The Thermodynamic Dissipation Theory of the Origin and Evolution of Life argues that the escence of the origin of life was the microscopic dissipative structuring under UVC light of organic pigments (now known as the fundamental molecules of life - those common to all three domains) and their proliferation over the entire Earth surface, driven by the thermodynamic imperative of dissipating this part of the Archean solar spectrum into heat. With time, dissipative structuring led to ever more complex biosynthetic pathways for creating pigments and their support structures (and processes) which could dissipate not only the UVC region but also other UV regions and the visible wavelengths, until today reaching the "red edge" (at approximately 700 nm). The heat of dissipation of photons absorbed on organic pigments in water then catalyzes a host of coupled secondary dissipative processes such as; the water cycle, ocean and wind currents, hurricanes, etc. pushing the limit for dissipation of the incident light even further towards the infrared.
The thermodynamic dissipation theory thus assgins an explicit thermodynamic function to life; the dissipative structuring, proliferation, and evolution of molecular pigments and their complexes from common precursor carbon based molecules under the impressed short wavelength solar photon potential to perform the explicit thermodynamic function of dissipating this light into long wavelength infrared light (heat). In a general sense, the origin of life is no different than the origin of other dissipative structuring processes like hurricanes and the water cycle, except that these latter processes deal with structuring involving hydrogen bonding while life deals with structuring involving covalent bonding. The external photon potential supplied continuously by the environment (our Sun), and its dissipation into heat by the assembly of dissipative structures, are, therefore, both integral components necessary for understanding life.
Difficult problems related to the origin of life, such as enzyme-less replication of RNA and DNA, homochirality of the fundamental molecules, and the origin of amino acid -codon assignments (information encoding in RNA and DNA), also find simple explanations within this same dissipative thermodynamic framework once the existence of a relation between primordial RNA and DNA replication and UV-C photon dissipation is established.
- Origin of Life
- Dissipative Structuring
- Non-equilibrium thermodynamics
- Homochirality
- Origin of translation
- Stereochemical era
- Nucleic acid
- Amino acids
- Fundamental molecules
The 19th-century physicist Ludwig Boltzmann first recognized that the struggle for existence of living organisms was neither over raw material nor energy, but instead had to do with entropy production derived from the conversion of the solar spectrum into heat by these systems.[1]. Boltzmann thus realized that living systems, like all irreversible processes, were dependent on the dissipation of a generalized chemical potential for their existence. In his book "What is Life", the 20th-century physicist Erwin Schrödinger [2] emphasized the importance of Boltzmann's deep insight into the irreversible thermodynamic nature of living systems, suggesting that this was the physical and chemical foundation of the origin and evolution of life.
However, irreversible processes, and much less living systems, could not be conveniently analyzed under this perspective until Lars Onsager, [3] and later Ilya Prigogine,[4] and coworkers developed an elegant mathematical formalism for treating the "self-organization" of material under a generalized chemical potential. This formalism became known as Classical Irreversible Thermodynamics and Prigogine was awarded the Nobel Prize in Chemistry in 1977 for his "contributions to non-equilibrium thermodynamics, particularly the theory of dissipative structures". The analysis by Prigogine showed that if a system were left to evolve under an imposed external potential, material could "spontaneously" organize (lower its entropy) forming what he called "dissipative structures" which arose to increase the dissipation of this potential (effectively augmenting the global entropy production of the system plus its environment). This non-equilibrium thermodynamic theory of Prigogine and coworkers, known as Classical Irreversible Thermodynamics, has since been successfully applied to the analysis of living systems, from the origin of life [5] [6] [7], to the biosynthetic production of adenosine triphosphate [8], to understanding bacterial metabolic pathways [9], and even to understanding the evolution of complete ecosystems.[10].[11] [12].
The "Thermodynamic Dissipation Theory of the Origin and Evolution of Life",[5] [6] [7] postulated by Karo Michaelian takes the insight of Boltzmann and the work of Prigogine to its ultimate consequences regarding the origin of life. This theory suggests that the hallmark of the origin and evolution of life is the microscopic dissipative structuring of organic pigments, their proliferation over the entire Earth surface, and their complexation, and general evolution, towards more efficient dissipative systems. Present day life augments the entropy production of Earth in its solar environment by dissipating ultraviolet and visible photons into heat through organic pigments in water. This heat then catalyzes a host of secondary dissipative processes such as hurricanes, the water cycle,and ocean and wind currents, etc.[13].
Michaelian argues that if the thermodynamic function of life today is to produce entropy through photon dissipation in organic pigments, then this probably was its function at its very beginnings. It turns out that both RNA and DNA when in water solution are very strong absorbers and extremely rapid dissipaters of ultraviolet light within the 220–290 nm wavelength (UV-C) region, which is a part of the Sun's spectrum that penetrated the prebiotic atmosphere of Earth [14] (see figure 1). It is thus probable that the nucleobases of RNA and DNA (adenine, guanine, cytosine, thymine, uracil) were ancient organic pigments dissipatively structured on the surface of Earth's early oceans through the direct interaction of UVC light affecting directly the covalent bonding of more common precursor molecules such as HCN and H\( _2 \)O thereby transforming and combining these into the fundamental molecules [15].
In fact, not only RNA and DNA, but many fundamental molecules of life (those common to all three domains) are also pigments which absorb in the UV-C atmospheric window of the Archean (see figure 1), and many of these also dissipate their photon-induced electronic excitation energy into heat very rapidly (a process known as internal conversion) through what are known as "conical intersections". Of the fundamental molecules that do not have a conical intersection to internal conversion, many do, however, have chemical affinity to RNA and DNA.[16] which do have such a conical intersection. Nucleic acids may thus have acted as acceptor molecules to the UV-C photon-induced excitation energy of antenna pigment donor molecules, providing an ultrafast channel for dissipation through resonant energy transfer between molecules. The thermodynamic imperative to increase global photon dissipation, according to Michaelian, is thus the driving force behind the complexation of molecules. For example, during the stereochemical era [17] some amino acids (e.g. the aromatics; tryptophan, tyrosine, phenylalanine, histidine, and others - see figure 3) developed chemical affinity to sections of nucleic acds (their cognate codons). By acting as an antenna-dissipator system, this association of amino acids with nucleic acids improved global photon dissipation (as compared to the molecules acting separately) and this was the origin of the information content of nucelic acids [18].
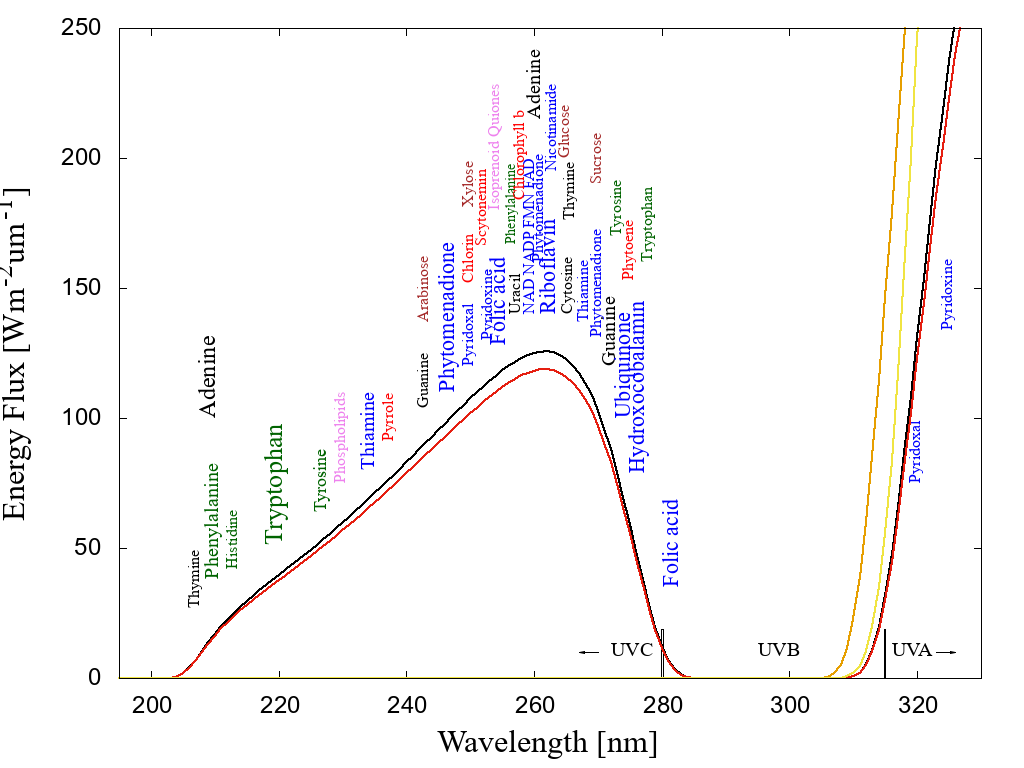
Figure 1: The spectrum of light available in the UV region at Earth’s surface before the origin of life at approximately 3.9 Ga and until at least 2.9 Ga (curves black and red respectively) during theArchean. CO2 and probably some H2S were responsible for absorption at wavelengths shorter than∼205 nm and atmospheric aldehydes (comon photochemical products of CO2 and water) absorbed between about 285 and 310 nm, approximately corresponding to the UVB region. Around 2.2 Ga (yellow curve), UVC light at Earth’s surface had been extinguished by oxygen and ozone resulting from organisms performing oxygenic photosynthesis. The green curve corresponds to the present surface spectrum. Energy fluxes are for the sun at the zenith. The names of the fundamental molecules of life are plotted at their wavelengths of maximum absorption; nucleic acids (black), amino acids (green),fatty acids (violet), sugars (brown), vitamins, co-enzymes and co-factors (blue), and pigments (red) (the font size of the letter roughly corresponds to the relative size of their molar extinction coefficient).Indications that dissipative structuring occurred at the origin of life are that the absorption wavelengths of these fundamental molecules coincide with the Archean UV surface spectrum and that most have a conical intersection to internal conversion. Adapted from Michaelian and Simeonov [16].
Michaelian has shown, using the formalism of non-linear irreversible thermodynamics,[4] that there would have existed during the Archean a thermodynamic imperative to the abiogenic UV-C photochemical synthesis and proliferation of these UVC pigment (fundamental) molecules over the entire Earth surface, as well as to their complexation, if this improved the efficacy of global photon dissipation.[15].[19] (see Fig. 2).
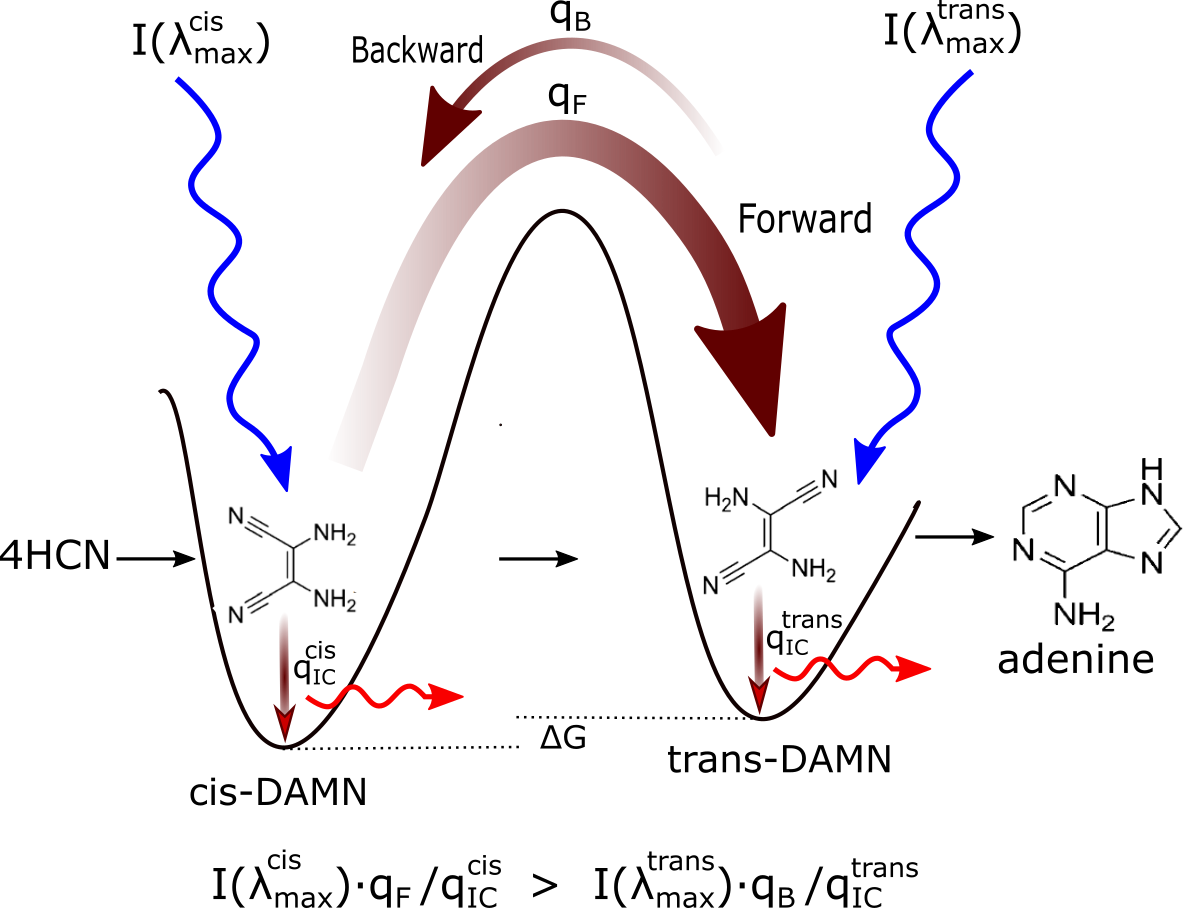
Figure 2: Mechanism for the evolution of molecular structures towards ever greater photon dissipative efficacy (microscopic dissipative structuring) on route to the fundamental molecules (in this case adenine). The high activation barriers between configurations (because of covalent bonding) mean that reactions will not proceed spontaneously but only through coupling to photon absorption events. Forward and backward rates depend on photon intensities at the different wavelengths of maximum absorption I(λmax) for the two structures, and on the phase space widths of paths on their excited potential energy surface leading to the conical intersection giving rise to the particular transformation; implying different quantum efficiencies for the forward (qF) and backward (qB) reactions. Since the intensity of the incident spectrum is assumed constant, and since \( q_F+···q^{cis}_{IC} = 1 \) and \( q_B+···q^{trans}_{IC} = 1 \) (where the “···” represents quantum efficiencies for other possible molecular transformations), those configurations (and also macroscopic concentration profiles) with greater photon dissipative efficacy (higher quantum efficiency for internal conversion (\( q_{IC} \)) will therefore gradually become more predominant under a continuously impressed UVC photon flux, independently of the sign or size of the difference in the Gibb’s free energies ∆G of the molecules. Taken from Michaelian [15].
By the end of the Archean (2.5 Ga), when oxygenic photosynthesis had saturated all available oxygen sinks, life-produced oxygen would begin to accumulate in the atmosphere and be converted into ozone. The global dissipation of UVC light would thus be transfered from the surface to the upper atmosphere and it would have become ever more improbable for completely new life forms to emerge on the surface that did not rely on the rather complex molecules and metabolic pathways already existing, since now the free energy in the only photons surviving to Earth's surface (the near UV and visible) would have been insufficient for directly transforming carbon covalent bonds in molecules. Molecules (pigments and their support structures) could still be dissipatively structured under this surviving light, however, but now indirectly by employing biochemical pathways leading to chemical sources of free energy such as ATP which could transform covalent bonds, or even directly by dissipative structuring employing the weaker hydrogen and Van der Waals bonding between molecules, since now no UVC photons survived to the surface to destroy complexations held together by these weaker bondings. The latter structuring amounting to the complexation of the existing fundamental molecules.
A similar change in the surface flux of ultraviolet radiation due to a geochemical event (the freezing of the inner metalic core of Earth) affecting the atmosphere through increadsing Earth's protective magnetic field, and thereby affecting the surface UV light spectrum, could also have promoted a further development in the complexity of life, giving rise to the Cambrian explosion around 0.54 Ga [20].
Some of the most difficult problems concerning the origin of life find relatively simple solutions within such a photon dissipative freamework once a relation is established between primordial replication and photon dissipation (see Fig. 3). These problems which find solution are; enzyme-less replication of RNA and DNA [21], homochirality of the fundamental molecules [22], and the origin of information encoding in RNA and DNA [18] .
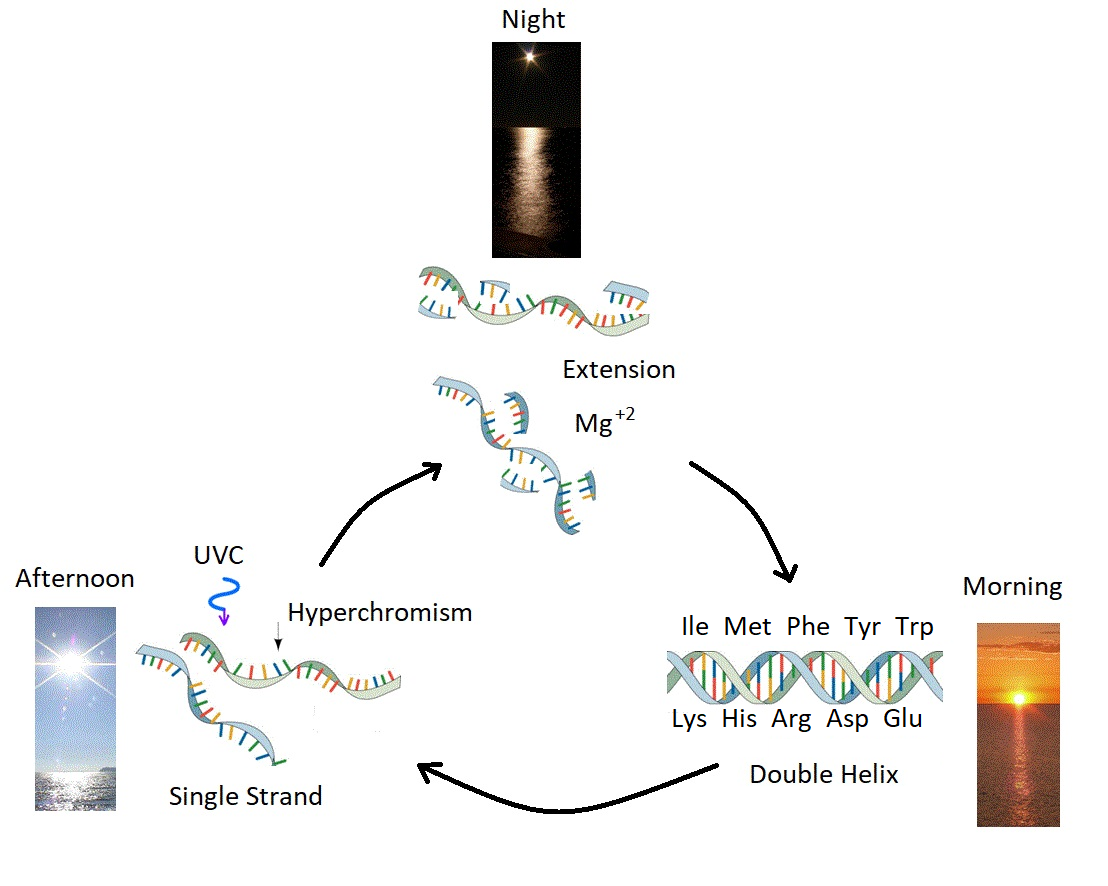
Figure 3: Ultraviolet and Temperature Assisted Reproduction (UVTAR) of RNA and DNA. A mechanism proposed for the enzyme-less reproduction of RNA and DNA assisted by the absorption and dissipation of the prevailing UVC light flux and the high temperatures of the ocean surface during the late Hadean or early Archean, including a day/night diurnal warming and cooling cycle of the water surface due to the absorption of solar infrared light. Most denaturing would occur in the afternoon when UVC light was most intense (photon-induced denaturing [21]) and when ocean surface temperatures were highest. Extension occurs overnight with the aid of Mg\( ^{+2} \) ions, UVC activated nucleotides, and colder surface temperatures. ``Hyperchromism" refers to an increase (\( \sim \) 35\( \% \)) in the absorption of photons at UVC wavelengths (\( \sim \) 260 nm) once RNA or DNA are denatured into single strands. Oligos which had chemical affinity to the 10 amino acids listed in the figure (all of which have photon absorption and dissipation fomenting characteristics), would have had a greater chance of denaturing during daylight hours as the surface water cooled, and could therefore be replicated overnight. This selection based on greater photon dissipation we have termed ``thermodynamic selection'' [5] [6] [7] [15]. The important aspect of this auto-catalytic mechanism is that replication is tied to photon dissipation, providing a thermodynamic imperative for proliferation.Taken from Mejía and Michaelian [18].
Dissipative structuring under light, as the fundamental creative force in biology, appears to have been ongoing, from the initial creation and dissipation at the UVC wavelengths during the Archean of the fundamental molecules of life, to the creation and dissipation of organic pigments today at wavelengths up to the red edge (700 nm). Beyond the red edge, starting at about 1200 nm, water in the leaf or the ocean surface microlayer absorbs strongly and dissipates photons into heat efficiently. There is, therefore, still a wavelength region between approximately 700 and 1200 nm which remains to be conquered by future evolution of pigments.The simultaneous coupling of biotic with abiotic irreversible processes, such as the water cycle and ocean and air currents, culminating in an efficient global dissipating system known as the biosphere, increases further the efficacy of solar photon dissipation into the far infrared much beyond 1200 nm.
Empirical evidence for selection in nature towards states of increased photon dissipation exists on vastly different size and time scales. For example, the increase in photon absorption and dissipation efficacy of a plant leaf over its life-cycle [23], the proliferation of photon absorbing pigments over the entire surface of Earth, the correlation between ecosystem succession and increased dissipation [24] [25], and the general increase of biosphere efficacy in photon dissipation over evolutionary history, including, for example, the plant-induced increases in the water cycle [26][27] and animal dispersal of nutrients required for pigment synthesis [28]. There is also evidence for this at the microscopic scale, for example in the increased rates of energy dissipation per unit biomass of the living cell over its evolutionary history [29]. Evolutionary increases in dissipation occur even at the nanoscale, for example, the sequential increase in photon dissipation at each step along the dissipative synthesis of the nucleobases from common precursor molecules under a UVC photon potential [30].
Any planet around any star giving off light in the long wavelength UVC region, but with protection against shorter wavelength light, which could destroy carbon based molecules through successive ionization, should therefore harbor its own concentration profile of dissipatively structured carbon based fundamental molecules (UVC pigments) who’s characteristics would depend on the exact nature of the local UV environment and the primordial precursor and solvent molecules available. Examples may include the sulfur containing UV pigments found in the clouds of Venus [31], the UV absorbing thiophenes [32] and the red chlorophyll-like pigments [33] found on the surface of Mars, the UVC and UVB absorbing poly-aromatic hydrocarbons (PAHs) found in the atmosphere and on the surface of Titan [34], on the surface of asteroids, and in interstellar space [16]. The observation that thiophenes and PAHs found on mars, on asteroids, and in space are of generally large size, and increase in size with greater photo-processing,[35] can be understood from within this non-equilibrium thermodynamic perspective since, without the possibility of vibrational dissipation through hydrogen bonding to solvent molecules, these molecules would have “grown” to large sizes, through the same kind of thermodynamic dissipative selection presented in figure 2, giving it many low frequency vibrational modes which would increase dissipation by pushing the emitted photon energy towards the infrared.
Dissipative structuring, dissipative proliferation, and dissipative selection are the necessary and sufficient ingredients for an explanation in physical-chemical terms of the synthesis, proliferation, and evolution of organic molecules on planets, comets, asteroids, and interstellar space, and, in particular, for contributing to an understanding of the origin and evolution of life on Earth.
References
- Boltzmann, L.. The Second Law of Thermodynamics, in: Ludwig Boltzmann: Theoretical physics and Selected writings; McGinness, B., Eds.; D. Reidel: Dordrecht, Netherlands, 1974; pp. 1.
- Schrödinger, Erwin . What is Life? The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, 1944; pp. 1.
- Onsager, Lars; Reciprocal Relations in Irreversible Processes I and II. Phys. Rev. 1931, 37, 38, 405, 2265.
- Prigogine, I. . Introduction to the Thermodynamics of Irreversible Processes; Wiley: New York, 1967; pp. 1.
- K. Michaelian, Thermodynamic Origin of Life, Cornell ArXiv, (2009), arXiv:0907.0042v3 [physics.gen-ph]
- K. Michaelian; Thermodynamic dissipation theory for the origin of life. Earth System Dynamics 2011, 2, 37-51, 10.5194/esd-2-37-2011.
- Karo Michaelian; Microscopic dissipative structuring and proliferation at the origin of life. Heliyon 2017, 3, e00424, 10.1016/j.heliyon.2017.e00424.
- R.C. Dewar; D. Juretić; P. Županović; The functional design of the rotary enzyme ATP synthase is consistent with maximum entropy production. Chemical Physics Letters 2006, 430, 177-182, 10.1016/j.cplett.2006.08.095.
- Pornkamol Unrean; Friedrich Srienc; Metabolic networks evolve towards states of maximum entropy production. Metabolic Engineering 2011, 13, 666-673, 10.1016/j.ymben.2011.08.003.
- Zotin, A.I.. Bioenergetic trends of evolutionary progress of organisms", in: ''Thermodynamics and regulation of biological processes"; Lamprecht, I. and Zotin, A.I., Eds.; De Gruyter: Berlin, 1984; pp. 451–458.
- Eric D Schneider; James J Kay; Life as a manifestation of the second law of thermodynamics. Mathematical and Computer Modelling 1994, 19, 25-48, 10.1016/0895-7177(94)90188-0.
- K. Michaelian; Thermodynamic stability of ecosystems. Journal of Theoretical Biology 2005, 237, 323-335, 10.1016/j.jtbi.2005.04.019.
- K. Michaelian; HESS Opinions "Biological catalysis of the hydrological cycle: life's thermodynamic function". Hydrology and Earth System Sciences 2012, 16, 2629-2645, 10.5194/hess-16-2629-2012.
- Carl Sagan; Ultraviolet selection pressure on the earliest organisms. Journal of Theoretical Biology 1973, 39, 195-200, 10.1016/0022-5193(73)90216-6.
- Karo Michaelian; The Dissipative Photochemical Origin of Life: UVC Abiogenesis of Adenine. Preprints 2021, 0, 2021010500, 10.20944/preprints202101.0500.v1.
- K. Michaelian; A. Simeonov; Fundamental molecules of life are pigments which arose and co-evolved as a response to the thermodynamic imperative of dissipating the prevailing solar spectrum. Biogeosciences 2015, 12, 4913-4937, 10.5194/bg-12-4913-2015.
- Michael Yarus; Jeremy Joseph Widmann; Rob Knight; RNA–Amino Acid Binding: A Stereochemical Era for the Genetic Code. Journal of Molecular Evolution 2009, 69, 406-429, 10.1007/s00239-009-9270-1.
- Julián Mejía Morales; Karo Michaelian; Photon Dissipation as the Origin of Information Encoding in RNA and DNA. Entropy 2020, 22, 940, 10.3390/e22090940.
- K Michaelian; A non-linear irreversible thermodynamic perspective on organic pigment proliferation and biological evolution. Journal of Physics: Conference Series 2013, 475, 012010, 10.1088/1742-6596/475/1/012010.
- C. Doglioni; Johannes Pignatti; Max Coleman; Why did life develop on the surface of the Earth in the Cambrian?. Geoscience Frontiers 2016, 7, 865-873, 10.1016/j.gsf.2016.02.001.
- Karo Michaelian; Norberto Santillán Padilla; UVC photon-induced denaturing of DNA: A possible dissipative route to Archean enzyme-less replication. Heliyon 2019, 5, e01902, 10.1016/j.heliyon.2019.e01902.
- Karo Michaelian; Homochirality through Photon-Induced Denaturing of RNA/DNA at the Origin of Life. Life 2018, 8, 21, 10.3390/life8020021.
- Gates, D.M.; , 1980.. Biophysical Ecology; Springer-Verlag: New York, 1980; pp. 1.
- Ulanowicz, R. and Hannon, B.; Life and the production of entropy. Proc R Soc Lond B 1987, 232, 181-192.
- Schneider, E.D. and Kay, J.J; Complexity and thermodynamics: towards a new ecology. Futures 1994, 24, 626–647.
- Michaelian, K.. The biosphere: A thermodynamic imperative, in book "The Biosphere"; INTECH: New York, 2012; pp. 51.
- A. Kleidon; Entropy production by evapotranspiration and its geographic variation. Soil and Water Research 2008, 3, S89-S94, 10.17221/1192-swr.
- Michaelian, K.. Thermodynamic Dissipation Theory of the Origina and Evolution of Life: Salient characteristics of1216RNA and DNA and other fundamental molecules suggest an origin of life driven by UV-C light; CreateSpace: Mexico City, 2016; pp. 1.
- Zotin, A.I.. Bioenergetic trends of evolutionary progress of organisms. In Thermodynamics and regulation of biological proceses; Lamprecht, I. and Zotin, A.I., Eds.; De Gruyter: Berlin, 1984; pp. 451–458.
- Karo Michaelian; A Dissipative Photochemical Origin of Life: UVC Abiogenesis of Adenine. Preprints 2021, 1, 202101, 10.20944/preprints202101.0500.v1.
- Limaye, S.S.; Mogul, R.; Smith, D.J.; Ansari, A.H.; Słowik, G.P.; Vaishampayan, P.; Venus’1503Spectral Signatures and the Potential for Life in the Clouds. Asrtobiology 2018, 18, 1181–1198.
- Jacob Heinz; Dirk Schulze-Makuch; Thiophenes on Mars: Biotic or Abiotic Origin?. Astrobiology 2020, 20, 552-561, 10.1089/ast.2019.2139.
- Pershin, S. Correlation of “chlorophyll” and water index on mars surface. Microsymposium 36, MS079,15082002.
- M. López-Puertas; B. M. Dinelli; A. Adriani; B. Funke; M. García-Comas; Maria Luisa Moriconi; E. D’Aversa; C. Boersma; Louis J Allamandola; LARGE ABUNDANCES OF POLYCYCLIC AROMATIC HYDROCARBONS IN TITAN'S UPPER ATMOSPHERE. The Astrophysical Journal 2013, 770, 132, 10.1088/0004-637x/770/2/132.
- J. Bouwman; Pablo Castellanos; Michał Bulak; Jeroen Terwisscha Van Scheltinga; Jan Cami; Harold Linnartz; A. G. G. M. Tielens; Effect of molecular structure on the infrared signatures of astronomically relevant PAHs. Astronomy & Astrophysics 2019, 621, A80, 10.1051/0004-6361/201834130.