The brain-gut-microbiota axis is a bidirectional system enabling gut microorgan-isms to communicate with the central nervous system (CNS), and the CNS with the gut . The mechanisms of signal transmission are complex and not fully understood, but include neural, endocrine, immune and metabolic pathways.
- gut microbiota
- gut microbiome
- gut-brain axis
- functional bowel disorders
- irritable bowel syn-drome
- antibiotics
- probiotics
1. Introduction
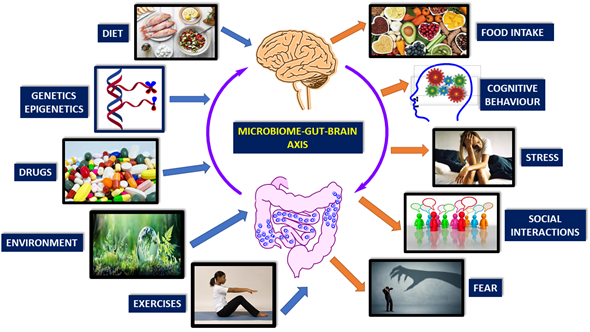
There are many factors affecting microbiome-gut-brain-axis. These are diet, genetics, drugs, environment, exercise, cognitive behavior, stress, social interactions, and fear (Figure 1) [1][2][3][4][5][6][7][8][9][20–28].
Gut microbes are capable of producing most neurotransmitters found in the human brain. While these neurotransmitters primarily act locally in the gut, modulating the enteric nervous system, there is also undeniable evidence indicating that gut microbes can influence CNS through multiple mechanisms. The treatment with probiotic Bifidobacteria for instance, can increase the amount of tryptophan, the precursor of serotonin [10][29]. Some Lactobacilli species alter gamma-aminobutyric acid (GABA) metabolism and change brain GABA receptor expression and behavior [11][30]. Preclinical studies show that the vagus nerve is the main route for exerting the effects of gut microbiota on CNS. Lactobacillus rhamnosus has a central effect in animals and this was ameliorated by vagotomy [12][31]. In fact, patients with a history of vagotomy have diminished risk for certain neurological diseases [13][32]. Synthesis and release of neurotransmitters from bacteria have been reported: Lactobacillus and Bifidobacterium species can produce GABA; Escherichia, Bacillus and Saccharomyces spp. can produce noradrenaline; Candida, Streptococcus, Escherichia and Enterococcus spp. can produce serotonin; Bacillus can produce dopamine; Lactobacillus can produce acetylcholine [14][15][16][33–35]. Although these neurotransmitters can cross inflamed intestinal mucosal barrier, they cannot cross blood–brain-barrier (BBB) in healthy conditions. Another way of gut-brain interaction is the stimulation of hypothalamic-pituitary-adrenal (HPA) axis, which induces cortisol secretion. This system is the main stressor system in the body and it is mainly regulated by gut-HPA axis [17][18][19][20][21][22][23][24][25][26][27][28][36–47]. Psychological or physical stress can affect HPA axis and subsequently gut microbiota/barrier function (e.g., IBS) [29][33].
Post-infectious IBS is a prototype for gut-brain axis disorders. Water-born gastroenteritis outbreak occurred in United States and people affected from this E.coli infection later developed IBS-like symptoms including co-morbid depression, anxiety disorder [30][48].
Figure 1. Brain-gut-microbiome axis is a dynamic, interactive network. Factors affecting the communication of these elements are mainly genetics, diet, and lifestyle (Modified from reference [31][8]).
2. Pathways of Communication
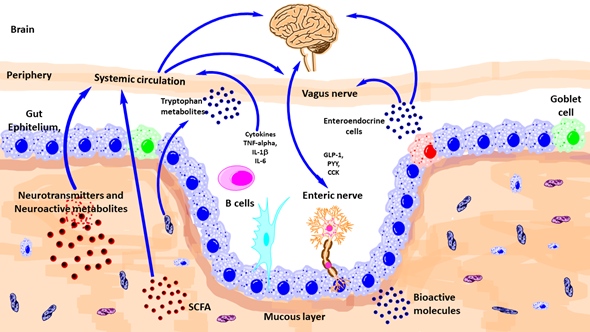
There are diverse ways of communication between gut microbiota and brain such as autonomic nervous system, vagus nerve, enteric nervous system, neurotransmitters, and immune system (Figure 2).
Figure 2. Schematic outlining the various known pathways of communication between the gut-microbiota and the brain. CCK, Cholecystokinin; GLp-1, glucagon-like peptide-1; IL, interleukin; PYY, peptide YY; TNF, tumor necrosis factor; SCFA, short-chain fatty acid. (Modified from reference [31][8]).
2.1. Autonomic Nervous System (ANS)
The autonomic nervous system (ANS) comprises the sympathetic and parasympathetic branches. Combined with activity from the enteric nervous system (ENS) and influenced by the CNS, the ANS is responsible for physiological homeostasis, as well as responding to endocrine, motor, autonomic, and behavioral areas. Gut microbiota communicate with ANS bidirectional both antagonistic and synergistically [32][33][49,50]. Afferent nerves carry information from visceral organs to CNS and from CNS, important survival messages sent towards peripheral organs. ANS acts as the most immediate responder in health and disease states [34][35][36][37][38][39][40][41][51–58]. Local GI autonomic activation can be stimulated by afferent feedback loops from the microbiota and CNS efferent modulation [42][59]. Microbiota-related metabolites such as tryptophan (and end products, e.g., serotonin-5HT), GABA, catecholamine’s mediate ANS related effects. Sympathetic innervation has post-ganglionic vasoconstrictor effects and also suppressive effects on gut secretions and motility. Intestinal mucus layer is regulated by sympathetic innervation, by modulating mucosal immune system, microbial composition, and function [43][52,54,60].
2.2. Vagus Nerve
It is the tenth cranial nerve and the longest in the body with extensive connections and networks with peripheral organs. The vagus exerts anti-inflammatory actions via medullary dorsal motor nucleus. The vagal modulation of macrophage action an important factor for the inflammation in inflammatory bowel disease (IBD) [44][53].
2.3. Enteric Nervous System (ENS)
At the interface of microbiota and host, there is a network of gut neurons called ENS. Anatomically divided as submucosal and myenteric plexus, ENS regulates gut motility and secretions [45][61]. Factors affecting neurodevelopment and health status of CNS may also affect ENS integrity. Gut microbiota influences development and function of ENS via pattern recognition receptors (PRR) and including Toll-like receptors (TLRs), especially TLR-2 and TLR-4. These TLRs are involved in the recognition of microbial molecules [46][62]. Bacteroides fragilis and the capsular exopolysaccharide, are good examples, which can influence ENS function [47][63]. L. rhamnosus strain (JB-1) performs this action via a G protein-coupled receptor-mediated pathway [31]. Recent data showed that stress-induced alterations in ENS activity, via stimulated acetylcholine release, were influenced by both maternal separation and the microbiota [48][64]. This proves that the microbiota may affect ENS-related gut dysfunction associated with early-life stress. ENS abnormalities are associated with life-threatening GI disorders including Hirsch sprung disease and chronic intestinal pseudo-obstruction [49][65]. Moreover, the ENS is also involved in disorders of the CNS, including ASD, Alzheimer’s disease (AD), and Parkinson’s disease (PD) [50][66].
2.4. Immune System
Gastrointestinal tract has the highest number of immune cells in the body and there is a delicate, complex communication with the gut microbiota [51][67]. Mucus produced by epithelial Goblet cells provide a barrier against contact with host cells and microbial elements. The gut microbiota influences regulation of subsets of immune cells including T helper (Th), T regulatory (Treg), natural killer (NK), mononuclear phagocytes, and innate lymphoid cells [52][68]. The mechanisms by which the gut microbiota influences innate and adaptive responses during health and disease is still being investigated.
Environmental factors affect immune function. The release of cytokines promotes the activation and recruitment of eosinophils, B cells, and mast cells. In addition to the traditional T-helper 2 pathway, secretion of IL-23 from antigen-presenting cells, such as dendritic cells, B cells, and macrophages, promotes T helper-cell 17 differentiation. Degranulation of these cells disrupt intestinal barrier and enteric nerves. This results in visceral hypersensitivity and dysmotility [53][19]. α4β7 gut homing T cells are a marker of intestinal inflammation in both functional dyspepsia and irritable bowel syndrome. The site and extend of intestinal immune activation can define the phenotype such as functional heartburn, functional dyspepsia, irritable bowel syndrome, functional constipation, or functional diarrhea [53][19] (Figure 3).
Figure 3. Mucosal immune system activation and functional gastrointestinal disorders. [53](Adapted from reference 19). CRF, corticotrophin-releasing factor; ACTH, adrenocorticotropic hormone; Ig, immunoglobulin; IL, interleukin; TH, T-helper cell.