Skin biopsy is a minimally invasive technique with the advantage, compared to sural nerve biopsy, of being suitable to be applied to any site in our body, of being repeatable over time, of allowing the identification of each population of nerve fiber through its target. In patients with symptoms and signs of small fiber neuropathy the assessment of IntraEpidermal Nerve Fiber density is the gold standard to confirm the diagnosis while the quantification of sudomotor, pilomotor, and vasomotor nerve fibers allows to evaluate and characterize the autonomic involvement. All these parameters can be re-evaluated over time to monitor the disease process and to evaluate the effectiveness of the treatments. Myelinated fibers and their receptors can also be evaluated to detect a “"dying back”" neuropathy early when nerve conduction study is still normal. Furthermore, the morphometry of dermal myelinated fibers has provided new insight into pathophysiological mechanisms of different types of inherited and acquired large fibers neuropathies. In genetic neuropathies skin biopsy has become a surrogate for sural nerve biopsy, no longer necessary in the diagnostic process, to study genotype–phenotype correlations.
- skin biopsy
- small fiber neuropathy
- autonomic neuropathy
- large fiber neuropathy
1. Introduction
In the last three decades, the analysis of cutaneous nerves sampled by means of 3 mm punch biopsy has provided an important contribution to our knowledge of peripheral sensory and autonomic neuropathies [1,2][1][2].
This because the skin includes a rich network of sensory nerves originating from the dorsal root ganglia and autonomic nerves originating from the postganglionic sympathetic endings.
The skin shares with the CNS the embryologic origin from the same layer and represents, beside a physical, chemical, and immunological barrier for the entire body surface, our interface with the outside. Due to the close relationship of epidermal nerves with keratinocytes and the presence of common sensory receptors, the epidermis can be considered as a huge sensory organ able to inform our brain on what is happening outside our body and how comfortable, or not, we feel with environmental changes. This implies a continuous flow of information from the periphery to the sensory brain cortex but also to limbic areas through the insula (interoception) and then to the central autonomic network that through the cutaneous autonomic nerves puts in place emotional and homeostatic responses to outer and inner changes.
2. Cutaneous Sensory and Autonomic Endings
2.1. Intraepidermal Nerve Fibers (IENFs)
First described by Paul Langerhans [3] and later by Arthur and Shelly [4] and Cauna [5], epidermal nerve fibers could be finally reliably and unequivocally demonstrated using the pan-neuronal marker protein gene product 9.5 (PGP 9.5) [6]. Thanks to PGP 9.5 antibodies, it has been possible to visualize in skin samples nerve bundles that from the subepidermal neural plexuses reach and penetrate the basement membrane, running with an ascending course between the keratinocytes. IENFs innervate the entire epidermis until the stratum corneum as naked axons, since they lose their Schwann cells ensheathment at the dermal–epidermal junction (Figure 1A). They are vertically oriented and may have different patterns [7]. Few of these fibers are immunoreactive (ir) to Sub P and CGRP.
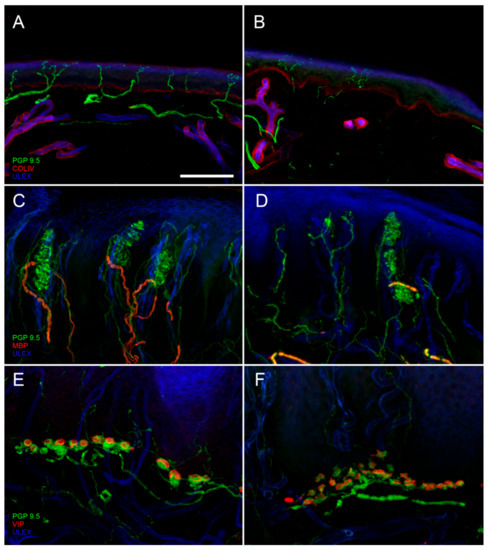
Digital confocal images from hairy (
,
) and glabrous skin (
–
) showing epidermal and dermal innervation in a healthy control (
,
,
) and in a patient with diabetic neuropathy (
,
,
). In the patient skin, a loss of Intraepidermal Nerve Fibers (IENF) is evident ((
) compared to (
)) as well as a loss of Meissner corpuscles and their myelinated afferences associated to morphological abnormalities of the surviving structures ((
) compared to (
)). Moreover, morphological abnormalities of a Merkel complex (axonal swelling and fragmentation) are present ((
) compared to (
)). In green: nerves marked with the pan-neuronal protein gene product 9.5 (PGP9.5) antibody. In blue: endothelia and epidermis marked with ULEX europaeus. In red: basement membrane and blood vessels marked with Collagen IV (Col IV) antibody in (
) and (
); myelined fibers marked with myelin basic protein (MBP) antibody in (
) and (
); Merkel cells marked with vasoactive intestinal peptide (VIP) antibody in (
) and (
). Scale bar: 100 µm in (
–
); 50 µm in (
,
).
IENFs are the last endings of C and A-delta fibers originating from dorsal root ganglia small neurons and mostly involved in the perception of pain and thermal stimuli. IENFs can be sub-divided into peptidergic and non-peptidergic fibers according to the expression of peptides [8]. Physiologically, peptidergic and non-peptidergic fibers terminate in different epidermal layers and have different projection patterns to the spinal cord [9].
IENFs can be found in any site of our body with higher density in proximal sites. Physiologically, the IENF density at distal leg is approximately 60% of the IENF density at thigh [10]. A reduction of IENFs density (number of fibers per linear mm) at distal leg (Figure 1B) is the hallmark to diagnose small fiber neuropathy (SFN) [11]. The advantages of applying skin biopsy compared to sural nerve analysis are the higher sensibility [12], the minimal invasiveness and then the possibility to perform it in any body site and to repeat it over time.
Two different methods of cutaneous nerves visualization are available, indirect immunofluorescence [6,13][6][13] with and without confocal analysis and bright-field immunohistochemistry [14]. For both techniques age and sex stratified normative datasets are available [10,15][10][15].
Moreover, IENFs can be analyzed using a bloodless and painless method, the skin blister [16]. Applying a capsula with a negative pressure on the skin, the epidermis separates from dermis and a blister will be obtained. The blister roof, composed only of epidermis, can be removed without damaging basement membrane and vessels. The whole sample then, formed by keratinocytes and IENFs can be processed to quantify nerve fibers with results comparable with skin biopsy [17].
2.2. Receptors and Myelinated Endings
In addition to free nerve endings, the skin is rich of corpusculated mechanoreceptors that are innervated by A-beta endings, provided, or not, with a capsula and able to respond with different modalities (rapidly or slowly adapting, respectively) to different kinds of stimuli. They are differently distributed in the skin and some of them are exclusively present in the hairy or glabrous skin (Meissner corpuscles, MC).
In hairy skin, hair follicle represents the main mechanoreceptor. This structure is innervated by several myelinated endings that with ascending vertical course reach the follicle, lose the myelin sheath, and envelop it constituting the characteristic “"palisade endings”".
In glabrous skin, MC are uniformly distributed at the apex of dermal papillae (Figure 1C). They are provided with a capsula formed by lamellar Schwann-like cells. Their myelinated afferences, usually in a number of 1–2, reach the corpuscle, leave the myelin sheath, and undergo several branchings, with neural expansions filling the spaces inside the capsula. MCs are rapidly adapting mechanoreceptors, responsible for the high tactile acuity of the fingertip where they are mostly concentrated. The assessment of their density in the skin represents a useful measure of large fiber involvement (Figure 1D) [18].
Merkel complexes (MK) are non-capsulated, slowly adapting mechanoreceptors, distributed at the base of dermal papillae predominately in glabrous skin (Figure 1E). Their myelinated afference, in its final route, assumes a typical sigmoidal aspect. After losing its myelin sheath, it gives off several branches, ending with neural expansions with different shapes and sizes. These expansions make synapse-like contact with vasoactive intestinal peptide (VIP)-ir Merkel cells present among keratinocytes of the basal layer of the epidermis. These cells display features of sensory receptors as they are excited by mechanical stimuli and drive neural depolarization [19]. Abnormalities of the MK structure can be appreciated in case of sensory neuropathy (Figure 1F).
Pacini, Ruffini, and Krause capsula are other mechanoreceptors that are more unevenly distributed in the dermis therefore their presence in skin samples may be occasional. They are different for function and morphology, but constituted by the large expansions of neural endings originating from a large myelinated fiber after losing the myelin and branching.
Using specific markers, it is possible to study the myelinated fibers direct to the mechanoreceptors (A-beta fibers), to assess their density in the papillary dermis and their morphometry (caliber, internode and node length, and paranodal–nodal architecture). These population has a mean caliber of 3.3 µm and an internodal length around 80 µm [18,20][18][20]. This marked reduction in diameter and internodal length, compared to parental fibers in the nerve [21], may be due to a repeated branching of A-beta fibers when they reach the skin. Reduced density and morphological abnormalities of dermal myelinated fibers can be found in peripheral neuropathy (Figure 1D).
2.3. Autonomic Innervation
Sudomotor nerves: Sweat glands present a dense PGP-ir network with fibers heavily encircling the sweat tubules (Figure 2A). Most of these fibers is VIP-ir, while some of them are CGRP-ir. VIP is co-expressed with cholinergic fibers and therefore it is used as surrogate cholinergic marker of sudomotor nerves. For routine purpose, sudomotor nerves can be assessed using a semiquantitative method, whereas for research different methods of quantification are available [22,23][22][23]. A loss of sudomotor nerves (Figure 2B) indicate a post-ganglionic damage of sudomotor pathway and correlates with sweating impairment.

Confocal digital images showing a loss of cutaneous autonomic nerves in a diabetic neuropathy patient (
,
,
) compared to a healthy control (
,
,
). In patient skin it is evident a loss of nerves around a sweat gland ((
) compared to (
)), along an arrector pili muscle ((
) compared to (
)) and around an arterio-venous anastomosis ((
) compared to (
)). In green: nerves marked with the pan-neuronal marker, protein gene product 9.5 (PGP 9.5) antibody. In red: basal membranes and vessels marked with Collagen IV antibody. In blue: endothelia marked with ULEX europaeus (
–
). Scale bar: 100 µm.
Pilomotor nerves: Arrector pili muscle is a small band of smooth muscle fibers connecting hair follicle to the basement membrane, with mostly vestigial thermoregulation role. Pilomotor muscle is innervated by nerve fibers that go parallel to the muscle fibers direction (Figure 2C). These fibers are prevalently noradrenergic, dopamine beta hydroxylase (DβH)-ir. However, cholinergic VIP-ir fibers are also present. Semi-quantitative and quantitative methods are available to assess the pilomotor nerve fibers [24]. A reduction of pilomotor nerve density (Figure 2D) can be observed in autonomic neuropathies.
Vasomotor nerves: Arteriovenous anastomosis (AVA) is a complex structure, predominately observed in glabrous skin, constituted by vessel segments that connect small arteries with small veins. These segments are rounded by a thick muscular wall that has the role to direct the cutaneous blood flow allowing or by-passing the capillary circulation, with the main role to regulate thermo-dispersion. Heavy innervation is present surrounding the structure (Figure 2E), whereas it is less abundant around the feeding artery and sparse around the draining vein. The abundant noradrenergic (DβH-ir) innervation around the lumen, suggests its role in blood flow regulation, whereas the cholinergic innervation (VIP-ir) forms a less dense network with a possible role in vasodilation. Interesting, a further CGRP- and SubP-ir component is also present, suggesting a sensitive role as well [25]. In patients with autonomic impairment sparsely innervated AVAs can be observed (Figure 2F).
3. Skin Biopsy in Small Fiber Neuropathies
3.1. Clinical Presentation of Small Fiber Neuropathy and the Role of Skin Biopsy
In patients affected by SFN usually both sensory and autonomic components of small fibers are affected, but sensory symptoms and particularly pain, are those which more frequently lead patients to seek for medical advice.
Sensory complaints are mainly represented by spontaneous pain that usually assumes a burning connotation. However, prickling, itching, or shock-like sensation may also coexist. Evoked and uncomfortable sensations, such as hyperalgesia and allodynia, and negative symptoms, such as a reduced sensitivity to thermal and noxious mechanical stimuli, may be part of the clinical picture.
Symptoms of SFN, are usually distal symmetrical, starting at distal lower limbs and progressing in a distal to proximal fashion. This progression leads to the sequential involvement of feet, ankles, and knees before involving distal upper arms and reveals a length dependent dying-back pathological mechanism affecting the nerve fibers. Sometimes symptoms have a patchy distribution, involving proximal sites such as head, trunk, or mouth. This different distribution reveals a non-length dependent pathological mechanism due to the involvement of the sensory neuron rather than its fiber.
The diagnosis of SFN can be suspected based on the presence of characteristic symptoms and signs. According to previously published criteria [26], diagnostic confirmation can be reached by demonstrating abnormalities at quantitative sensory testing. This test, however, is by its nature a psychophysical test, and therefore dependent on the collaboration of the subject. It is recommended for screening and monitoring purposes but less suitable as a stand-alone test [27]. Quantitative sensory testing, as well as additional tests, such as laser-evoked potentials [28], contact heat evoked potentials [29], Nociceptive Flexion Reflex [30], Cutaneous Silent Period [31], ultrasonography [32], and ultrasound assisted microneurography [33] can are useful tools to study SFN [34], but, according to current guidelines, the loss of IENFs represents the morphological hallmark and the only objective test that allows to confirm a suspect of SFN.
In addition to IENF loss, some morphological changes, such as varicosity [35] and abnormalities in the distribution of nerves in the epidermis [36], may predict fibers loss. Moreover, skin biopsy can help to differentiate length dependent neuropathy from non-length dependent ganglionopathy by assessing the IENF density ratio between distal and proximal site of the lower limb [37]. This feature may guide clinicians in the SFN screening; for example, a length-dependent SFN points toward a toxic-dysmetabolic etiology, while SFN ganglionopathy suggests a dysimmune etiology.
Pain is the symptom that characterizes SFN. However, it is not possible to discriminate between patients with painful and painless SFN based on their IENF density [38].
Contradictory results have been described using other morphological features such as fibers length [39], axonal swellings [40], or the presence of GAP43-ir regenerating fibers [41]. Recently, an increase density of dermal peptidergic fibers (CGRP- and SubP-ir) has been described in patients with painful compared to painless diabetic SFN [42].
3.2. Causes of Small Fiber Neuropathy and the Contribution of Skin Biopsy
The commonest cause of SFN is diabetes [43]. Patients with diabetes have been among the first populations of patients in which a reduced density of IENFs was demonstrated [44]. IENF loss in diabetes is usually length dependent and is associated to a sensory dysfunction as assessed by quantitative sensory testing [45], although the loss of nerve fibers poorly correlates with the increase of sensory thresholds, regardless of the sensory modality taken into account [46]. Skin biopsy represents a useful tool to monitor the progression of the diabetic SFN. In fact, an impairment of IENFs is evident since the earliest stages of the disease. The loss of fibers correlates with the severity of the disease [47,48][47][48]. However, an SFN can manifest in patients with impaired glucose tolerance as well as in early diabetes [49].
In the last three decades, skin biopsy contributed to demonstrate small fibers involvement in an increasing number of pathologies (Table 1). In 30–50% of patients with SFN, it is not possible to identify a cause and the condition remains idiopathic [50]. In the last years, a proportion of cases of idiopathic SFN was associated with mutations in gene encoding subunits of voltage-gated sodium channels (Nav1.7, Nav.18, Nav1.9) [51,52,53][51][52][53]. These genetic SFN channelopathies are characterized by gain of function mutation that alters excitability of sensory fibers and causes pain. These disorders, although very rare, have provided insight into the perception of pain as well as the identification of new therapeutic targets.
Table 1. Causes of small fiber neuropathy (SFN).
|
Immuno-Mediated Disorders |
Toxic and Drugs |
|
Systemic lupus erythematosus* |
Antibiotics (metronidazole, nitrofurantoin, linezolid, fluoroquinolones) |
|
Rheumatoid arthritis* |
Chemotherapy (vinca, taxanes, bortezomib) |
|
Sjögren’s syndrome* |
Other drugs (flecainide, antiretroviral HIV, colchicine, statin) |
|
Sarcoidosis |
Alcohol abuse |
|
Vasculitis* |
Vitamin B6 toxicity |
|
Inflammatory bowel disease |
Hematologic causes |
|
Paraneoplastic |
Monoclonal gammopathy* |
|
Celiac disease* |
Hereditary |
|
Metabolic and endocrine disorders |
Nav channelopathies |
|
Diabetes mellitus and glucose intolerance* |
Familial amyloid polyneuropathy |
|
Dysthyroidism* |
Fabry’s disease |
|
Vitamin B12 and folate deficiency* |
Tangier’s disease |
|
Dyslipidemia* |
Ehlers–Danlos syndrome |
|
Chronic kidney disease* |
Familial neuropathic chronic itch |
|
Infections |
|
|
HIV |
|
|
Hepatitis B |
|
|
Hepatitis C* |
|
|
Lyme disease |
|
|
Leprosy |
|
|
Systemic lupus erythematosus* |
|
|
Rheumatoid arthritis* |
|
|
Sjögren’s syndrome* |
|
|
Sarcoidosis |
|
|
Vasculitis* |
|
|
Inflammatory bowel disease |
|
|
Paraneoplastic |
|
|
Celiac disease* |
|
|
Metabolic and endocrine disorders |
|
|
Diabetes mellitus and glucose intolerance* |
|
|
Dysthyroidism* |
|
|
Vitamin B12 and folate deficiency* |
|
|
Dyslipidemia* |
|
|
Chronic kidney disease* |
* Causes that should be included in a first level screening.
Finally, growing evidence revealed a small nerve involvement in fibromyalgia, classically considered as prevalently central sensory processing disorder. A recent metanalysis showed that 49% of fibromyalgia patients presented a loss of IENFs, often described as non-length dependent [54]. This morphological evidence is further corroborated by functional studies that through microneurography demonstrated an abnormal ongoing activity of peripheral C nociceptors and increased mechanical sensitivity in fibromyalgia patients compared to controls [55]. The identification of SFN in fibromyalgia has several implications on pathogenetic, diagnostic and therapeutic aspects: (1) it shifted from central toward peripheral neuropathic pain condition; (2) it raised the question if all fibromyalgia patients need to be screened for SFN, including skin biopsy; (3) it opened the way to new potential treatments; for example, based on a possible dysimmune etiology, IVIg proved to be efficient with improvement of both symptoms and morphology [56].
3.3. Skin Biopsy as an Outcome Measure in Small Fiber Neuropathy
Interestingly, repeated skin biopsy over time can also be used to assess the efficacy of pharmacological and non-pharmacological treatments. In fact, a lifestyle change, including moderate physical activity and diet, showed to normalize IENF density in patients with SFN associated to impaired glucose tolerance [57]. Similarly, hormone replacement therapy has been shown to be effective for the recovery of SFN associated to hypothyroidism [58].
4. Skin Biopsy in Autonomic Neuropathy
Autonomic neuropathy is a disorder characterized by the selective or prevalent involvement of autonomic nerves. The onset may be acute, subacute, or chronic and from the clinical point of view it may manifest with symptoms due to the dysfunction of all domains (sudomotor, pupillomotor, gastrointestinal, genitourinary, and cardiovascular) or may be limited to only one or few of them. Abnormal pupillary reflexes, dry skin, dry mouth, dry eyes, reduced orthostatic tolerance, orthostatic hypotension, reduced gastrointestinal motility, slowing of urinary flow, and impotence may be the main signs of autonomic disfunction. The main tools available to study autonomic function are the cardiovascular reflexes with the assessment of changes in blood pressure and heart rate variability, and the cutaneous sudomotor and vasomotor function by means of Thermoregulatory Sweat test (TST), Sympathetic Skin Response (SSR), Quantitative Sudomotor Axon Reflex Test (QSART), Dynamic Sweat Test (DST), microneurography, and laser doppler flowmetry [59]. While TST [60] and SSR [61] explore the entire polysynaptic sudomotor pathway, QSART [62] and DST [63] are quantitative methods to assess postganglionic sudomotor function after pharmacological stimulation (acetylcholine or pilocarpine) by iontophoresis.
Morphological evidence of the postganglionic sudomotor damage may be obtained with the analysis of autonomic nerves in skin biopsy. This is useful since it provides information on the extent of autonomic involvement underlying autonomic dysfunction in patients with SFN. Moreover, it may allow to monitor over time such involvement through repeated biopsies.
5. Skin Biopsy in Large Fibers Neuropathies (LFN)
Although skin biopsy represents an important tool in SFN, it provided an important contribution also in LFN. It is well-known that nerve conduction study is the first-line assessment in LFN. However, electrical stimuli do not allow to explore the very distal endings of the sensory pathway (receptors and their myelinated afferences). This distal segment, precocious involved in dying-back neuropathy, is bypassed by standard electrodiagnostic test [80][64]. Instead, skin biopsy allows to visualize early abnormalities of receptors and their myelinated fibers. Moreover, this approach replaces the more invasive sural nerve biopsy [81][65] in the study of nodal and paranodal architecture and morphometry of myelinated fibers (Figure 3A,B), widening knowledge about disease phenotypes and pathomechanisms.
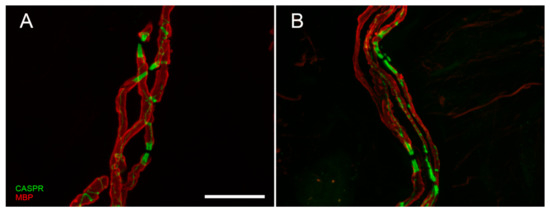
Confocal digital images of myelinated nerve fiber bundles in a control (
) and a diabetic patient (
). The double staining with anti-myelin basic protein (MBP, in red) and anti-contactin associated protein (CASPR, in green) allows to appreciate the nodal and paranodal architecture that is regular and symmetric in normal conditions, asymmetric and deranged in case of peripheral neuropathy. Scale bar: 20 µm.
Lastly, skin biopsy allowed to demonstrated small fiber involvement in LFN patients who may developed symptoms and signs of small fiber dysfunction, often overlooked when large fiber impairment is documented.
6. Highlights
1. Skin biopsy is a minimally invasive technique, repeatable over time and applicable in any part of our body.
2. It allows to reach a definite diagnosis of SFN.
3. It allows to study all nerve fibers population, according to their targets and their immuno‐histochemical characteristics.
4. It allows to study receptors and their afferences, morphometry, and nodal/paranodal architecture of myelinated fibers.
5. In the last decades, it allows to recognize a small fiber involvement in several hereditary and acquired conditions.
6. It is allowing to widen the clinical spectrum of peripheral neuropathy and provide new insight on the pathophysiological mechanisms.
7. It allows to monitor disease progression and to assess efficacy of pharmacological and non‐pharmacological treatments.
References
- Kennedy WR, Wendelschafer-Crabb G, Polydefkis MG and Wendelschafer G. Pathology and quantitation of cutaneous nerves. In: PJ Dyck, PK Thomas (Eds.), Peripheral Neuropathy. 4th edn (2005). Saunders, Philadelphia, pp. 869–896.
- Mellgren SI, Nolano M, Sommer C. The cutaneous nerve biopsy: technical aspects, indications, and contribution. Handb Clin Neurol. 2013;115:171-88.
- Langerhans P. Uber die Nerven der menschlichen Haut. Virchow’s Arch. 1868;44:325-337
- Arthur RP, Shelley WB. The innervation of human epidermis. J Invest Dermatol. 1959;32:397-411
- Cauna N. The free penicillate nerve endings of the human hairy skin. J Anat. 1973;115:277-288
- Wang L, Hilliges M, Jernberg T, Wiegleb-Edström D, Johansson O. Protein gene product 9.5-immunoreactive nerve fibres and cells in human skin. Cell Tissue Res. 1990;261:25-33.
- Wendelschafer-Crabb G, Kennedy WR, Walk D. Morphological features of nerves in skin biopsies. J Neurol Sci. 2006;242:15–21.
- Zylka MJ. Nonpeptidergic circuits feel your pain. Neuron. 2005;47:771–2.
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84.
- Provitera V, Gibbons CH, Wendelschafer-Crabb G, Donadio V, Vitale DF, Stancanelli A, Caporaso G, Liguori R, Wang N, Santoro L, Kennedy WR, Nolano M. A multi-center, multinational age- and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur J Neurol. 2016 Feb;23:333-8.
- Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, Sommer C, Valls-Solé J; European Federation of Neurological Societies; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. J Peripher Nerv Syst. 2010;15:79-92.
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634-40.
- Kennedy WR, Wendelschafer-Crabb G. The innervation of human epidermis. J Neurol Sci. 1993;115:184-90.
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848-55.
- Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, Smith AG, Hsieh ST, Mellgren SI, Umapathi T, Ziegler D, Faber CG, Merkies IS. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010;15:202-7.
- Kennedy WR, Nolano M, Wendelschafer-Crabb G, Johnson TL, Tamura E. A skin blister method to study epidermal nerves in peripheral nerve disease. Muscle Nerve. 1999;22:360-71.
- Panoutsopoulou IG, Wendelschafer-Crabb G, Hodges JS, Kennedy WR. Skin blister and skin biopsy to quantify epidermal nerves: a comparative study. Neurology. 2009;72:1205-10.
- Nolano M, Provitera V, Crisci C, Stancanelli A, Wendelschafer-Crabb G, Kennedy WR, Santoro L. Quantification of myelinated endings and mechanoreceptors in human digital skin. Ann Neurol. 2003;54:197-205.
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509:617-21.
- Provitera V, Nolano M, Pagano A, Caporaso G, Stancanelli A, Santoro L. Myelinated nerve endings in human skin. Muscle Nerve. 2007;35:767-75.
- Caruso G, Massini R, Crisci C, Nilsson J, Catalano A, Santoro L, Battaglia F, Crispi F, Nolano M. The relationship between electrophysiological findings, upper limb growth and histological features of median and ulnar nerves in man. Brain. 1992;115:1925-45.
- Gibbons CH, Illigens BM, Wang N, Freeman R. Quantification of sudomotor innervation: a comparison of three methods. Muscle Nerve. 2010;42:112-9.
- Provitera V, Nolano M, Caporaso G, Stancanelli A, Manganelli F, Iodice R, Selim MM, De Rosa A, Lanzillo B, Pellecchia MT, De Michele G, Santoro L. Postganglionic sudomotor denervation in patients with multiple system atrophy. Neurology. 2014 Jun 17;82:2223-9.
- Donadio V, Nolano M, Provitera V, Stancanelli A, Lullo F, Liguori R, Santoro L. Skin sympathetic adrenergic innervation: an immunofluorescence confocal study. Ann Neurol. 2006;59:376-81.
- Nolano M, Provitera V, Caporaso G, Stancanelli A, Vitale DF, Santoro L. Quantification of pilomotor nerves: a new tool to evaluate autonomic involvement in diabetes. Neurology. 2010 Sep 21;75(12):1089-97.
- Devigili G, Rinaldo S, Lombardi R, Cazzato D, Marchi M, Salvi E, Eleopra R, Lauria G. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain. 2019;142:3728-3736.
- Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH, Hansson P, Hatem SM, Krumova EK, Jensen TS, Maier C, Mick G, Rice AS, Rolke R, Treede RD, Serra J, Toelle T, Tugnoli V, Walk D, Walalce MS, Ware M, Yarnitsky D, Ziegler D. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154:1807-19.
- Lefaucheur JP, Wahab A, Planté-Bordeneuve V, Sène D, Ménard-Lefaucheur I, Rouie D, Tebbal D, Salhi H, Créange A, Zouari H, Ng Wing Tin S. Diagnosis of small fiber neuropathy: A comparative study of five neurophysiological tests. Neurophysiol Clin. 2015;45:445-55.
- Lagerburg V, Bakkers M, Bouwhuis A, Hoeijmakers JG, Smit AM, Van Den Berg SJ, Hordijk-De Boer I, Brouwer-Van Der Lee MD, Kranendonk D, Reulen JP, Faber CG, Merkies IS. Contact heat evoked potentials: normal values and use in small-fiber neuropathy. Muscle Nerve. 2015;51:743-9.
- Skljarevski V, Ramadan NM. The nociceptive flexion reflex in humans - review article. Pain. 2002;96:3-8.
- Svilpauskaite J, Truffert A, Vaiciene N, Magistris MR. Electrophysiology of small peripheral nerve fibers in man. A study using the cutaneous silent period. Medicina (Kaunas). 2006;42:300-13.
- Ebadi H, Siddiqui H, Ebadi S, Ngo M, Breiner A, Bril V. Peripheral Nerve Ultrasound in Small Fiber Polyneuropathy. Ultrasound Med Biol. 2015;41:2820-6.
- Dunham JP, Sales AC, Pickering AE. Ultrasound-guided, open-source microneurography: Approaches to improve recordings from peripheral nerves in man. Clin Neurophysiol. 2018 Nov;129(11):2475-2481.
- Chiaramonte R, Romano M, Vecchio M. A Systematic Review of the Diagnostic Methods of Small Fiber Neuropathies in Rehabilitation. Diagnostics (Basel). 2020 Aug 20;10(9):613.
- Lauria G, Morbin M, Lombardi R, Borgna M, Mazzoleni G, Sghirlanzoni A, Pareyson D. Axonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathies. Neurology. 2003;61:631-6.
- Piscosquito G, Provitera V, Mozzillo S, Caporaso G, Borreca I, Stancanelli A, Manganelli F, Santoro L, Nolano M. The analysis of epidermal nerve fibre spatial distribution improves the diagnostic yield of skin biopsy. Neuropathol Appl Neurobiol. 2020 Aug 4. doi: 10.1111/nan.12651. Epub ahead of print.
- Provitera V, Gibbons CH, Wendelschafer-Crabb G, Donadio V, Vitale DF, Loavenbruck A, Stancanelli A, Caporaso G, Liguori R, Wang N, Santoro L, Kennedy WR, Nolano M. The role of skin biopsy in differentiating small-fiber neuropathy from ganglionopathy. Eur J Neurol. 2018;25:848-853.
- Bönhof GJ, Strom A, Püttgen S, Ringel B, Brüggemann J, Bódis K, Müssig K, Szendroedi J, Roden M, Ziegler D. Patterns of cutaneous nerve fibre loss and regeneration in type 2 diabetes with painful and painless polyneuropathy. Diabetologia. 2017;60:2495-2503.
- Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56:2148–54.
- Cheung A, Podgomy P, Martinez JA, Chan C, Toth C. Epidermal axonal swellings in painful and painless diabetic peripheral neuropathy. Muscle Nerve. 2015;51:505–13.
- Scheytt S, Riediger N, Braunsdorf S, Sommer C, Üçeyler N. Increased gene expression of growth associated protein-43 in skin of patients with early-stage peripheral neuropathies. J Neurol Sci. 2015;355:131-7.
- Karlsson P, Provitera V, Caporaso G, Stancanelli A, Saltalamacchia AM, Borreca I, Manganelli F, Santoro L, Jensen TS, Nolano M. Increased peptidergic fibers as a potential cutaneous marker of pain in diabetic small fiber neuropathy. Pain. 2020 Aug 21. doi: 10.1097/j.pain.0000000000002054. Epub ahead of print.
- Oaklander AL, Nolano M. Scientific Advances in and Clinical Approaches to Small-Fiber Polyneuropathy: A Review. JAMA Neurol. 2019 Sep 9. doi:10.1001/jamaneurol.2019.2917. Epub ahead of print.
- Levy DM, Terenghi G, Gu XH, Abraham RR, Springall DR, Polak JM. Immunohistochemical measurements of nerves and neuropeptides in diabetic skin: relationship to tests of neurological function. Diabetologia. 1992 Sep;35:889-97
- Vlckova-Moravcova E, Bednarik J, Belobradkova J, Sommer C. Small-fibre involvement in diabetic patients with neuropathic foot pain. Diabet Med. 2008;25:692-9.
- Nebuchennykh M, Løseth S, Lindal S, Mellgren SI. The value of skin biopsy with recording of intraepidermal nerve fiber density and quantitative sensory testing in the assessment of small fiber involvement in patients with different causes of polyneuropathy. J Neurol. 2009 Jul;256(7):1067-75. Erratum in: J Neurol. 2009 Jun;256(6):1034. PMID: 19252773.
- Kennedy WR, Wendelschafer-Crabb G, Quantitation of epidermal nerves in diabetic neuropathy. Johnson T. Neurology. 1996;47:1042-8.
- Shun CT, Chang YC, Wu HP, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain. 2004;127:1593-1605.
- Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology. 2001;57:1701-4.
- Farhad K, Traub R, Ruzhansky KM, Brannagan TH 3rd. Causes of neuropathy in patients referred as “idiopathic neuropathy”. Muscle Nerve 2016;53:856–61.
- Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, Dib-Hajj S, Drenth JP, Waxman SG, Merkies IS. Gain of function Naν1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71:26-39
- Faber CG, Lauria G, Merkies IS, et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci USA. 2012;109:19444-49.
- Huang J, Han C, Estacion M, et al. Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain. 2014;137:1627–42.
- Grayston R, Czanner G, Elhadd K, Goebel A, Frank B, Üçeyler N, Malik RA, Alam U. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: Implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Serra J, Collado A, Solà R, Antonelli F, Torres X, Salgueiro M, Quiles C, Bostock H. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol. 2014;75:196-208.
- Metyas S, Chen C, Quismorio A, Abdo N, Kamel K. Improvement in Fibromyalgia Symptoms and Skin Biopsy Results in Patients with Fibromyalgia Related Small Fiber Neuropathy. Curr Rheumatol Rev. 2019 Nov 6. doi:10.2174/1573397115666191106120622. Epub ahead of print.
- Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294-1299.
- Penza P, Lombardi R, Camozzi F et al. (2009). Painful neuropathy in subclinical hypothyroidism: clinical and neuropathological recovery after hormone replacement therapy. Neurol Sci 30: 149–151.
- Freeman R, Chapleau MW. Testing the autonomic nervous system. Handb Clin Neurol. 2013;115:115-36.
- Low PA. Evaluation of sudomotor function. Clin Neurophysiol. 2004;115:1506-13.
- Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13:256-70.
- Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983;14:573-80.
- Provitera V, Nolano M, Caporaso G, Stancanelli A, Santoro L, Kennedy WR. Evaluation of sudomotor function in diabetes using the dynamic sweat test. Neurology. 2010;74:50-6.
- Caruso G, Nolano M, Lullo F, Crisci C, Nilsson J, Massini R. Median nerve sensory responses evoked by tactile stimulation of the finger proximal and distal phalanx in normal subjects. Muscle Nerve. 1994 Mar;17(3):269-75.
- Li J, Bai Y, Ghandour K, Qin P, Grandis M, Trostinskaia A, Ianakova E, Wu X, Schenone A, Vallat JM, Kupsky WJ, Hatfield J, Shy ME. Skin biopsies in myelin-related neuropathies: bringing molecular pathology to the bedside. Brain. 2005;128:1168-77.
