Tannins are polyphenolic compounds historically utilized in textile and adhesive industries, but also in traditional human and animal medicines or foodstuffs.
- tannins
- pharmacological
- medicinal
- veterinary
- nutritional
- traditional application
- traditional use
- human and animal health
1. Introduction
Tannins have been used throughout history for their pharmacological properties as part of plants and herbs in traditional medicine. Also, they have been extensively used since the 18th Century by leather manufacturers to improve leather resistance in the dyeing or tanning process, as they can precipitate gelatin adhered to animal skin and provide a brownish color. Hence, the name of this group of phytochemicals [1]. Tannins are a heterogeneous group of polyphenols, secondary metabolites in plants synthesized in response to biotic and abiotic stress inducers. The phenolic rings and hydroxyl groups present in their chemical structures confer them antioxidant and protein-binding properties, as they have a wide range of molecular weight (500–20,000 Da) that it also showed in their broad structure diversity [2]. By their nature and abundance of hydroxyl radicals, tannins are highly hydrophilic molecules, soluble in aqueous solvents as well as exhibiting a high tendency to stably bond with proteins and carbohydrates [3]. This feature is common to all tannins, yet it seems that their link with polysaccharides lowers the probability of bonding and interacting with proteins [4]. They also share other properties, as the precipitation of colored complexes with iron salts or oxidation by potassium permanganate in alkaline media. Tannins are ubiquitously present in barks, seeds or fruit peels of many vegetable species, but also in brown algae [5]. Although several categorizations have been made on tannins regarding their molecular weight, properties and source, tannins are widely accepted to be classified under their functional units. As such, hydrolyzable tannins (HT), proanthocyanidins or condensed tannins (CT) and complex tannins (CoT) can be found on terrestrial plants, while phlorotannins (PT) have only been reported in brown macroalgae [4]. Among tannin diversity, the most abundant are terrestrial tannins, of which CT are generally most common. Even though their concentration and class differ in the different fractions of plants, tannins seem to have similar properties such as antioxidant, antimicrobial or predator-deterrent (i.e., against helminths or herbivores) [6].
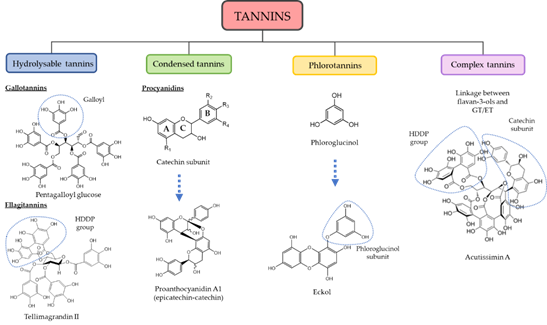
Regarding tannins structure, galloyl units are the bricks that form HT, but depending on their chemical unions and radicals, they may be differentiated as gallotannins (GT) or ellagitannins (ET), relying upon the presence of gallic acid (GA) or ellagic acid (EA) subunits on degradation [7]. As such, they are synthesized from the shikimate pathway. In general terms, GT are polymers of galloyl coupled with polyol, catechin or triterpenoid units, frequently found in the form of pentagalloyl glucose (PGG). The complexity of HT grows as more galloyl units are coupled through meta- or para-depside bonding, forming a chained structure of ester (oxidative) bonds [8]. ET are mainly galloyl units organized through C–C bonds such as in hexahydroxydiphenol (HHDP), HHDP-esters or nonahydroxytriphenoyl (NHTP) esters subunits [6]. The hydrolyzation of the HHDP subunit prompts EA subunits. The hydrolyzable label of HT indicates its low resistance to be hydrolyzed by high temperatures, acids, bases and specific enzymes such as tannase, commonly resulting in pyrogallol or GA products [9]. However, many ET are much more resistant to hydrolyzation because of the additional C–C bonding of their polyphenolic residue with the polyol unit [10].
The CT structure is regularly built upon catechins or epicatechins, the most common being (2,3-trans)-(±)-catechin and (2,3-cis)-(±)-epicatechin, which are flavan-3-ols moieties [11]. They are thus originated on the flavonol pathway [12]. The polymerization of CT is usually formed by bonding other catechins through C4-C8 bonds, but C4-C6 bonds may also be created, albeit less frequently [5]. The position of hydroxyl groups gives away a variation on their hydroxylation pattern in the A and B ring of the flavanol-3-ol unit, which in turn provides the classification of several groups of CT such as procyanidins (3,5,7,3′,4′–OH), prodelphidins (3,5,7,3′,4′,5′–OH), propelargonidins (3,5,7,4′–OH), profisetinidins (3,7,3′,4′–OH), prorobinetinidins (3,7,3′,4′,5′–OH) or proteracacinidins (3,7,8,4′–OH) among others. Among these groups, procyanidins are the most abundant in nature, which can be sorted on the linkage between flavanyl units in A (double), B or C (single) class [13][14].
On the other hand, CoT are tannins of high molecular weight resulting from the bonding of flavan-3-ols with either GT or ET via a C–C bond. Some examples of CoT are acutissimins A and B, which can be isolated from Quercus sp. and Castanea sativa or camelliatannin A from Camelia japonica [3].
PT are common tannins present in algae and constituted upon molecules of phloroglucinol (PG, aromatic ring with 1,3,5 hydroxyl groups) that polymerize with ease between C1-C3. They are grouped into three distinctive classes based on the coupling between subunits: fucols (C–C), phloroetols (C-O-C) and fucophloroteols (C–C and C-O-C). Increasing complexity in their structure is correlated to a higher presence of PG subunits (3 to 7 subunits) [15]. As well as terrestrial tannins, PT exert, in some cases, antimicrobial protection while their potent antioxidant properties confer protection against UV-A and UV-B radiation. A general perspective of tannin classification attending to their structure is presented in Figure 1.
Figure 1. Classification and general representative structures of tannins. Functional groups are circled. Rings in catechin molecule are labeled as A, B and C. R = radical, H, OH; GT = gallotannins; ET = ellagitannins; HHDP = hexahydroxydiphenol.
Generally, tannins are accumulated in vegetable cells in a special vacuole of recent discovery called tannosome, from which they are secreted to tissues. The inclusion of tannins in this vacuole avoids a disruptive binding of tannins with metabolic proteins or polysaccharides. In the case of ET, it is worth noting that they show much lower protein binding activity at low/neutral pH, in contrast with the rest of the tannin groups [6]. Yet, this is not always the case, as they may also be embedded in cell walls. This is most prominent in the case of PT since they are integrated into the cell wall and bound to algal polysaccharides like alginate, laminarin or fucoidan [16]. Correlating to their biosynthesis pathways, different classes of tannins do not tend to accumulate simultaneously in the same tissue, as their concentrations may change with environmental conditions (i.e., seasonal changes) and plant tissue structural characteristics [17].
Tannins have rather undesirable organoleptic properties, as they are bitter and give a brownish color to foods. Nevertheless, they show remarkable antioxidant properties that justify their use as food additives to improve food-shelf life and safety, an issue that has made several tannins undergo trials for their legal approval as such additives. Furthermore, their precipitation properties are accounted for their decades-long use as clarification agents in the beverage industry (i.e., in beer, juices and wines) [18]. For instance, in the case of wines, ET from oak or chestnut are transferred to the wine during barrel aging, which is an appreciated feature in aged wines. As another example, tannic acid, a common GT found in many species, is approved as a flavoring agent in the EU [19]. However, it has also been reported alongside many other tannins to provide further oxidative and antimicrobial protection when added to foods. In the same sense, several in vitro, in vivo and clinical studies researching the bioactive properties of tannins have been developed throughout the years [20]. Thus, it is evidenced by their polyvalent potential, whether as additives, nutraceuticals or pharmaceutics. The mentioned findings are paired with increased consumer demand for natural products, with a preference to avoid or replace synthetic compounds in food, for example [21]. Furthermore, the feasibility of tannin extraction and acquisition proves that it is affordable and may be carried out with little difficulties even from by-products of the agri-food industry, such as barks, leaves, peels or seeds that are not exploitable for other uses, since these fractions are accounted for the highest tannin concentrations. Among these plant tissues, some remarkable examples involve peels and/or seeds from several fruits (i.e., grape, pomegranate), citrus, nuts (i.e., chestnut, walnut), herbs (i.e., tea, basil, cinnamon), legumes and barks of trees (i.e., Acacia spp., Castanea spp., Quercus spp.) [22][23][24]. Some common sources of currently commercialized tannin extracts are trees such as chestnut (C. sativa) for HT and quebracho (Schinopsis balansae & Schinopsis lorentzii) for CT [25]. In the case of these woody trees, tannin content in barks may be as high as 38% of dry matter in quebracho or as much as 16% in chestnut, and their use in traditional medicine is well recorded. Other usual sources of tannins are black wattle (Acacia mearnsii) for CT or valonea (Quercus macrolepsis) and tara (Tara spinosa) for ET and GT, respectively [26]. Furthermore, as stated, brown algae (i.e., Arame, Eisenia bicyclis, Sargassum sp.) are also considered an adequate source of tannins, as well as other bioactive compounds, as they are easily harvested and currently underutilized while being the focus of research on bioactive compounds in recent years [27].
Taking into account the mentioned properties of tannins, it may be possible to explain the effectiveness of tannin-rich medicinal plants used in traditional medicine while these medicinal properties are also related to the synergy of tannins with other bioactive polyphenols present in these plants [28]. The recorded medicinal use of tannin extracts and tannin-rich plants will be addressed together with the study of the main chemical profile of the mentioned tannin-rich plants.
2. Traditional Applications of Rich-Tannins Plants
In the following sections and in Table 1, some examples of the traditional uses of plants and other sources of tannins will be explained. The selection of these species has been made according to their well-known content in tannins, their extensive recorded traditional applications (paying special attention to those orally administrated), their reported bioactivities and the availability of quantitative and qualitative studies that determined their chemical profile and their high levels of tannins.
Table 1. Traditional applications of plants containing tannins. Selection of species and tissues rich in tannins traditionally applied under diverse administration ways (admin.) for treating different affections or diseases and the potential mechanism of action of their biomolecules.
|
Traditional Use of Plants and Macroalgae Rich in Tannins |
|||||||
|
Plant |
Admin. |
Treatment, Remedy, Uses |
Mechanism of Action |
Ref. |
|||
|
PLANTS |
|||||||
|
Acacia |
|||||||
|
A. nilotica |
O, T |
Gastrointestinal, respiratory, inflammatory, parasitic, neurological diseases, sexual disorders, skin issues, diabetes. Aphrodisiac, chemo-preventive, antimutagenic |
Antioxidant, anti-inflammatory, anti-nociceptive, and antipyretic |
||||
|
A. arabica |
O (G, S) |
Used for sweetmeats (G) or roasted (S, India) |
|||||
|
A. tortilis |
O, T |
Gastrointestinal disorders in camelids, skin issues (edema, allergic dermatitis, wound/burns healing) |
Antiparasitic and anti-inflammatory |
[34] |
|||
|
Betula |
|||||||
|
B. pendula |
O (B in I/D) |
Urinary, respiratory affections. Systematic diseases |
Anti-viral |
||||
|
Juglans |
|||||||
|
J. regia |
O (N), T |
Hemorrhoids, rheumatism, varicose veins, skin wounds, fever, cough, toothache, infecundity. Local analgesic. Hypercholesterolemic, antidiabetic, cardiotonic, vasodilator. Aromatizer. Antiparasitic |
Anti-platelet, cardioprotective, antiatherogenic and anti-inflammatory |
||||
|
Picea |
|||||||
|
P. abies |
O (Sp/L/F/R/B) |
Food ingredients or supplements (Sp, L, F, R). Bread-preparing flour or thicker in soups (B) |
Antioxidant, antimicrobial, preservative |
[43] |
|||
|
Pistacia |
|||||||
|
P. lentiscus |
O, T (St, FR-oil) |
Improvement of gastrointestinal function. Infected wounds, scabies, bloat, constipation |
Antiparasitic, anti-inflammatory |
||||
|
Phyllanthus |
|||||||
|
P. niruri |
O (L and FR) |
Liver diseases (jaundice), urinary infections, inflammatory processes and malaria |
Anti-inflammatory, antioxidant, hypoglycemic, hypolipidemic, hepatoprotective |
||||
|
Quercus |
|||||||
|
Quercus sp. |
O, T (R/S in D/FR) |
Skin injuries (burn, boil wound). Respiratory affections (cold and flu). Diabetes |
Antioxidant, antidiabetic |
||||
|
Rhus |
|||||||
|
Rhus sp. |
O |
Gastrointestinal diseases (diarrhea, ulcers, hemorrhoids), dysentery, or stroke |
Antimicrobial, anti-inflammatory, antiapoptotic, immunomodulatory, healing |
||||
|
Schinopsis |
|||||||
|
Schinopsis sp. |
O, T (I/D of L/B/Rs/FR/Br/C/W/S) |
Anti-inflammatory, antimicrobial, antipyretic, astringent and cicatrizing. Respiration affections (cold, cough, asthma), stomachache, headache, dysentery or fractures |
Antioxidant, antimicrobial, anthelmintic |
||||
|
Smilax |
|||||||
|
S. aspera |
O, T (D) |
Urinary retention, antiseptic in cows, enhancing health state of rabbits, treatment of purulent vesicles |
Antioxidant, anti-inflammatory, diuretic |
[61] |
|||
|
Umbilicus |
|||||||
|
U. rupestris |
O, T (minced L) |
Infected wounds, diarrhea, fever, intoxications, antiparasitic in hens |
Anti-inflammatory, antiparasitic |
||||
|
Urtica |
|||||||
|
U. dioica |
O, T (I, direct application) |
Arthritis, lumbago, rheumatism, muscular or limb paralysis. Rubefacient, blood circulation stimulant. Relief allergic rhinitis symptoms. Revitalizing. In animal promotes weight gain, growth and increases galactagogue production (ruminants) |
Antioxidant, anti-inflammatory, antimicrobial, analgesic, anti-diabetic, antimutagenic. Emulsifier, gelling agent |
||||
|
Vitis |
|||||||
|
V. vinifera |
O (raw sp, vinegar) |
Gastrointestinal diseases, headaches, and colds. Thirst-quenching, revitalizing and anti-inflammatory |
Antioxidant, anti-obesity, anti-inflammatory |
[69] |
|||
|
Combination of plants |
|||||||
|
“Triphala” |
Oral |
Restorative, revitalizing, boosting of the immune system, treatment for chronic gastrointestinal diseases |
- |
[70] |
|||
|
MACROALGAE |
|||||||
|
Sargassum |
|||||||
|
Sargassum sp. |
O, T |
Nutritional value. Treatment for inflammations, goiter, dropsy, edema, dysuria, respiratory affections, angina pectoris, high blood pressure, skin diseases, neurosis, pregnancy-related depression and diabetes mellitus |
Antioxidant, antibacterial, antiproliferative, anti-inflammatory. Gelling hydrocolloid, emulsifier |
||||
|
Ecklonia |
|||||||
|
E. cava |
Oral |
Common food ingredient, attenuation of goiter, treatment for mammary hyperplasia and diuretic |
Antioxidant, anti-inflammatory |
||||
Definitions: preparation modes: D: decoction; I: infusion; Pp: plant parts: B: bark; Br: branches; Bu: buds; C: cortex; F: flowers; FR: fruits; G: gum; J: jam; L: leaves; N: nuts; Rs: resin; R: roots; S: sap; Sp: sprouts; St: stems; W: wood.
Among the species of the genus Acacia, A. nilotica is the most relevant from a medicinal point of view. Different parts of the plant have been used for very diverse affections. Even though all tissues have been described to possess activity, leaves, pods and bark present more healing properties. In general terms, this species has been described to treat gastrointestinal disorders or diseases (diarrhea, congestion, anthelmintic, diuretic, emetic, for burning sensation and it is also considered as nutritive), respiratory affections (pharyngitis, bronchitis, cough, cold, expectorant and for sore throat), skin issues (eczema, ulcers, leukoderma, wounds), variable inflammatory processes (toothache, conjunctivitis, menstrual pain, hemorrhoids, smallpox, biliousness) or diabetes. Its sedative and narcotic properties were applied for nervous system disorders, Alzheimer’s disease and its antimicrobial capacity was exploited as a remedy for dysentery, leprosy, tuberculosis or even malaria. It also possesses aphrodisiac properties, it can be used for treating spermatorrhoea and sexually transmitted diseases, but it was also claimed to possess chemo-preventive and antimutagenic activity . The properties recognized for major tannins in A. nilotica include antioxidant, anti-inflammatory, anti-nociceptive, and antipyretic activities . Another plant belonging to this genus with recognized properties is A. tortilis sap, whose seeds and bark recovered have been used for gastrointestinal ailments (stomachache, mild diarrhea or indigestion), eye conditions (treat white stains in the cornea or used in incipient cases of eye entropy), respiratory issues, as antipyretic, for jaundice, malaria, as wound healing and injuries disinfectant, as liver detoxifying and for bone strengthening. Roots soaked in water and crushed can be orally administrated to treat diphtheria . A. tortilis also has been used for treating gastrointestinal disorders especially described for camelids. Their seeds, suckers, stipules and young spines have been found to be a remedy against sand colic that mostly to dromedaries. Moreover, its chewing gum has wound and burns healing properties, and when seeds or bark from A. tortilis are mixed with seeds from Vigna unguiculata, this mixture can be applied for treating skin issues (edema or allergic dermatitis) or as antiparasitic, respectively . Pods from other species, like A. arabica or A. catechu, have also been reported for confectioning fodder for animals, particularly for sheep and goats [77]. In fact, A. arabica has been widely utilized in humans as a treatment for multiple affections and diseases, very similar to those already cited. The bark is considered a powerful astringent, and its extract has been used to allay irritation in acute gonorrhea and leucorrhea, cystitis, vaginitis and anal or uterus prolapsed. Decoctions or dry powder were used for treating hemorrhages, skin wounds, ulcers or leukoderma, diarrhea, dysentery, leprosy, diabetes, bronchitis, seminal weakness, as diuretic or anthelmintic agent. Noteworthy, its leaves have also been used for diarrheal disorders. Gargles were applied for cancerous and syphilitic affections, sore-throat, cough or toothache since it has been described as tonic, demulcent, aphrodisiac and anti-viral. The ground bark of A. arabica mixed with seeds of Sesamum indicum have been used as food and the juice of their bark mixed with milk is dropped into the eye for treating conjunctivitis. Pods, fruits, flowers, roots, leaves and gum present very similar applications; additional ones include the treatment of eczema and abscess with leaves, the use of fried gum for preparing sweetmeats or flowers as antipyretic. Moreover, the gum obtained from this species can be fried using ghee, a kind of clarified butter traditionally confectioned in India, for preparing sweetmeats and roasted seeds which served as food during acute scarcity periods [33]. Bark decoctions of another species, Acacia catechu, also has been reported to cure cold and cough, severe diarrhea or piles (applied with lemon slice), as tonic for women after delivery (with cardamom) while heartwood can be used as antipyretic, for cold during the pregnancy and to cure ulcers both in skin and mouth/tongue [77].
Several plants have been used in labor and delivery, such as the fern Asplenium ceterach (accepted name of Ceterach officinarum), used in cows and ewes after delivery as depurative. This effect is attributed to the astringent tannins present in its composition. Another example is the plant Capsella bursa-pastoris, known as shepherd’s purse, which presents anti-hemorrhaging properties associated with the presence of tannins [78]. In this case, a decoction of the plant was given to pregnant animals to avoid hemorrhage.
Different pharmacopeias worldwide, including Russia, India and some European countries (France and Deutschland), have described the healing properties of several tannin-based rich plants like Betula species. Most texts point to bark as the main plant target to prepare decoction- or infusion-based extracts. Nevertheless, other parts like leaves, flowers, stems, roots or even sap or resin have also been exploited. Betula pendula is the species that has been further used and reported to have pharmacological properties. The principal applications of the extracts of B. pendula are aimed to treat or prevent urinary affections such as infections of the urinary tract or bladder, renal inflammation, renal stones or hindered dieresis. It also has been widely used for treating systematic diseases (rheumatism or arthritis), blood system disorders, respiratory tract ailments, or as wound healing, antipyretic or even anti-alopecic agent. Other minor utilizations included it as a remedy for spleen affections, hypercholesterolemia, headache or even as anti-helminthic. Betulinic acid, a triterpenoid acid extracted from B. pendula, is well-known for its antiviral, tested against HIV, and anti-inflammatory activity, which may act in synergy with the tannins present.
Castanea sativa has been referenced as alimentary or medicinal with applications as laxative or stomach regulator when chestnuts are consumed or even as hemoptysis agents. C. sativa episperma was, however, described as astringent. Nevertheless, its most relevant value has been underlined as a source of nutrients even though it also has been cited as a possible antidote to lip and esophagus lacerations caused by Colchicum autumnale poison [79].
Edible nuts from Juglans regia had been recognized since ancient times, but also other tissues were exploited with diverse purposes, such as its leaves, which have been used to wrap cheese in order to provide aroma, but also antiparasitic properties [38,42]. Moreover, it has been traditionally applied as an anti-inflammatory for rheumatism and hemorrhoids, antipyretic, antifungal, antitussive and for skin affections. Specific applications of each plant part include bark decoction to gargle it for toothache, direct application of fresh leaves to reduce varicose veins, mild skin inflammations and its use as a local analgesic, and immature fruits to color hair. Moreover, a dosage of one teacup of J. regia twice or three times a day during one month has been described to be able to provide antidiabetic, hypercholesterolemic (HDL cholesterol), cardiotonic and vasodilator properties and reduce infecundity. In fact, scientific reports have described tannins from walnut to possess anti-platelet, cardioprotective, antiatherogenic and anti-inflammatory properties.
The genus Lotus has been traditionally utilized as forage for different ruminants
[80,81]. Lotus species, rich in CT, have been demonstrated to provide different benefits, such as favoring the weight gain, the growth of wool, improvement of the production and composition of milk and reducing the number of anthelmintic products in farming animals [81]. In cattle, the production of methane was reduced and enhanced ruminal fermentation when the animals were fed with Lotus corniculatus, which was attributed to the CT [82]. The administration of L. corniculatus has shown positive effects on milk production and gastrointestinal function of sheep [80]. In addition, L. cornicatus, Lespedeza cuneata and Hedysarum coronarium have shown anti-helminthic effects on ewes, reducing the presence of fecal eggs and worms and inhibit the development of larvae [83][84].
The genus Phyllanthus has a long clinical application in Asia. The fruit of P. niruri, P. amarus, P. fraternus, P. debilis and P. maderaspatensis and the leaves of P. polyphyllus has been applied for their tonic properties to liver diseases such as jaundice in India while for urinary affection were used P. simplex, P. reticulatus, and P. acidus. In India, these plants have also been utilized for wound healing, as antipyretic, anti-inflammatory or for treating diabetes. Similarly, this genus has been used as antipyretic and antitussive in China and for treating blood, bile disease, hypertension and anuria in Tibetan medicine. P. urinaria has been described to possess detoxifying properties and was also used for liver-based diseases (jaundice or hepatitis B), gastrointestinal (enteritis, diarrhea), or systematic affections (dropsy). As in India, other species like P. reticulatus, P. niruri and P. simplex were applied for treating urinary infection, among other inflammatory processes like rheumatism. In Thailand, P. emblica is used as the previously cited “Triphala” for chronic gastrointestinal diseases. However, other species are utilized for treating the same affections P. amarus, P. urinaria and P. virgatus are aimed at treating liver diseases, diabetes or gonorrhea. P. acidus was the remedy for slightly different affections like hypertension, constipation, fever or skin issues, whereas urinary infections or malaria are treated with P. taxodiifolius, P. niruri, and P. reticulates. However, in Africa, P. muellerianus is the most used species, and the one applied for malaria (and P. reticulates), tetanus, as antipyretic and wound healing. Instead, P. polyanthus is applied in Kenya for treating sexually transmitted diseases. Similar applications are found in South America, where leaves of P. tenellus have diuretic properties, P. amarus and P. sellowianus are used for treating diabetes, but also for jaundice and urinary infection, respectively. For P. niruri, different bioactivities have been demonstrated, such as antioxidant, anti-inflammatory, anti-nociceptive, analgesic, hypoglycemic, lypolipidemic and hepatoprotective.
Different P. abies plant parts have been also applied in traditional culinary art. Young sprouts are used as food ingredients, while leaves (needles), flowers, pinecones and resin are utilized as food supplements. A Finnish novel food called “pettu” is prepared from the bark that is roasted and scratched of oozed substances or just boiled for 2 to 3 h. Then, the bark is dried, grounded and mixed with some cereals-based flour at equal parts since the consumption of pure bark can induce stomachache and constipation. This product can be used as bread-preparing flour; it can also be mixed with milk or animal fat, or blood or to prepare soup to where it provides a thickening effect. This polyvalent flour was used in the 1860 s, during the famine, in Finland [85]. Other uses of different tissues of P. abies include animal treatments. Twigs serve for feeding calves, resin heals skin afflictions, and sores and ointments were used to treat or prevent respiratory affections like cough or pneumonia. This ointment of P. abies, when combined with other species like Rumex obtusifolus provides a remedy for mastitis [86]. Indeed, the veterinarian use of P. abies has been recognized by the European Medicines Agency through Veterinary Medicines and Inspections. The final preparation to administrate to animals is named Piceae turiones recentes extractum and can be obtained from boiling 10 to 15 cm long shoots, collected in spring, of fresh P. abies. This extract is then mixed with starch and an herbal powder. The final product that can be orally administrated (dosage: 0.6–6.4 mL solution, equivalent to 3.1 mg to 30. 6 mg spruce-tips extract, per kg body weight) is aimed to treat diarrhea in cattle, horses, pigs, sheep and poultry [87].
Plants such as Parietaria officinalis, Pistacia lentiscus, and Prunus spinosa, rupestris, also present tannins in their chemical composition, have been traditionally used to treat different disorders of domestic animals. The main use described for Parietaria officinalis is the treatment of diarrhea in domestic animals. Pistacia lentiscus and Prunus spinosa present a high content of tannins, and they have been reported to exert beneficial effects on the protein metabolism of ruminants, improving the absorption of amino acids in the small intestine. P. lentiscus, known as lentisk, has also shown antiparasitic activity against intestinal helminths and coccidia on sheep and goats, which have been attributed to the presence of tannins and other compounds. Additional traditional uses of this plant include the treatment of scabies, diarrhea, constipation, dermal affections and infected wounds [88]. In the case of P. spinosa or plum tree, this plant has also been applied externally to treat wounds infected by worms and also to control diarrhea [89]. Similarly, P. officinalis was also effective in controlling diarrhea, which is attributed to the presence of astringent compounds such as tannins. In addition, this plant was used to elaborate tisanes with anti-inflammatory and antiseptic properties in combination with other traditional plants, such as Pisum sativum, Beta vulgaris, Lavandula latifolia or Malva sylvestris.
Punica granatum has been used as itself or in combination with other plants to treat very variable affections. When used as a unique herb, mainly bark and roots were used to treat intestinal worms, decoctions of pomegranate hulls were described as strong astringents and a remedy for treating dysentery, diarrhea, and stomatitis. Pomegranate hulls and/or root extracts were also administrated orally and intravaginally to minimize fertility and treat gynecological affections. Alternative uses include the application of pomegranate extracts for snakebite, diabetes, burns and leprosy, while the fresh fruit has been used as a refrigerant to ameliorate fever processes [90][91]. When used in combination, very assorted plants were used such as Achillea millefolium, Artemisia sp., Emblica officinalis, Nepeta sp., Tanacetum sp., Taraxacum officinale, Terminalia chebula, or Zingiber officinale, among many others. These mixtures of plants were mostly administrated to treat cold, cough and fever [92].
Among the genus Quercus, few examples of traditional medicinal or pharmacological uses can be found in the literature. A decoction of roots from Q. cerris and Q. coccifera can be applied as lotion twice a day for 2–3 weeks to treat skin burn, boil and wound. Fruits of Q. coccifera are edible and can represent a remedy for controlling diabetes. A teacup of a decoction of seeds from Q. ithaburensis administrated 2–3 times a day for one week can improve cold and flu processes. A decoction of oak-apples from Q. pubescens together with other plants like mallow or chamomile was also used for healing wounds in newborn infants. Tannins from Quercus had been described to have antioxidant and anti-diabetic activity.
Several species belonging to Rubus have been considered for their properties in different traditional medicines. Bud, fruits, leaves and roots of R. canescens can be eaten raw or drunk as an infusion (teacup twice a day for 2–3 weeks) or applied as a decoction for treating gastrointestinal issues (as carminative, dyspepsia and intestinal spasm) or diabetes. Similarly, fruits of R. idaeus can be eaten twice or three times a day for 2 to 5 days for mouth sores and as antiemetic. However, R. sanctus has wider applications, including treatment for atherosclerosis, stomachache, diabetes, eye diseases, nephralgia, kidney gravels, rheumatism, cold and flu, bronchitis, burn, boil and wound care, or as anti-hemorrhagic. Leaves from R. ulmifolius can be topically administrated for hemostatic for cuts and for removing thorns; the extract of tender tips is useful for skin cuts and bruises, and jam obtained from fruits relief cough and sore throat. Moreover, R. ulmifolius sprouts can be mixed with walnut kernels, Verbena officinalis leaves, Sambucus nigra bark, cyclamen tubers and bramble buds to prepare a cream with beeswax and oil basis that can be administrated for inflammation processes.
Rhus genus has been traditionally applied and named as sumac. Its main uses included it as a food condiment, but also for the treatment of gastrointestinal diseases such as diarrhea, intestinal ulcers, rectal prolapse and hemorrhoids, oral diseases, dysentery, or stroke. Sumac has been described to possess anti-inflammatory, immunomodulatory, antimicrobial, antiviral, antioxidant, antifungal and antiapoptotic effects.
Several species from the genus Sapium have been described to be used as part of traditional medicine in different cultures. Most of the Sapium species were used to treat skin-related diseases such as eczema, dermatitis, wounds or snake bites. However, additional uses were pointed to this genus as a remedy for overstraining, lumbago, constipation and hernia. For instance, Sapium baccatum has been used for treating eczema, roots bark and leaf from Sapium japonicum were applied for treating overstrain, lumbago and knee pain and similarly, the resin from Sapium glandulosum was used for hernias. Other uses included the treatment of digestive and urinary ailments (Sapium sebiferum root bark and seed), skin affections and antiparasitic (bark juice of Sapium insigne). Different plant tissues of Sapium ellipticum were applied with different purposes such as for respiratory complications (root decoction and dried stems), abdominal swelling, eye diseases and mumps (leaves), malaria (root decoction), anemia, fever, guinea worms, elephantiasis and rheumatic problems (stem bark decoction) [93].
Smilax aspera or sarsaparrille, another plant that has been reported to contain tannins in its chemical composition, has been administered orally as a diuretic and urinary antiseptic in cows and has been used in the alimentation of rabbits due to its beneficial effects on the health of the animals. In addition, decoctions of this plant were applied to eliminate purulent vesicles.
To our knowledge, the traditional uses of the Schinopsis genus as medicinal and pharmacological remedies are few. Leaves, bark, resin and fruits from S. brasiliensis were used in the popular medicine as a general anti-inflammatory, for treating cold, cough, fever, diarrhea, dysentery, fractures and as antimicrobial. S. lorentzii has been reported to be traditionally used for treating stomachache, headache or cough when prepared as infusion or decoction using leaves and tender branches. Nevertheless, leaves have been described to be useful as a cicatrizing agent and to relieve bruises while the bark may have anti-asthmatic properties. The cortex of S. balansae also has cicatrizing properties accompanied by anti-inflammatory and antiseptic capacity. The wood of S. balansae has been referred to as astringent, and fresh sap may remove moles. The main properties associated with Schinopsis are antioxidant, antimicrobial, anthelmintic.
Similarly, in the case of the genus Terminalia, many species had been contemplated as medicinal plants such as Terminalia bellirica, T. chebula, T. arjuna, T. catappa, etc. Among them, T. bellirica has been studied for being considered edible and for its multiple properties to treat edema, diarrhea, leprosy, bile congestion, indigestion, headache, fever, cough, dysentery or skin diseases [94]. Different plant structures have been suggested to have diverse applications. For instance, fruits can be utilized for respiratory tract affections like cough (decoction), hoarseness, asthma or bronchitis; for digestive issues (indigestion, diarrhea, edema or hemorrhoids that can be treated with pulp fruit), menstrual disorders, hepatitis, as purgative or even as a hair tonic. Fruit kernel has been described as narcotic, and its oil was purgative like bark gum. Seed oil has anti-rheumatic activity while leaves improve health status by improving immunity, acting as anti-aging, enhance appetite, relieve hemorrhoids and can reduce cholesterol and blood pressure. Extracts obtained from the bark of T. arjuna are aimed as cardioprotective and antihyperlipidemic, but also as a remedy for muscle sores, contusions, fractures, ulcers, treatment of bile infection, dysentery or as poison antidote [94][95]. For T. chebulla, the fruit has also been widely used for digestive alterations to improve appetite, as an astringent, antiemetic, stomach tonic, mild laxative, for hemorrhoids or as antispasmodic [96]. T. chebulla can also be applied for infertility, asthma, sore throat, dental caries, urticaria, dysentery, bleeding, ulcers, gout and bladder disease [94]. A paste obtained by mixing grounded T. chebulla with water has anti-inflammatory, analgesic and wound healing capacities while its decoction helps to treat oral ulcers or sore throat [96]. A combination of dried fruits obtained from these two species, T. chebula and T. bellirica, and Phyllanthus emblica, is known as “Triphala,” being long-used as a restorative and revitalizing natural formulation that boosts the immune system against infectious diseases .
The nutritional value of Urtica dioica has been recognized from ancient times for both humans and animals. It was administrated as a revitalizing agent for humans. In animals, it is also restorative and promotes weight gain and growth of chicks, Turkey cocks and pigs, and it was also described to increase the production of galactagogue (a substance that promotes lactation) in ruminants. Currently, U. dioica is consumed as part of curry, soup, vegetable complement or as the main ingredient of an omelet. Within its chemical composition, tannins have been quantified in 0.93 mg/100 g, 38% of proteins, 9% of crude fiber and 0.2% for both calcium and iron. The fresh plant has been applied both directly and as an infusion for treating arthritis, lumbago, rheumatism, muscular or limb paralysis. The direct use of U. dioica has been described as a rubefacient; thus, it was utilized for the stimulation of blood circulation, which helped the warmth of joints and extremities. This plant was also stated to relieve symptoms of allergic rhinitis and to provide vitality to people. Indeed, scientific works have described antioxidant, anti-inflammatory, antimicrobial, analgesic, antidiabetic, and antimutagenic activities for U. dioica extracts. Another plant used in animal feeding is U. dioica (traditionally named nettle), which has been given to chicks, Turkey cocks and pigs as restorative to promote weight gain and the growth of the animals. It has also been described to increase the production of galactagogue (a substance that promotes lactation) in ruminants. Different compounds have been identified in U. dioica, including tannins.
The traditional veterinarian applications of the species Umbilicus rupestris, also known for its common name navelwort, are well documented. This plant, which has been described to possess tannins, has been used externally to treat wounds of animals and orally to control diarrhea, fever, intoxications and has been used as an antiparasitic in hens.
Vitis vinifera has been widely and repeatedly used as food and beverage ingredients, and its consumption has been associated with different beneficial health effects. It has been used for gastrointestinal diseases; sprouts can be eaten as thirst-quenching, but also for treating headaches (as vinegar), colds (mixed with honey, cinnamon and cloves), and wine baths were used for children to strengthen them or to treat inflammations. Most of their traditionally and currently exploited bioactivities are directly related to their high content in molecules with antioxidant properties such as proanthocyanidins and anthocyanins [97]. Among their recognized benefits, extracts obtained from seed grapes have been described to possess anti-obesity and antidiabetic capacity by downregulating the lipid metabolism. The administration of grape seed extracts rich in procyanidins revealed a high increase in the expression of several genes involved in β-oxidation, involved in lipid catabolism, and which ultimately suggests their potential to prevent fat accumulation [98]. These extracts demonstrated to increase energy expenditure, and thermogenesis was also found to diminish the expression of TNF-α, a proinflammatory factor augmented in chronic diseases, such as obesity [99]. Procyanidins present in V. vinifera, besides inhibiting fat gain, have shown the potential to alter the small intestinal gut microbiota. This has been suggested to be related to their capability to improve glucose tolerance and insulin sensitivity, and hence described as antidiabetic [100]. Other tannin classes present in several species that possess antidiabetic or hypoglycemic properties are valoneic acid dilactone, TGG and PGG from C. sativa, both HTs (TGG, PGG) and CTs (catechin derivatives) from S. lorentzii or chebulanin, chebulagic acid and chebulinic acid from T. chebula.
Another source of tannins recently demonstrated is brown macroalgae with a high content in phlorotannins. Macroalgae have been used for nutritional purposes since ancient times, especially in the Far East Asiatic cultures, such as Sargassum and Ecklonia. Sargassum, among other algae species, has been historically used as edibles and folk medicine. Seaweed consumption was supported for its nutritional value but also because its regular ingestion was related to an effective reduction of depressive symptoms during pregnancy and with a diminution of suicide rate in Japan, while in Korea was associated with minor diabetes mellitus incidence. Additionally, these algae have been used as hydrocolloids, emulsifiers and gelling agents in various food product preparations. Different species belonging to the genus Sargassum have been used in traditional Chinese medicine. S. pallidum, S. confusum, S. fusiforme, S. fulvellum, S. siliquastrum, S. thunbergii, S. muticum or S. hornerii have served as a treatment for goiter, inflammation-based diseases like scrofula, arteriosclerosis, hepatosplenomegaly or testes swelling, dropsy, edema due to retention of phlegm and morbid fluids, dysuria, respiratory affections like sore throat, cough, acute esophagitis or chronic bronchitis, angina pectoris and high blood pressure, skin diseases like furuncle, and even neurosis. The potential mechanism through which Sargassum may exert its activity includes its capacity as antioxidant, antibacterial, antiproliferative, and anti-inflammatory. Ecklonia cava is a highly valued edible brown seaweed in Japan, China and South Korea, where it is consumed daily. It is foremost intake as part of salads, miso soup, or powdered as a condiment in rice cakes, candies or kimchi [101][102][103]. It is recorded in Chinese Pharmacopoeia as part of preparations with other seaweeds like Sargassum sp. as “Laminaria Thallus”, and it is attributed to attenuate goiter, diuretic and treatment for mammary hyperplasia. Additionally, it is allegedly held as “health-promoting” in Korea [104]. E. cava ethanolic extract, highly rich in PT (> 90%), has been approved for use as a food ingredient by both Food & Drug Administration (FDA) and European Food Safety Authority (EFSA). This approval is justified on the evidence regarding its antioxidant, antiviral or anti-inflammatory, as well as antidiabetic and anti-obesity properties [105[105,106][106]. In fact, an in vivo experiment determined significantly lower carbohydrate absorbance and metabolization in rats when administered said E. cava extract [107].
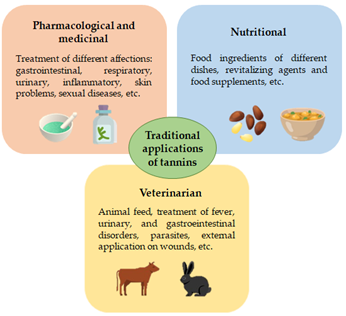
As a final mention to this section, in Figure 2, an illustrative description of the traditional uses of tannins with pharmacological, medicinal, nutritional, veterinarian and botanical applications is briefly depicted.
Figure 2. Brief description of the traditional uses of tannins with pharmacological, medicinal, nutritional, and veterinarian applications.
References
- Falcão, L.; Araújo, M.E.M. Vegetable tannins used in the manufacture of historic leathers. Molecules 2018, 23, 8–10, doi:10.3390/molecules23051081.
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128147757.
- Okuda, T.; Ito, H. Tannins of constant structure in medicinal and food plants-hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217, doi:10.3390/molecules16032191.
- Molino, S.; Casanova, N.A.; Rufián Henares, J.Á.; Fernandez Miyakawa, M.E. Natural Tannin Wood Extracts as a Potential Food Ingredient in the Food Industry. J. Agric. Food Chem. 2020, 68, 2836–2848, doi:10.1021/acs.jafc.9b00590.
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary in-take and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262, doi:10.1111/bph.13630.
- Salminen, J.P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338, doi:10.1111/j.1365-2435.2010.01826.x.
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological application of tannin-based extracts. Molecules 2020, 25, 1–27, doi:10.3390/molecules25030614.
- Hagerman, A.E. Hydrolyzable Tannin Structural Chemistry. Tann. Handb. 2010, 1–8.
- Jiménez, N.; Esteban-Torres, M.; Mancheño, J.M.; De las Rivas, B.; Muñoza, R. Tannin degradation by a novel tannase en-zyme present in some Lactobacillus plantarum strains. Appl. Environ. Microbiol. 2014, 80, 2991–2997, doi:10.1128/AEM.00324-14.
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649, doi:10.1039/b101061l.
- Rousserie, P.; Rabot, A.; Geny-Denis, L. From Flavanols Biosynthesis to Wine Tannins: What Place for Grape Seeds? J. Agric. Food Chem. 2019, 67, 1325–1343, doi:10.1021/acs.jafc.8b05768.
- Díaz, A.M.; Caldas, G.V.; Blair, M.W. Concentrations of condensed tannins and anthocyanins in common bean seed coats. Food Res. Int. 2010, 43, 595–601, doi:10.1016/j.foodres.2009.07.014.
- Sieniawska, E.; Baj, T. Tannins; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128020999.
- Macáková, K.; Kolečkář, V.; Cahlíková, L.; Chlebek, J.; Hošt’álková, A.; Kuča, K.; Jun, D.; Opletal, L.; Hoštálková, A.; Kuča, K.; et al. Tannins and their Influence on Health. In Recent Advances in Medicinal Chemistry; Elsevier: Amsterdam, The Nether-lands, 2014; Volume 1, pp. 159–208, ISBN 9780128039618.
- Cuong, D.X.; Hoan, N.X.; Dong, D.H.; Thuy, L.T.M.; Van Thanh, N.; Ha, H.T.; Tuyen, D.T.T.; Chinh, D.X.; Dang, X.C.; Ho-an, N.X.; et al. Tannins: Extraction from Plants. In Tannins—Structural Properties, Biological Properties and Current Knowledge; IntechOpen: London, UK, 2019; pp. 1–20.
- Venkatesan, J.; Keekan, K.K.; Anil, S.; Bhatnagar, I.; Kim, S.-K. Phlorotannins. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 515–527, ISBN 9780081005965.
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218, doi:10.1080/10408398.2011.650336.
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2019, 0, 1–13, doi:10.1080/15569543.2019.1662813.
- EC, E.C. Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substanc-es provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334. Off. J. Eur. Communities 2.10. 2012, L 2012, 267, 1–161.
- Sieniawska, E. Activities of tannins-From in vitro studies to clinical trials. Nat. Prod. Commun. 2015, 10, 1877–1884, doi:10.1177/1934578X1501001118.
- Rana, J.; Paul, J. Consumer behavior and purchase intention for organic food: A review and research agenda. J. Retail. Con-sum. Serv. 2017, 38, 157–165, doi:10.1016/j.jretconser.2017.06.004.
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of solid wastes from chestnut industry processing: Extraction and opti-mization of polyphenols, tannins and ellagitannins and its potential for adhesives, cosmetic and pharmaceutical industry. Waste Manag. 2016, 48, 457–464, doi:10.1016/j.wasman.2015.11.019.
- Grenda, K.; Arnold, J.; Hunkeler, D.; Gamelas, J.A.F.; Rasteiro, M.G. Tannin-based coagulants from laboratory to pilot plant scales for coloured wastewater treatment. BioResources 2018, 13, 2727–2747, doi:10.15376/biores.13.2.2727-2747.
- Laddha, A.P.; Kulkarni, Y.A. Tannins and vascular complications of Diabetes: An update. Phytomedicine 2019, 56, 229–245, doi:10.1016/j.phymed.2018.10.026.
- Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Fernandez Miyakawa, M.E. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014, 5, 1–7, doi:10.3389/fmicb.2014.00118.
- Hassanat, F.; Benchaar, C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. J. Sci. Food Agric. 2013, 93, 332–339, doi:10.1002/jsfa.5763.
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and po-tential incorporation into functional foods. Food Res. Int. 2020, 137, 109676.
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B.; Kitts, D.D. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156, doi:10.3390/molecules201219753.
- Subhan, N.; Burrows, G.E.; Kerr, P.G.; Obied, H.K. Chapter 9—Phytochemistry, Ethnomedicine, and Pharmacology of Acacia. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 57, pp. 247–326, ISBN 1572-5995.
- Rather, L.J.; Shahid-ul-Islam; Mohammad, F. Acacia nilotica (L.): A review of its traditional uses, phytochemistry, and phar-macology. Sustain. Chem. Pharm. 2015, 2, 12–30, doi:10.1016/j.scp.2015.08.002.
- Xiong, J.; Grace, M.H.; Esposito, D.; Wang, F.; Lila, M.A. Phytochemical Characterization and Anti-inflammatory Properties of Acacia mearnsii Leaves. Nat. Prod. Commun. 2016, 11, 1934578X1601100524, doi:10.1177/1934578X1601100524.
- Safari, V.Z.; Kamau, J.K.; Nthiga, P.M.; Ngugi, M.P.; Orinda, G.; Njagi, E.M. Antipyretic, antiinflammatory and antinocicep-tive activities of aqueous bark extract of Acacia nilotica (L.) Delile in albino mice. Pain Manag. Med 2016, 2, 2.
- Roqaiya, M.; Begum, W.; Jahufer, R. Acacia arabica (Babool)-A Review on Ethnobotanical and Unani Traditional Uses as well as Phytochemical and Pharmacological Properties. Int. J. Pharm. Phytopharm. Res. 2015, 4, 315–321.
- Jaouadi, W.; Mechergui, K.; Ammari, Y.; Hamrouni, L.; Hanana, M.; Khouja, M.L. Étude ethnobotanique et ethnopharma-cologique d’Acacia tortilis (Forssk) Hayne subsp. raddiana (Savi) de la steppe arborée du Nord de l’Afrique. Phytothérapie 2016, 14, 285–292, doi:10.1007/s10298-015-0951-1.
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal plants of the genus Betula--traditional uses and a phytochemi-cal-pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83, doi:10.1016/j.jep.2014.11.010.
- Wang, Q.; Li, Y.; Zheng, L.; Huang, X.; Wang, Y.; Chen, C.-H.; Cheng, Y.-Y.; Morris-Natschke, S.L.; Lee, K.-H. Novel Betu-linic Acid–Nucleoside Hybrids with Potent Anti-HIV Activity. ACS Med. Chem. Lett. 2020, doi:10.1021/acsmedchemlett.0c00414.
- Sargin, S.A.; Selvi, S.; López, V. Ethnomedicinal plants of Sarigöl district (Manisa), Turkey. J. Ethnopharmacol. 2015, 171, 64–84, doi:10.1016/j.jep.2015.05.031.
- Quave, C.L.; Plano, L.R.W.; Pantuso, T.; Bennett, B.C. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2008, 118, 418–428, doi:10.1016/j.jep.2008.05.005.
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348, doi:10.1016/j.foodchem.2013.11.158.
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.A.; Moutsatsou, P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br. J. Nutr. 2008, 99, 715–722, doi:10.1017/S0007114507837421.
- Sun, Y.; Qi, G.; Li, D.; Meng, H.; Zhu, Z.; Zhao, Y.; Qi, Y.; Zhang, X. Walnut (Juglans regia L.) Kernel Extracts Protect Against Isoproterenol-Induced Myocardial Infarction in Rats. Rejuvenation Res. 2018, 22, 306–312, doi:10.1089/rej.2018.2140.
- Guarrera, P.M.; Forti, G.; Marignoli, S. Ethnobotanical and ethnomedicinal uses of plants in the district of Acquapendente (Latium, Central Italy). J. Ethnopharmacol. 2005, 96, 429–444, doi:10.1016/j.jep.2004.09.014.
- Raitanen, J.-E.; Järvenpää, E.; Korpinen, R.; Mäkinen, S.; Hellström, J.; Kilpeläinen, P.; Liimatainen, J.; Ora, A.; Tupasela, T.; Jyske, T. Tannins of Conifer Bark as Nordic Piquancy—Sustainable Preservative and Aroma? Molecules 2020, 25, doi:10.3390/molecules25030567.
- Landau, S.; Azaizeh, H.; Muklada, H.; Glasser, T.; Ungar, E.D.; Baram, H.; Abbas, N.; Markovics, A. Anthelmintic activity of Pistacia lentiscus foliage in two Middle Eastern breeds of goats differing in their propensity to consume tannin-rich browse. Vet. Parasitol. 2010, 173, 280–286, doi:10.1016/j.vetpar.2010.07.006.
- Markovics, A.; Cohen, I.; Muklada, H.; Glasser, T.A.; Dvash, L.; Ungar, E.D.; Azaizeh, H.; Landau, S.Y. Consumption of Pistacia lentiscus foliage alleviates coccidiosis in young goats. Vet. Parasitol. 2012, 186, 165–169, doi:10.1016/j.vetpar.2011.11.072.
- Bullitta, S.; Piluzza, G.; Viegi, L. Plant resources used for traditional ethnoveterinary phytotherapy in Sardinia (Italy). Genet. Resour. Crop Evol. 2007, 54, 1447–1464, doi:10.1007/s10722-006-9130-4.
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of tra-ditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247, doi:10.3109/13880209.2010.501083.
- Mao, X.; Wu, L.-F.; Guo, H.-L.; Chen, W.-J.; Cui, Y.-P.; Qi, Q.; Li, S.; Liang, W.-Y.; Yang, G.-H.; Shao, Y.-Y.; et al. The Genus Phyllanthus : An Ethnopharmacological, Phytochemical, and Pharmacological Review. Evidence-Based Complement. Altern. Med. 2016, 2016, 7584952, doi:10.1155/2016/7584952.
- Lee, N.Y.S.; Khoo, W.K.S.; Adnan, M.A.; Mahalingam, T.P.; Fernandez, A.R.; Jeevaratnam, K. The pharmacological potential of Phyllanthus niruri. J. Pharm. Pharmacol. 2016, 68, 953–969, doi:10.1111/jphp.12565.
- Muccilli, V.; Cardullo, N.; Spatafora, C.; Cunsolo, V.; Tringali, C. α-Glucosidase inhibition and antioxidant activity of an oe-nological commercial tannin. Extraction, fractionation and analysis by HPLC/ESI-MS/MS and 1H NMR. Food Chem. 2017, 215, 50–60, doi:10.1016/j.foodchem.2016.07.136.
- Horvathova, M.; Orszaghova, Z.; Laubertova, L.; Vavakova, M.; Sabaka, P.; Rohdewald, P.; Durackova, Z.; Muchova, J. Effect of the French oak wood extract robuvit on markers of oxidative stress and activity of antioxidant enzymes in healthy volunteers: A pilot study. Oxidative Med. Cell. Longev. 2014, 2014, doi:10.1155/2014/639868.
- Natella, F.; Leoni, G.; Maldini, M.; Natarelli, L.; Comitato, R.; Schonlau, F.; Virgili, F.; Canali, R. Absorption, metabolism, and effects at transcriptome level of a standardized french oak wood extract, Robuvit, in healthy volunteers: Pilot study. J. Agric. Food Chem. 2014, 62, 443–453, doi:10.1021/jf403493a.
- Isik, S.; Tayman, C.; Cakir, U.; Koyuncu, I.; Taskin Turkmenoglu, T.; Cakir, E. Sumac (Rhus coriaria) for the prevention and treatment of necrotizing enterocolitis. J. Food Biochem. 2019, 43, e13068, doi:10.1111/jfbc.13068.
- Khalilpour, S.; Sangiovanni, E.; Piazza, S.; Fumagalli, M.; Beretta, G.; Dell’Agli, M. In vitro evidences of the traditional use of Rhus coriaria L. fruits against skin inflammatory conditions. J. Ethnopharmacol. 2019, 238, 111829, doi:10.1016/j.jep.2019.111829.
- Saraiva, A.M.; Saraiva, C.L.; Cordeiro, R.P.; Soares, R.R.; Xavier, H.S.; Caetano, N. Atividade antimicrobiana e sinérgica das frações das folhas de Schinopsis brasiliensis Engl. frente a clones multirresistentes de Staphylococcus aureus. Rev. Bras. Plantas Med. 2013, 15, 199–207, doi:10.1590/S1516-05722013000200006.
- Del Carrizo, E.V.; Palacio, M.O.; Roic, L.D. Uso medicinal de algunas especies nativas en Santiago del Estero (República Ar-gentina). Dominguezia 2005, 21, 25–32.
- Barboza, G.E.; Cantero, J.J.; Núñez, C.; Ariza Espinar, L.; del Pacciaroni, A.V. Medicinal plants: A general review and a phytochemical and ethnopharmacological screening of the native Argentine Flora. Kurtiziana 2009, 34, 7–365.
- Venter, P.B.; Sisa, M.; Van Der Merwe, M.J.; Bonnet, S.L.; Van Der Westhuizen, J.H. Analysis of commercial proanthocya-nidins. Part 1: The chemical composition of quebracho (Schinopsis lorentzii and Schinopsis balansae) heartwood extract. Phyto-chemistry 2012, 73, 95–105, doi:10.1016/j.phytochem.2011.10.006.
- Cardullo, N.; Muccilli, V.; Cunsolo, V.; Tringali, C. Mass Spectrometry and 1H-NMR Study of Schinopsis lorentzii (Quebracho) Tannins as a Source of Hypoglycemic and Antioxidant Principles. Molecules 2020, 25, doi:10.3390/molecules25143257.
- Fruet, A.P.B.; Giotto, F.M.; Fonseca, M.A.; Nörnberg, J.L.; De Mello, A.S. Effects of the Incorporation of Tannin Extract from Quebracho Colorado Wood on Color Parameters, Lipid Oxidation, and Sensory Attributes of Beef Patties. Foods 2020, 9, 667, doi:10.3390/foods9050667.
- Bonet, M.À.; Vallès, J. Ethnobotany of Montseny biosphere reserve (Catalonia, Iberian Peninsula): Plants used in veterinary medicine. J. Ethnopharmacol. 2007, 110, 130–147, doi:10.1016/j.jep.2006.09.016.
- Bullitta, S.; Re, G.A.; Manunta, M.D.I.; Piluzza, G. Traditional knowledge about plant, animal, and mineral-based remedies to treat cattle, pigs, horses, and other domestic animals in the Mediterranean island of Sardinia. J. Ethnobiol. Ethnomed. 2018, 14, 1–26, doi:10.1186/s13002-018-0250-7.
- Adhikari, B.M.; Bajracharya, A.; Shrestha, A.K. Comparison of nutritional properties of Stinging nettle ( Urtica dioica ) flour with wheat and barley flours. Food Sci. Nutr. 2015, 119–124, doi:10.1002/fsn3.259.
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.-H.; Bhat, T.M.; Sharma, P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180, doi:10.3109/13880209.2012.715172.
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging nettle (Urtica dioica L.): A reservoir of nutrition and bioactive components with great functional potential. J. Food Meas. Charact. 2017, 11, 423–433, doi:10.1007/s11694-016-9410-4.
- Guarrera, P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25, doi:10.1016/j.fitote.2004.09.006.
- Uncini Manganelli, R.E.; Camangi, F.; Tomei, P.E. Curing animals with plants: Traditional usage in Tuscany (Italy). J. Eth-nopharmacol. 2001, 78, 171–191, doi:10.1016/S0378-8741(01)00341-5.
- Viegi, L.; Pieroni, A.; Guarrera, P.M.; Vangelisti, R. A review of plants used in folk veterinary medicine in Italy as basis for a databank. J. Ethnopharmacol. 2003, 89, 221–244, doi:10.1016/j.jep.2003.08.003.
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape seeds proanthocyanidins: An overview of in vivo bioactivity in animal models. Nutrients 2019, 11, 1–18, doi:10.3390/nu11102435.
- Gupta, A.; Kumar, R.; Bhattacharyya, P.; Bishayee, A.; Pandey, A.K. Terminalia bellirica (Gaertn.) roxb. (Bahera) in health and disease: A systematic and comprehensive review. Phytomedicine 2020, 77, 153278, doi:10.1016/j.phymed.2020.153278.
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619, doi:10.1016/j.jep.2012.05.046.
- Casas, M.P.; Rodríguez-Hermida, V.; Pérez-Larrán, P.; Conde, E.; Liveri, M.T.; Ribeiro, D.; Fernandes, E.; Domínguez, H. In vitro bioactive properties of phlorotannins recovered from hydrothermal treatment of Sargassum muticum. Sep. Purif. Tech-nol. 2016, 167, 117–126, doi:10.1016/j.seppur.2016.05.003.
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargas-sum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, doi:10.3390/md15020049.
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263.
- European Commission Commission Implementing Regulation (EU) 2018/460 of 20 March 2018 authorising the placing on the market of Ecklonia cava phlorotannins as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commiss. Off. J. Eur. Union 2018, 2016, 48–119.
- Park, J.; Kim, J.H.; Kwon, J.M.; Kwon, H.; Jeong, H.J.; Kim, Y.M.; Kim, D.; Lee, W.S.; Ryu, Y.B. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg. Med. Chem. 2013, 21, 3730–3737, doi:10.1016/j.bmc.2013.04.026.
- Singh, K.N.; Lal, B. Notes on traditional uses of khair (Acacia catechu Willd.) by inhabitants of shivalik range in Western Himalaya. Ethnobot. Leafl. 2006, 2006, 12.
- Ghalandari, S.; Kariman, N.; Sheikhan, Z.; Mojab, F.; Mirzaei, M.; Shahrahmani, H. Effect of Hydroalcoholic Extract of Capsella bursa pastoris on Early Postpartum Hemorrhage: A Clinical Trial Study. J. Altern. Complement. Med. 2017, 23, 794–799, doi:10.1089/acm.2017.0095.
- Conedera, M.; Krebs, P.; Tinner, W.; Pradella, M.; Torriani, D. The cultivation of Castanea sativa (Mill.) in Europe, from its origin to its diffusion on a continental scale. Veg. Hist. Archaeobot. 2004, 13, 161–179.
- Aguerre, M.J.; Duval, B.; Powell, J.M.; Vadas, P.A.; Wattiaux, M.A. Effects of feeding a quebracho–chestnut tannin extract on lactating cow performance and nitrogen utilization efficiency. J. Dairy Sci. 2020, 103, 2264–2271, doi:10.3168/jds.2019-17442.
- Meagher, L.P.; Lane, G.; Sivakumaran, S.; Tavendale, M.H.; Fraser, K. Characterization of condensed tannins from Lotus species by thiolytic degradation and electrospray mass spectrometry. Anim. Feed Sci. Technol. 2004, 117, 151–163, doi:10.1016/j.anifeedsci.2004.08.007.
- Christensen, R.G.; Eun, J.S.; Yang, S.Y.; Min, B.R.; MacAdam, J.W. In vitro effects of birdsfoot trefoil (Lotus corniculatus L.) pasture on ruminal fermentation, microbial population, and methane production. Prof. Anim. Sci. 2017, 33, 451–460, doi:10.15232/pas.2016-01558.
- Lange, K.C.; Olcott, D.D.; Miller, J.E.; Mosjidis, J.A.; Terrill, T.H.; Burke, J.M.; Kearney, M.T. Effect of sericea lespedeza (Lespedeza cuneata) fed as hay, on natural and experimental Haemonchus contortus infections in lambs. Vet. Parasitol. 2006, 141, 273–278, doi:10.1016/j.vetpar.2006.06.001.
- Katiki, L.M.; Ferreira, J.F.S.; Gonzalez, J.M.; Zajac, A.M.; Lindsay, D.S.; Chagas, A.C.S.; Amarante, A.F.T. Anthelmintic effect of plant extracts containing condensed and hydrolyzable tannins on Caenorhabditis elegans, and their antioxidant capacity. Vet. Parasitol. 2013, 192, 218–227, doi:10.1016/j.vetpar.2012.09.030.
- Jyske, T.; Kuroda, K.; Keriö, S.; Pranovich, A.; Linnakoski, R.; Hayashi, N.; Aoki, D.; Fukushima, K. Localization of (+)-Catechin in Picea abies Phloem: Responses to Wounding and Fungal Inoculation. Molecules 2020, 25, 2952, doi:10.3390/molecules25122952.
- Disler, M.; Ivemeyer, S.; Hamburger, M.; Vogl, C.R.; Tesic, A.; Klarer, F.; Meier, B.; Walkenhorst, M. Ethnoveterinary herbal remedies used by farmers in four north-eastern Swiss cantons (St. Gallen, Thurgau, Appenzell Innerrhoden and Appenzell Ausserrhoden). J. Ethnobiol. Ethnomed. 2014, 10, 32, doi:10.1186/1746-4269-10-32.
- Agency, E.M.; Medicines, V. EMA 2004 Committee for Medicinal Products for Veterinary Use; EMA: Amsterdam, The Nether-lands, 2005; pp. 1–3.
- Landau, S.Y.; Muklada, H.; Abu-Rabia, A.; Kaadan, S.; Azaizeh, H. Traditional Arab ethno-veterinary practices in small ruminant breeding in Israel. Small Rumin. Res. 2014, 119, 161–171, doi:10.1016/j.smallrumres.2014.01.004.
- Piluzza, G.; Virdis, S.; Serralutzu, F.; Bullitta, S. Uses of plants, animal and mineral substances in Mediterranean eth-no-veterinary practices for the care of small ruminants. J. Ethnopharmacol. 2015, 168, 87–99, doi:10.1016/j.jep.2015.03.056.
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206, doi:10.1016/j.jep.2006.09.006.
- Lansky, E.; Shubert, S.; Neeman, I. Pharmacological and therapeutic properties of pomegranate. In Production, Processing and Marketing of Pomegranate in the Mediterranean Region: Advances in Research and Technology; CIHEAM: Zaragoza, Spain, 2000; Options Méditerranéennes: Série A. Séminaires Méditerranéens; pp. 231–235.
- Ballabh, B.; Chaurasia, O.P. Traditional medicinal plants of cold desert Ladakh—Used in treatment of cold, cough and fever. J. Ethnopharmacol. 2007, 112, 341–349, doi:10.1016/j.jep.2007.03.020.
- Al Muqarrabun, L.M.R.; Ahmat, N.; Aris, S.R.S. A review of the medicinal uses, phytochemistry and pharmacology of the genus Sapium. J. Ethnopharmacol. 2014, 155, 9–20, doi:10.1016/j.jep.2014.05.028.
- Zhang, X.-R.; Kaunda, J.S.; Zhu, H.-T.; Wang, D.; Yang, C.-R.; Zhang, Y.-J. The Genus Terminalia (Combretaceae): An Eth-nopharmacological, Phytochemical and Pharmacological Review. Nat. Prod. Bioprospect. 2019, 9, 357–392, doi:10.1007/s13659-019-00222-3.
- Fahmy, N.M.; Al-Sayed, E.; Singab, A.N. Genus Terminalia: A phytochemical and biological review. Montin.) species. Med. Aromat Plants 2015, 4, 1–22.
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244–252, doi:10.1016/S2221-1691(13)60059-3.
- Ćurko, N.; Tomašević, M.; Bubalo, M.C.; Gracin, L.; Redovniković, I.R.; Ganić, K.K. Extraction of proanthocyanidins and anthocyanins from grape skin by using ionic liquids. Food Technol. Biotechnol. 2017, 55, 429–437, doi:10.17113/ftb.55.03.17.5200.
- Caimari, A.; del Bas, J.M.; Crescenti, A.; Arola, L. Low doses of grape seed procyanidins reduce adiposity and improve the plasma lipid profile in hamsters. Int. J. Obes. 2013, 37, 576–583, doi:10.1038/ijo.2012.75.
- Zhou, F.; Yin, M.; Liu, Y.; Han, X.; Guo, J.; Ren, C.; Wang, W.; Huang, W.; Zhan, J.; You, Y. Grape seed flour intake decreases adiposity gain in high-fat-diet induced obese mice by activating thermogenesis. J. Funct. Foods 2019, 62, 103509, doi:10.1016/j.jff.2019.103509.
- Griffin, L.E.; Witrick, K.A.; Klotz, C.; Dorenkott, M.R.; Goodrich, K.M.; Fundaro, G.; McMillan, R.P.; Hulver, M.W.; Ponder, M.A.; Neilson, A.P. Alterations to metabolically active bacteria in the mucosa of the small intestine predict anti-obesity and anti-diabetic activities of grape seed extract in mice. Food Funct. 2017, 8, 3510–3522, doi:10.1039/C7FO01236E.
- Cardullo, N.; Muccilli, V.; Saletti, R.; Giovando, S.; Tringali, C. A mass spectrometry and 1H NMR study of hypoglycemic and antioxidant principles from a Castanea sativa tannin employed in oenology. Food Chem. 2018, 268, 585–593, doi:10.1016/j.foodchem.2018.06.117.
- Senthilkumar, G.P.; Subramanian, S.P. Biochemical studies on the effect of Terminalia chebula on the levels of glycoproteins in streptozotocin-induced experimental diabetes in rats. J. Appl. Biomed. 2008, 6, 105–115, doi:10.32725/jab.2008.014.
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather‐Tait, S.; Heinonen, M.; Hirsch‐Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Ecklonia cava phlorotannins as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, doi:10.2903/j.efsa.2017.5003.
- Li, Y.; Qian, Z.J.; Ryu, B.M.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorganic Med. Chem. 2009, 17, 1963–1973, doi:10.1016/j.bmc.2009.01.031.
- Kim, S.K.; Lee, D.Y.; Jung, W.K.; Kim, J.H.; Choi, I.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; Yea, S.S.; et al. Effects of Eck-lonia cava ethanolic extracts on airway hyperresponsiveness and inflammation in a murine asthma model: Role of suppressor of cytokine signaling. Biomed. Pharmacother. 2008, 62, 289–296, doi:10.1016/j.biopha.2007.07.009.
- Kang, M.C.; Ahn, G.; Yang, X.; Kim, K.N.; Kang, S.M.; Lee, S.H.; Ko, S.C.; Ko, J.Y.; Kim, D.; Kim, Y.T.; et al. Hepatoprotec-tive effects of dieckol-rich phlorotannins from Ecklonia cava, a brown seaweed, against ethanol induced liver damage in BALB/c mice. Food Chem. Toxicol. 2012, 50, 1986–1991, doi:10.1016/j.fct.2012.03.078.
- Roy, M.C.; Anguenot, R.; Fillion, C.; Beaulieu, M.; Bérubé, J.; Richard, D. Effect of a commercially-available algal phlorotan-nins extract on digestive enzymes and carbohydrate absorption in vivo. Food Res. Int. 2011, 44, 3026–3029, doi:10.1016/j.foodres.2011.07.023.