Microplastics (MPs) are tiny plastic particles (<5 mm). They have been classified as contaminants of emerging concern (CECs) recently. Microplastics is a commonly used term, the more detailed classification includes: mesoplastics (1–5 mm), microplastics (0.0001–1 mm), and nanoplastics (<0.1 µm).
- microplastics
- freshwater
- ecotoxicology
- (micro)organisms
1. Introduction
The global production of plastic materials increased tremendously; from ~1.5 million tons (MT) in 1950 up to ~187 MT in 2000, then to ~265 MT in 2010, while in 2018 production was 360 MT [1][2][1,2]. Reasons for such exponential increase rate are cost-effective production and wide applicability of plastic materials relying on their excellent properties: lightness, strength, and resistance/durability [3]. However, it should be noted that anthropogenic litter of plastic material in the environment ranges from 60–80% of the produced amount, which means that large quantities of plastic waste dramatically increased over the last decades as well [4].
Special attention has been recently given to tiny plastic particles (<5 mm) called microplastics (MPs) that have also been detected in the environment [5]. The boundary of 5 mm represents the upper limit for particles that can be readily ingested by organisms [3][6][7][8][3,6,7,8]. Although microplastics is a commonly used term, the more detailed classification includes: mesoplastics (1–5 mm), microplastics (0.0001–1 mm), and nanoplastics (<0.1 µm) [9].
MPs discharge in the environment occurs through various transport media: sewage sludge, urban runoff and dust, industrial and municipal wastewater. Accordingly, MPs are detected in almost all environmental components/constituents: seas [10][11][12][13][10,11,12,13], freshwaters [14][15][14,15], sediments [12][13][16][12,13,16], soils [17][18][17,18] and even atmosphere [19][20][19,20]. The major MPs sources identified are synthetic textiles (34%), tire wear (29%), city dust (24%), road markings/dust (7%), marine coatings (4%), microbeads (2%), and plastic pellets (0.3%) [19][21][19,21]. MPs distribution, abundance, and occurrence in the environment are affected by various factors: type of environment, MPs properties (type, density, size, and shape), climate regions (air turbulence, waves), industrialization, urbanization (proportional to MP’s concentrations), waste management, development in general, and the living standard of gravitating society [14][15][14,15]. MPs are insoluble in water and have low susceptibility to degradation; therefore, they remain in the environment for long period [6]. MPs possess large bioaccumulation potential [17]. Studies showed that MPs exhibited harmful effects to organisms, particularly aquatic ones due to the direct contact. Hence, these may be reflected as behavioral effects, oxidative stress, neurotoxicity, genotoxicity, reproductive impairment, and hepatic damage [9][22][23][9,22,23]. MPs intake by humans is mostly through food and beverages, such as fish [17], mollusks [24], sugar [25], sea salt [26], beer [27], or even tap and bottled water [7][28][7,28]. Hence, it is not surprising that many countries recognize MPs as contaminants of emerging concern (CECs) with severe hazard potential [29][30][29,30].
Understanding MPs’ behavior in the environment and related impact on abiotic and biotic qualities of ecosystems (including the possible impact on humans) is the basis for correct risk assessment. European Chemicals Agency (ECHA) considers that current information is insufficient to derive a robust predicted non-effective MPs concentration, i.e., a threshold value that could be used as proof that risks are adequately controlled. This means that any MPs release in the environment should be considered as risk [31]. Therefore, ECHA proposed the restriction of MPs usage in different professional, agricultural, industrial, and consumer products. The 1st restriction phase is planned for 2021 with a 5-year transition period; ECHA considered that is sufficient time for the industry to substitute MPs. The estimation is that the proposed restriction would yield a reduction of MPs emission of ~400,000 tones within the next 20 years [31]. Additional devastating fact is that developed world economies recycle < 30% of plastic-waste [32]. European Commission brought European Strategy for Plastics in a Circular Economy [33] presenting the vision for confronting MPs pollution, including better design of plastic products, a higher rate of plastic-waste recycling, and better quality recyclates—all boosting the recycled-plastics market.
2. Microplastics Types and Properties
Plastics is a term, derived from Greek plastikos—meaning fit for molding [34], commonly used for a wide range of (semi-)synthetic polymers of high molecular weight. The most common types are: polyethylene (PE), polypropylene (PP), polyvinyl-chloride (PVC), polystyrene (PS), and polyethylene-terephthalate (PET) (Figure 1) [6][35][6,35], possessing specific physical, chemical, mechanical, optical, and electrical properties (Table 1).
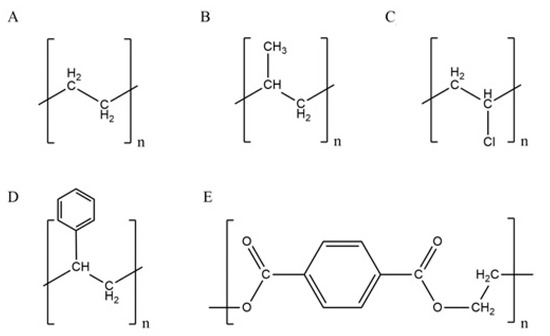
Table 1. Physical and chemical properties of most used plastics.
| Types of Plastic | PE | PP | PVC | PS | PET | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Density | (g cm −3) | 0.91–0.92 | (23/4 °C) | [ ] |
0.85–0.94 [9] | 0.89–0.92 | [ 36], | 0.94–0.97 | (23/4 °C) | [ ] |
1.38 [9], | 1.30–1.58 | [ 36], | 1.35–1.45 | (23/4 °C) | [ ] |
0.96–1.05 [9], | 1.04–1.05 | [ 36], | 0.90–0.91 | (23/4 °C) | [ ] |
1.34–1.39 [9], | 1.29 | [ 36], | 1.03–1.09 | (23/4 °C) | [ ] |
|||||||||||||||||||
Melting | point (°C) | 90–110 [38], | 98–130 | [ 36] |
168–175 [36] | 115–245 [39] | none (atactic), | 240 (isotactic), | 270 (syndiotactic), | [ ] |
245 [36] | ||||||||||||||||||||||||||||||||||||
Glass transition | temperature (°C) | −25 [36] | −10 [36], | −20 | [ 41] |
75–105 [36] | 80–85 | [ 41] |
74–105 [36], | > 80 | [ 41] |
73 [36], | 70 | [ 41] |
|||||||||||||||||||||||||||||||||
Tensile strength (MPa) | 8–32 [36] | 31–41 [36] | 41–52 [36] | 36–52 [36] | 48 [36] | ||||||||||||||||||||||||||||||||||||||||||
Crystallinity (%) | 45–95 [9], | 50 | [ 37] |
50–80 [9], | 65 | [ 41], | 50 | [ ] |
High [9], | 5–15 | [ 41], | 0 | [ ] |
Low [9], | 0 | [ 37] |
High [41], | 30–40 | [ 42], | 0–50 | [ ] |
||||||||||||||||||||||||||
Lifespan (year) | 10–600 [37] | 10–600 [37] | 50–100 [37] | 50–80 [37] | 450 [37] |
Applications of polymers are determined, but not limited, by their properties. Applicative properties can be enhanced by the addition of accelerants, cross-linking additives, UV stabilizers, antidegradants, antioxidants, antiozonants, photosensitizers, surfactants, pigments, flame retardants, or plasticizers with biocidal additives [9]. Generally, plastics consist of crystalline and amorphous phases, affecting mechanical properties such as strength and elasticity. At a lower amount of amorphous phase, crystallinity and density increase. With the increase in the latter property, the elastic modulus, tensile strength, stiffness, and surface hardness also increase, but impact strength decreases [43].
PE is homopolymer consisting of long hydrocarbon repeated chains of ethylene monomer (Figure 1) and is used in a variety of products, e.g., plastic bags; bottles for milk, water, shampoo, or motor oils; toys; food packaging like yogurt cans, margarine containers or cereal box liners; irrigation and drainage pipes; various medical and cosmetic products [38][44][38,44]. It is not surprising that most commonly found plastics debris in freshwater ecosystems pertains to PE [6]. PE is classified as: (i) ultra-high, high or ultra-low molecular weight PE (UHMWPE, HMWPE or ULMWPE, respectively), (ii) high, high cross-linked, medium, linear low, low or very low-density PE (HDPE, HDLPE, MDPE, LLDPE, LDPE or VLDPE, respectively), or (iii) chlorinated PE [38]. PE is a good electrical insulator with low hardness, rigidity, and melting point, and high ductility and impact strength (Table 1). It has a lower density comparing to water (Table 1); thus, it is uncommon to be present in deep layers of aquatic ecosystems [6]. PE is very persistent in the environment due to its non-reactive C-C and C-H bonds, high molecular weight, hydrophobicity (allowing sorption on sediments or activated sludge) [6], and lack of functional groups that can be “recognized” by microbial enzymatic systems [45].
PP is a crystalline thermoplastic polymer with methyl-group on every subunit of polymer backbone (Figure 1); this improves its mechanical properties and thermal resistance. Linear PP has various structural isomers: isotactic, syndiotactic, and atactic. Isotactic PP has a greater degree of crystallinity than the other two; thus, it is less susceptible to biodegradation. A high degree of tacticity (iso- or syndio-) provides higher crystallinity, better mechanical properties, and chemical stability. A low degree of tacticity means lower hardness, strength, density, rigidity, stability, and melt fluidity. Commercial PP is approximately 90% isotactic with 60–70% crystallinity [43]. Comparing to HDPE, PP has some similar properties (permeability of gasses and vapor is almost the same), although differs in hardness, tensile strength, elasticity, transparency, and gloss (higher than HDPE). PP is highly resistant to water, inorganic chemicals, organic solvents, and lubricants; degradation occurs by strong oxidants [43]. PP is also a very good electrical insulator. Accordingly, it has numerous applications: as packaging material; medical and electronic equipment; furniture production; textile and automotive industries [6][10][38][44][46][6,10,38,44,46]. Hydrophobic surface and high molar mass of PP limit its biodegradation [37][47][37,47]. The insertion of hydrophilic groups onto the polymer surface via physical or chemical processes (e.g., degradation) is highly required to enable the attachment of microorganisms to such partially destructed polymer surface [48]. Accordingly, microorganisms can use low-molecular-weight fragments (oligomers), dimers, or monomers as carbon and energy sources [49], providing biodegradation. However, small oligomers may also diffuse into the organism and get assimilated, providing adverse effects [37].
PVC structure (Figure 1) contains heteroatom: chlorine, on every second C-atom of polymer backbone. PVC usage in Europe for packaging only exceeds 500,000 tons annually [43][44][43,44]. Due to its extreme versatility, PVC is irreplaceable for medicine equipment (blood bags, tubs for catheters, surgical gloves) and wall and floor coverings [46]. It is also used for construction purposes: piping, electrical cable insulation, doorframes, and windows [6][44][6,44]. PVC comes in two forms: (i) the rigid, which is used for food packaging, pharmaceutical, and medical products, and (ii) the flexible, which is used as a film for food wrappings due to its stretchability [43]. PVC is an excellent water and oxygen barrier; thus, packaged food may last longer ensuring its taste. PVC is resistant to chemicals but very sensitive to photodegradation [6]. Since its inception, PVC is a known polymer with exceptional resistance to biodegradation, making it an environmental issue; from production to waste disposal.
PS has an amorphous structure with a phenyl ring linked on every second C-atom of polymer backbone (Figure 1). It is well-known in its expanded form as Styrofoam and is used for packaging, bowls, containers, rigid trays, lids, and tumblers [43][44][50][43,44,50]. Global production of PS in 2013 was ~21 MT [50]. PS is hard, stiff, durable, and transparent thermoplastic polymer, which is available in four forms: (i) general-purpose PS (GPPS), (ii) high impact PS (HIPS), (iii) PS foam, and (iv) expanded PS foam (EPS) [50][51][50,51]. Its foamed form is lightweight (it floats on the water surface), bulky, and recyclable [51]. PS is brittle and flammable with relatively low melting temperature and with a glass transition temperature of >100 °C; thus, it softens in boiling water. PS is poor oxygen and vapor barrier and sensitive to UV irradiation (it yellows) [6][43][6,43].
PET (Figure 1) is, along with PE, one of the main food packaging materials. It is very common in the textile industry as well, where is commonly used in the following forms: (i) fiber filling in insulated clothing, furniture, and pillows, (ii) fine filaments in artificial silk, and (iii) large-diameter filaments in carpets production [7][43][7,43]. PET is used as/in yarns of vehicle tires; conveyer, drive or seat belt; reinforcement for fire and garden hoses; disposable medical clothing; synthetic fibers, bottles, containers, insulators, 3D printing filaments, and trays [7][43][44][7,43,44]. PET is transparent and colorless semi-crystalline thermoplastic polyester, strong and resistant to chemicals, and characterized by low vapor and gas permeability (Table 1) [52]. It is a good alternative for glass; thus, it is used for the production of bottles/containers for beverages [43]. PET is a major environmental pollution issue due to its high durability and low biodegradability. PET, similarly to PP and PS, has a hydrophobic surface, thus attenuating effective adsorption and access of hydrolytic enzymes (hydrolases, lipases, esterases, and cutinases) to accomplish polymer degradation [53].
3. Sources of Microplastics in the Environment
There are primary and secondary sources of MPs in the environment [5]. Primary include MPs deliberately manufactured for some products; i.e., textile (laundering of synthetic clothes providing ~35% of primary microplastics), cosmetics (e.g., microbeads in facial scrubs (~2%) as intentionally added MPs in personal care products), electronic equipment [13][35][54][13,35,54]. Furthermore, abrasion of tires through driving (28%), city dust (24%), road making (7%), marine coating (3.7%), and plastic pellets (0.3%) are also considered as the main sources of primary MPs. Most of these enter the environment through wastewater generated during the production or usage phases (Figure 2).

Figure 2. Sources of microplastics in the environment.
Secondary sources include a breakdown of larger plastic fragments such as plastic bags, bottles, or fishing nets introduced in the environment; the breakdown is caused by UV radiation, physical abrasion, chemical oxidation, and possibly biodegradation [13][35][55][56][13,35,55,56].
Agriculture is one of the major entry points for MPs in the environment [57]; applications of sewage sludge, fertilizers/compost, soil conditioners, and vinyl coverings are the main sources. LDPE films, used in large volumes to protect agricultural crops, suppress weeds, increase temperature, and retain irrigation water in the soil, can be degraded into small fragments and end up in the soil or water resources through irrigation channels. The expanded PS flakes and polyurethane foam are used in horticulture to improve soil quality and as composting additive, respectively [58][59][60][58,59,60]. However, significant MPs amounts originate from various industries (through wastewaters or plastic residues disposal) and households (fibers from washing clothes, personal care products usage). Once released into the environment, MPs can be transported by wind, washed from land to surface waters during rainfall (stormwater run-off especially), and be transported in freshwater and seawater [3][6][8][10][15][35][61][3,6,8,10,15,35,61]. Industrial and municipal wastewaters are also main MPs sources, containing synthetic fibers (e.g., polyester, PES, produced in 2013 at >44.6 MT) [62]. Browne et al. [63] quantified that >1900 PES fibers are released during a single wash. Therefore, wastewaters containing MPs should be treated prior to discharging into the environment. However, only ~60% of municipal wastewaters are treated [64]. Besides, wastewater treatment plants (WWTPs), commonly based on primary and secondary treatment units, are not designed to remove MPs. The tertiary treatment, including disinfection, is optional and irrelevant for MPs removal. After primary treatment application, including removal of material that either floats or readily settle out by gravity, wastewater may contain > 20% of MPs [65]. Secondary treatment is biological; mixed consortium of microorganisms has a key role in forming activated sludge flocs. If MPs are retained within, there is a possibility to be released from flocs due to their instability in the aqueous phase. Although the efficiency of common WWTP for MPs removal is up to 95%, a large MPs fraction is transferred into the activated sludge [65]. Moreover, WWTPs are not effective in removing particles < 10 μm [15]; these remain in effluent and are consequently discharged into recipient natural aquatic systems. Besides technological, there are legislative issues too; Directive 91/271/EEC [66], which does not consider MPs monitoring in treated effluents or activated sludge, is still in force. Approximately 4–5 MT of activated sludge is applied to agricultural lands annually [14], thus providing additional MPs quantities in the environment. Upon entering freshwater sources, a fraction of MPs would be suspended in the water body, while others would deposit at the bottom of the water column (i.e., sediment). This process depends on the density of MPs; low-density MPs would float, while those with higher density would submerge [59]. The creation of biofilms may increase MPs density and consequently reduce their hydrophobicity, yielding their deposition to a higher extent. However, biofilms can carry pathogenic microorganisms (e.g., Vibrio or fecal coliforms) as well, which may present adverse effects on water biota and humans [67]. Moreover, MPs can also adsorb heavy metals and various CECs; e.g., polycyclic aromatic hydrocarbons, polybrominated diphenyl ethers, polychlorinated biphenyls, and dichlorodiphenyltrichloroethane [68][69][68,69]. MPs distribution may also be affected by particle aggregation and activity of animals [70]. MPs can be easily ingested by aquatic organisms of various trophic levels and can undergo biomagnification along the food chain [71]. MPs shapes and densities affect their availability to organisms; e.g., pelagic organisms (phytoplankton and zooplankton) are more likely to encounter less dense, floating MPs, while benthic organisms (amphipods, polychaete worms, tubifex worms, mollusks, and echinoderms) would encounter more dense MPs [56][70][72][73][56,70,72,73].
4. Ecotoxicological Effects on Microplastics
Ecotoxicological studies on MPs can be distinguished according to: (i) test organisms, (ii) type, shape, and size of the polymer, and (iii) the site of analysis (in situ or in laboratory). There are numerous organisms that can be used in MPs ecotoxicological assessment; however, >75% of studies were performed on marine (micro)organisms. The most common testing organisms applied are: fish, mollusks, small and large crustaceans, annelids, mammals and echinoderms, birds and cnidarians, sponges, reptiles, and rotifers. Fish is mostly used in in situ studies, while small crustaceans prevail among organisms tested in a laboratory [4][19][22][23][29][74][75][76][77][78][79][80][4,19,22,23,29,104,105,106,107,108,109,110]. The most studied MPs shapes include spherical particles, fibers, and fragments. Although PE and PS are the most studied MPs types (due to their ubiquitous presence in aquatic ecosystems), investigations also comprehended ecotoxicological effects of other MPs types such as PP, PES/PET, PVC, polyamide, acrylic, polyether, cellophane, and polyurethane [22].
Regardless of MP types, both direct and indirect toxic effects on aquatic organisms have been investigated. Results of ecotoxicological studies are commonly expressed as a concentration of tested compounds causing certain effects (in percentage) on measured population. The most common effect considered within these studies was the percentage of population mortality. Hence, in lethal toxicity testing, LD50 represents the median lethal dose, while LC50 represents the median lethal concentration. If a test end point is an adverse response other than death, an effective concentration (EC) or effective dose (ED) is used toxicity parameter. Concentration causing 50% of adverse effect in the tested population is commonly used (EC50), although other levels (i.e., EC10 [81][111] or EC20 [82][112]) can be applied if necessary. Effective and lethal values can be expressed per mass of tested organism; ED and LD are used for higher organisms. Besides, it is also possible to determine the No Observed Adverse Effect Level (NOAEL) and Lowest Observed Adverse Effect Level (LOAEL). NOAEL is the highest exposure level at which there are no biologically significant increases in the frequency or severity of adverse effect between the exposed population and its appropriate control; some effects may be produced at this level, but they are not considered as adverse. LOAEL is the lowest exposure level at which there are biologically significant increases in frequency or severity of adverse effects between the exposed population and its appropriate control group. Growth inhibition test is used for the ecotoxicological purpose as well [83][113]. Table 2 summarizes ecotoxicological studies performed including (micro)organisms and MPs types used.
Table 2. Parameters given by different ecotoxicological studies.
Microorganism/Organism | Type of MPs | Size of MPs | Concentration of MPs | Parameters Value | Effects | References | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Vibrio fischeri | PE | 1.00–3.00 µm | 1000.0 mg/L | EC20 = 3600.0 mg/L | (5 min) | EC 20 = 2600.0 mg/L | (30 min) | decrease of bioluminescence | [82] | [112] | |||||||||||||||||||||||||||||||||||||
PS-PEI a | 0.06 µm | 3.0–1000.0 mg/L | EC50 = ≥ 1000.0 mg/L | (30 min) | [83] | [113] | |||||||||||||||||||||||||||||||||||||||||
0.11 µm | EC50 = ≥ 1000.0 mg/L | (30 min) | |||||||||||||||||||||||||||||||||||||||||||||
Algae | Pseudokirchneriella subcapitata | PS-PEI a | 0.06 µm | 0.1–1.0 mg/L | EC50 = 0.58 ± 0.04 mg/L (72 h) | inhibition of algal growth | [83] | [113] | |||||||||||||||||||||||||||||||||||||||
0.11 µm | 0.1–0.8 mg/L | EC50 = 0.54 ± 0.06 mg/L (72 h) | |||||||||||||||||||||||||||||||||||||||||||||
Chlorella sp. | PS | 0.02 µm | 80.0–800.0 mg/L | kb = 3918.0 (mg/L)1−n | adsorption of particles on algae, reduction of photosynthesis, oxidative stress | [84] | [114] | ||||||||||||||||||||||||||||||||||||||||
Scenedesmus sp. | kb = 4309.0 (mg/L)1−n | ||||||||||||||||||||||||||||||||||||||||||||||
Scenedesmus sp. | PS | 0.10 µm | 10.0, 50.0 and 100.0 mg/L | IR c = 21.0, 29.0, 38.5% | inhibition of algal growth, morphological changes, oxidative stress | [85] | [115] | ||||||||||||||||||||||||||||||||||||||||
1.00 µm | IR c = 20.9, 28.4, 38.1% | ||||||||||||||||||||||||||||||||||||||||||||||
Chlorella pyrenoidosa | PP | 64.0–236.0 µm | 5.0, 10.0, 50.0, 100.0, 250.0, 500.0 mg/L | IRa d = 10.61, 15.86, 22.10, 31.08, 25.53, 24.57% | decrease of chlorophyll a content and photosynthetic | activity | [86] | [116] | |||||||||||||||||||||||||||||||||||||||
PVC | 111.0–216.0 µm | IRa d = 20.39, 37.67, 49.46, 48.49, 55.23, 55.17% | |||||||||||||||||||||||||||||||||||||||||||||
Microcystis flos-aquae | PP | 64.00–236.00 µm | IRa d = 11.13, 1.29, 10.52, 13.13, 13.06, 16.92% | ||||||||||||||||||||||||||||||||||||||||||||
PVC | 111.0–216.0 µm | IRa d = 9.55, 24.92, 23.97, 18.61, 32.20, 46.93% | |||||||||||||||||||||||||||||||||||||||||||||
Chlamydomas reinhardtii | PP | 400.0–1000.0 µm | 400.0 mg/L | IR c = ~18.0% (78 days) | inhibition of algal growth, formation of hetero-aggregates | [87] | [117] | ||||||||||||||||||||||||||||||||||||||||
Daphnia magna | PS-PEI a | 0.06 µm | 0.33–3.30 mg/L | EC50 = 0.77 ± 0.10 mg/L | (48 h) | immobilization rate | [83] | [113] | |||||||||||||||||||||||||||||||||||||||
0.11 µm | EC50 = 0.66 ± 0.17 mg/L | (48 h) | |||||||||||||||||||||||||||||||||||||||||||||
PS | 1.0 µm | 0.1–600.0 mg/L | EC50 = 66.97 mg/L (48 h) | LC 50 = 87.83 mg/L (48 h) | immobilization rate, oxidative stress, mortality | [88] | [118] | ||||||||||||||||||||||||||||||||||||||||
10.0 µm | 0.01–40.0 mg/L | EC50 = 199.94 mg/L (48 h) | LC 50 = 291.69 mg/L (48 h) | ||||||||||||||||||||||||||||||||||||||||||||
PE | 1.0 µm | 12.5, 25.0, 50.0, 100.0, 200.0 and 400.0 mg/L | ID e = 25.00% ± 1.91, 35.00% ± 1.00, 55.00% ± 1.00, 50.00% ± 2.58, 75.00% ± 1.00, 35.00% ± 1.91 (96 h) | immobilization rate, accumulation in gut | [89] | [119] | |||||||||||||||||||||||||||||||||||||||||
PE | 1.00–5.00 µm | 3.0, 4.0, 5.0, 6.0 and 7.0 f particles/mL | LC50 f = 32.0 particles/mL (48 h, 18 °C) | LC 50 f = 18.0 particles/mL (96 h, 18 °C) | LC 50 f = 10.0 particles/mL (48 h, 22 °C) | LC 50
f = 5.8 particles/mL (96 h, 22 °C) | LC 50
f = 8.0 particles/mL (48 h, 26 °C) | LC 50
f = 4.0 particles/mL (96 h, 26 °C) | immobilization rate, mortality | [90] | [120] | ||||||||||||||||||||||||||||||||||||
PET | fibers of 20.0 µm thickness | 12.5–100.0 mg/L | EC50 = 1.34 mg/L (24 h) | mortality was in the range 20.0–40.0% for 12.5–100.0 mg/L | immobility, accumulation in gut, mortality | [91] | [121] | ||||||||||||||||||||||||||||||||||||||||
Fish | Danio rerio | PE | 140.6 ± 80.0 µm | 100 mg/L | 75.0% deformed embryos (96 h, aged MP in WWTP effluent) | EC 50 ≤ 1.0% | (96 h, aged MP in landfill leachate) | impact on development | [92] | [122] | |||||||||||||||||||||||||||||||||||||
PE, PP, PS, and PVC | 0.10, 1.0 and 5.0 µm | 0.001–10.0 mg/L | SP g = 73.0% ± 24.0 (PP, after 10 days, at 10.0 mg/L) SP g = 83.0% ± 24.0 (PVC, after 10 days, at 10.0 mg/L) | morphological deformations, damage of the intestine, mortality | [93] | [123] | |||||||||||||||||||||||||||||||||||||||||
PS | 45.0 µm | 0.05 µm | 1.0 mg/L | 22.0%, 6.1% suppressed locomotor ability, body length during exposure at 0.05 µm particles, respectively | suppressed locomotor activity, decrease of body length, deterioration of nervous and visual systems | [94] | [124] | ||||||||||||||||||||||||||||||||||||||||
PS | 0.07 µm | 5.0 µm | 0.002, 0.2 and 2.0 mg/L | 5.7 × 10−4, 1.25 × 10−3 and 8.9 × 10−4 mg/mg fish (5.0 µm particles accumulated in gill, liver, and gut, respectively) | accumulation of particles in fish gill, gut, and liver, deterioration of liver metabolism, oxidative stress | [95] | [125] | ||||||||||||||||||||||||||||||||||||||||
20.0 µm | - | ||||||||||||||||||||||||||||||||||||||||||||||
Cyprinus carpio | Carassius auratus | Hypophthalmichthys molitrix | Pseudorasbora parva | Megalobrama amblycephala | Hemiculter bleekeri | 49.1% cellophane | 76.3% particles <5 mm | - | 2.5 ± 1.3 particles/fish | 1.9 ± 1.0 particles/fish | 3.8 ± 2.0 particles/fish | 2.5 ± 1.8 particles/fish | 1.8 ± 1.7 particles/fish | 2.1 ± 1.1 particles/fish | accumulation in stomach and intestine | [96] | [126] | ||||||||||||||||||||||||||||||
Bagre bagre | Bagre marinus | Caranx hippos | Lutjanus analis | Polydactylus oligodon | Cynoscion leiarchus | Sphyrna tiburo | Trichiurus lepturus | 97.4% of polyamide | 0.38–4.16 mm | - | 12.8 particles/fish | 7.8 particles/fish | 30.7 particles/fish | 1.0 particle/fish | 3.0 particles/fish | 2.0 particles/fish | 9.0 particles/fish | 2.0 particles/fish | accumulation in stomach and intestine | [97] | [127] |
a PS-PEI = polyethyleneimine polystyrene; b Freundlich coefficient k; c inhibition ratio of algal growth; d inhibition ratio of chlorophyll a content; e means of immobilized daphnids; f log-transformed values; g survival percentages of zebrafish.
MPs toxicological effects on freshwater aquatic (micro)organisms are still scarce since most studies applied marine (micro)organisms. Therefore, future studies should be focused on freshwater microorganisms such as algae and bacteria or other surface water and sediment organisms. Current data summarized in Table 2 reveal that researches are performed using a wide range of concentrations and types of MPs to study adverse effects to the range of organisms (from bacteria to fish). Accordingly, the graphical presentation in Figure 3 shows the range of ecotoxicological concentrations established for different organism levels studied, thus providing an insight into the sensitivity of tested organisms to changes in MPs concentrations affecting different trophic levels.
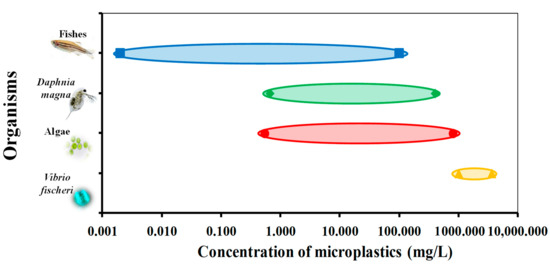
Figure 3. The range of ecotoxicological concentrations for different organism levels.
