that ingestion of the L. rhamnosus strain regulated emotional behavior and central GABA receptor expression [139][196]. Mice fed with L. rhamnosus exhibited decreased GABA mRNA expression in the amygdala, along with lower levels of stress-induced corticosterone and reduced anxiety- and depression-related behavior [139][196]. Another study replicated these results [140][197], and showed that the increase in GABA metabolites in the brain was evident after about four weeks, a lag that is comparable with the onset of other pharmaceutical interventions, such as serotonin-reuptake inhibitors [141][198]. Importantly, it was pointed out that re-colonization of gut bacteria in adolescent GF mice was not sufficient to reverse anxiety-like behavior, further supporting the idea that early deficits in gut microbiota may not be reversible. With regards to human studies, Pärtty and colleagues administered L. rhamnosus to seventy-five infants aged six months and followed up with them for 13 years. The results suggested that supplementation with this probiotic strain may reduce the incidence of ADHD and Autism Spectrum disorders. However, the underlying mechanisms of action need further clarification, as no consistent microbial patterns could be identified [142][199]. In a randomized double-blind trial including 55 adult participants, it was found that the consumption of probiotics led to reduced measures of mood and distress, as well as decreased levels of urinary free cortisol, reflecting a decreased stress response [143][200]. Similarly, the results of a placebo-controlled, four-week probiotic food-supplement intervention study with multispecies probiotics in 20 healthy participants without mood disorders revealed a significantly reduced overall cognitive reactivity to sad mood (assessed by the revised Leiden index of depression sensitivity scale) as compared to the placebo group [144][201]. Lastly, consumption of a fermented milk product with probiotic for a period of four weeks by healthy women resulted in altered activity of brain regions involved in the control of emotion processing and regulation [145][202]. In addition to probiotics, prebiotics have more recently been classified as psychobiotics. Prebiotics are specific non-digestible food components, which selectively feed beneficial gut bacteria, consequentially stimulating their growth and activity with remarkable effects on brain development and function [136][193]. Prebiotics include oligosaccharides, fructans, unsaturated fatty acids, polyphenols and dietary fibers. To date, fructooligosaccharides (FOS) and galactooligosaccharides (GOS) have been studied the most, showing promising effects in animal models [146][147][203,204]. For example, milk oligosaccharides administration has been shown to prevent stress-induced dysbiosis and anxiety-like behavior in mice. Similarly, chronic combined FOS and GOS supplementation exhibited anxiolytic and antidepressant effects, as well as a reduction in the corticosterone stress response in mice [147][204]. In addition, prebiotics have been shown to modulate hippocampal and hypothalamus gene expression, and induce changes in SCFA concentration, which positively correlate with the behavioral effects. Further supporting the beneficial impact of prebiotics, a recent study in mice demonstrated that combined FOS-GOS supplementation from birth was associated with reduced anxiety-like and improved social behavior. Importantly, supplementation of short-chain GOS and long-chain FOS also affected serotonergic brain network regions comprising the prefrontal cortex (PFC) and the somatosensory cortex, and increased BDNF mRNA expression in the PFC [148][205]. In infants 12 months of age, administration of a combination of B. longum (BL999), L. rhamnosus, inulin, fructo-oligosaccharides (FOS), and LCPUFAs for one year resulted in higher, albeit not significantly different, scores in cognition and adaptive behavior [149][206]. More in-depth research on the potential beneficial effects of psychobiotics within such a critical developmental time window is needed, especially in light of the promising findings in older subjects. Therefore, an increase in processing of positive versus negative attentional vigilance and a significantly lower cortisol awakening response were observed in healthy adults after 3 weeks daily B-GOS intake, compared to the placebo group [150][207]. In a very recent study, the effects of prebiotic intake for four weeks on psychological and behavioral emotion regulation trait indices and the underlying brain networks involved were investigated in 60 girls in late adolescence [151][208]. The results showed a significant decrease in self-reported anxiety levels in the prebiotic group, along with a change in overt emotional processing in the dot-probe task. Moreover, the analyses of the pre- and postintervention stool samples showed a significant increase in beneficial Bifidobacteria in the gut microbiome. Together, these results suggest that four weeks of prebiotic intake is sufficient to induce changes in the microbial composition that lead to reduced anxiety levels in late adolescence. Psychobiotics can also act on the brain via modification of metabolic dynamics. For example, they can modulate tryptophan availability [152][153][209,210], which might have an impact on the kynurenine pathway that is responsible for 90% of tryptophan metabolization [154][113]. The downstream metabolites -kynurenic acid and quinolinic acid- of this pathway have recently been identified as relevant for the nervous system, as they exert neuroprotective, and excitotoxic effects, respectively, through their interaction with N-methyl-D-aspartate (NMDA) receptors [155][156][211,212]. Notably, research has suggested that dysfunctions of NMDA receptors during early development might cause CNS disorders, such as autism spectrum disorder (ASD) and attention deficit disorder (ADD), later in life [157][158][213,214]. This further emphasizes the importance of proper nutrition early in life, and indicates that psychobiotic supplementation might be an effective approach to influence tryptophan-kynurenine metabolism and prevent atypical developmental trajectories. In conclusion, we propose that in addition to the more traditionally recognized nutrients discussed in this review, emerging nutrients such as polar lipids, high quality protein/specific amino acids, and psychobiotics, are of critical importance for normal neurodevelopment in young children (see Figure 2, adapted from [159][215]).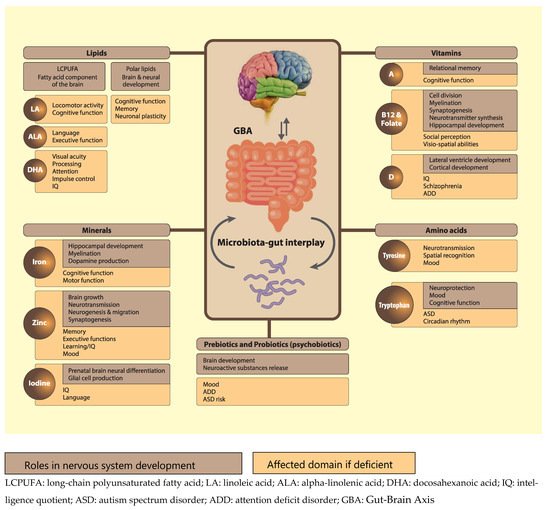 Figure 2. Functions and effect of some nutrients on brain and neuronal development. It also includes pre-and probiotics and tryptophan-based interactions through the gut brain axis.
Figure 2. Functions and effect of some nutrients on brain and neuronal development. It also includes pre-and probiotics and tryptophan-based interactions through the gut brain axis.
