This entry describes the basics of photocatalysis. It also presents properties and applications of C-,N- and S-Doped TiO2 as a photocatalyst.
- TiO2
- photocatalyst
- photocatalysis
- carbon
- nitrogen
- sulfur
- doped
- co-doped
- tri-doped
1. Introduction
More than ever before, environmental problems have become a major concern. Urbanization and rapid growth of industries generate abundant amounts of pollutants which are released into the environment. Among them, there are highly hazardous materials such as pharmaceuticals [1][2][1,2], dioxins [3], pesticides [4], herbicides [1], phenols [4][5][4,5], and textile dyes [1][4][5][6][1,4,5,6]. This increasing occurrence of organic pollutants in the environment is a serious danger for health and the lives of humans and other living beings. Conventional treatment methods very often fail in the removal of these kinds of residues entirely, because of their high (bio)chemical stability. Moreover, a conventional approach is associated with the operational problems and high costs. Hence, the development of new and efficient methods of the removal of organic contaminants is a matter of growing interest [1][2][4][1,2,4].
In recent decades, semiconductor photocatalysis has been proved to be an efficient approach for organic compounds decomposition and degradation. TiO2 has been widely and successfully used as a photocatalyst in many different areas (Figure 1) due to its advantages, such as low cost and good chemical stability. However, it requires employing relatively high photon energy to be activated. For this reason, many methods of narrowing of the band gap of TiO2 have been proposed, aimed at the direct usage of sunlight [7][8][7,8]. Amongst them, doping of TiO2 with non-metals such as carbon, nitrogen and sulfur is often reported as one of the most effective ways of increasing its photocatalytic activity under visible light [1][9][10][1,9,10]. Non-metal doping of TiO2 leads to changes in the electronic band structure, resulting in a smaller band gap energy value, and thus an improved response in the visible light [7][8][11][7,8,11].
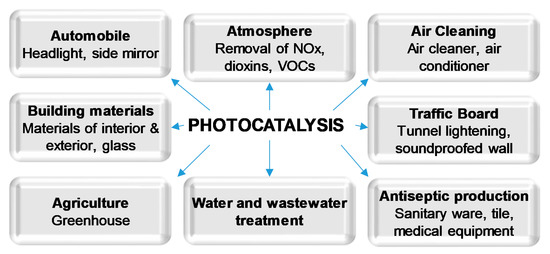
Figure 1. Applications of photocatalysis.
The idea of non-metal doping of TiO2 has been discussed in numerous reviews through the years [1][9][10][12][13][14][15][16][17][18][19][20][21][22][23][1,9,10,12,13,14,15,16,17,18,19,20,21,22,23]. Most of the recent reviews referred to nitrogen only, which is one of the most frequently used non-metal dopants [17][24][25][26][27][28][29][30][31][32][33][34][17,24,25,26,27,28,29,30,31,32,33,34]. On the other hand, the reviews devoted exclusively to S-doped TiO2 are very limited [35]. In some papers, the modifications of TiO2 with carbon are also summarized [1][10][36][37][1,10,36,37]. Shi et al. [36] presented various carbon-based (nano)composites, including C-doped TiO2. Moreover, diverse, more complex configurations, e.g., with multi-walled carbon nanotubes (MWCNT) in TiO2-SiO2/MWCNT [37], with carbon dots (CDs) in CDs-N-TiO2 [38], and Ag-modified g-C3N4/N-doped TiO2 [24] have been reported. There is only one review referring to the beneficial effects and challenges of tri-doping of TiO2 with carbon, nitrogen, and sulfur, which was published in 2017 [39]. Moreover, recently, a review on single doping of TiO2 with various non-metals, including C, N, and S was published [1].
2. TiO2 Photocatalysis
The discovery of the photocatalytic splitting of water on TiO2 electrodes in 1972 heralded a new era of heterogeneous photocatalysis [40]. Despite several decades having passed since then, the most popular photocatalyst is still TiO2. Amongst the different structures of titania, anatase and rutile are commonly used in photocatalysis, with anatase displaying a higher photocatalytic activity.
In heterogeneous photocatalysis, a reaction takes place on the surface of a photocatalyst. The general mechanism of photocatalytic decomposition of organic compounds is summarized in Figure 2.
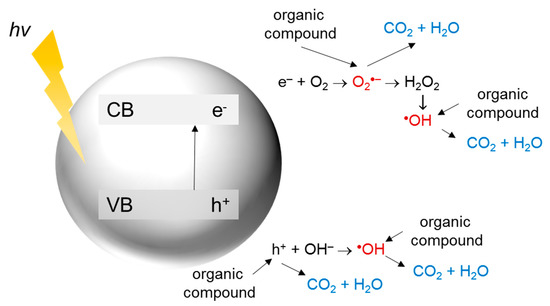
Figure 2.
The mechanism of photocatalytic decomposition of organic compounds.
First, the energy higher than the band gap energy of the semiconductor is required for photon absorption and excitation of an electron (e−) from the valence band (VB) to the conduction band (CB), resulting in hole (h+) generation. The holes in the VB can react with the surface adsorbed water or hydroxyl ions to form hydroxyl radicals, which are extremely strong oxidants (oxidation potential around +2.7 V). The photoexcited electrons in the CB can generate superoxide radicals due to the reaction with oxygen, being the main electron acceptor in the system. Further reactions lead to the formation of other reactive oxygen species (ROS) such as hydrogen peroxide, hydroperoxyl radicals, or hydroxyl radicals. These species participate in the degradation of organic contaminants [41][42][43][44][41,42,43,44].
The effectiveness of the photodegradation of pollutants on the semiconductor surface is influenced by: (i) the chemical composition, structure and concentration of pollutants, (ii) the radiation intensity, (iii) the exposure time, (iv) the amount of photocatalyst used, (v) the oxygen content in the reaction medium, (vi) the pH value of the solution, (vii) the properties of a photocatalyst (specific surface area, crystallographic structure, number of surface defects, presence of additives and dopants, etc.) [45][46][45,46].
3. C-,N- and S-Doped TiO2
The most widespread approaches of the synthesis of the C, N or S-doped TiO2 photocatalysts are sol-gel, hydrothermal, solvothermal and wet impregnation methods, while the major precursors of TiO2, carbon, nitrogen and sulfur are titanium alkoxides, sugars, urea, and thiourea, respectively. However, great ambiguity in the case of the application of thiourea as a modifying agent exists. Thiourea has been used as the source of sulfur in S-doped, C,S-co-doped, and N,S-co-doped TiO2 or as the source of carbon and sulfur in the C,S-co-doped TiO2, while for the C,N,S-tri-doped TiO2 it was the precursor of all three non-metals. Thus, it remains vague as to what the role of thiourea is and which of the three mentioned above non-metals are built in the structure of the modified TiO2 photocatalysts. This leads to the conclusion that further thorough research regarding the effect of not only thiourea, but also other C, N and S sources on the structure and properties of the doped TiO2 is essential.
Numerous studies have shown that the incorporation of non-metals into TiO2 usually results in the narrowing of the band gap due to the formation of new impurity levels (C 2p, N 2p, S 2p) above the VB of the semiconductor. As a result, a red shift of optical absorption leading to an enhancement of the visible light photocatalytic activity is commonly reported. Moreover, the presence of C, N, or S could also contribute to the increase in the specific surface area or the improvement in crystallinity, thus additionally enhancing the photocatalytic performance.
The most common method of doping of TiO2 is modification with nitrogen. This issue has been widely investigated over the years. Presently, there is growing interest in the doping of TiO2 with more than one non-metal, including C,N-, C,S-, and N,S-co-doping or C,N,S-tri-doping. Two possible modes of nitrogen incorporation can occur, depending on the preparation conditions, i.e., interstitial (Ti-O-N) and substitutional (O-Ti-N) doping. In the case of sulfur, anionic (as S2−) or cationic (as S4+ or S6+) doping is possible, with the latter case being more energetically favorable and, thus, more commonly reported. The modification with carbon includes the widest range of species that could possibly be formed, such as Ti-C, C-C, C-N, C=N, C=O or C-O, etc. The reported pathways of C-doping include: (i) substitution of lattice oxygen with carbon (formation of Ti-C bonds); (ii) replacement of Ti by C (formation of C-O bonds); or (iii) stabilization of C at the interstitial position. The various mechanisms of doping affect the properties of the photocatalysts, although clear correlations between the modification procedure and the type of doping difficult to find. Therefore, more extensive investigations regarding this issue are necessary, especially when the photocatalysts with designed properties are considered.
It is not possible to unambiguously indicate the most advantageous mode of non-metal doping of TiO2. Each of them has some advantages and disadvantages. Moreover, a clear correlation between the doping mode and the physicochemical properties or photoactivity of the modified TiO2 is difficult to find. Co-doping and tri-doping of TiO2 can result in combining the properties of the particular single doped TiO2, leading to the enhancement of photoactivity. However, such improvement usually requires the employment of more reagents and more complicated synthesis procedures. Moreover, only a few authors compared the co-doped or tri-doped photocatalysts with single doped ones, while most of the others referred their results to an undoped TiO2. On the other hand, comparing the photocatalysts obtained by different authors would not be reliable since various conditions of experiments were applied, e.g., light sources and intensity, type and concentration of model pollutant, or dose of photocatalysts. Therefore, simple and reliable standard methods for testing of photocatalytic activity that will be widely applied by scientists around the world are a key issue to enable the comparison of the results obtained in different laboratories. That would contribute to defining the correlations and development of methods for designing the highly active photocatalysts.
A majority of applications of doped photocatalysts refer to the removal of pollutants from water and wastewater. The C-, N-, and S-doped photocatalysts were applied mainly for the decomposition of various dyes. Considering that the main aim of modification of TiO2 with the non-metals is the enhancement of its visible light photoactivity, such an attempt is not appropriate, as was already widely discussed in the literature. This is because of the dye sensitization effect. Therefore, it is very important to study the photocatalytic activity with the application of colorless compounds, such as phenols, pharmaceuticals, etc. Moreover, the determination of mineralization efficiency apart from decomposition rates is also important. A detailed evaluation of the mechanisms of the visible light photocatalysis, with reference to the by-product formation or the role of various ROS is also of high significance. Finally, the toxicity of the treated solutions should be carefully examined as one of the most important parameters reflecting the treatment efficiency and environmental safety. The important research should also focus on applications of doped photocatalysts other than water/wastewater treatment. The visible light photocatalytic air treatment, hydrogen production, CO2 photoreduction, and bacterial inactivation are still not thoroughly examined.
Reference (Editors will rearrange the references after the entry is submitted)
- Mittal, A.; Mari, B.; Sharma, S.; Kumari, V.; Maken, S.; Kumari, K.; Kumar, N. Non-metal Modified TiO2: A Step towards Visible Light Photocatalysis. J. Mater. Sci. Mater. Electron. 2019, 30, 3186–3207.
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2019, 12, 102.
- Saibu, S.; Adebusoye, S.A.; Oyetibo, G.O. Aerobic Bacterial Transformation and Biodegradation of Dioxins: A Review. Bioresour. Bioprocess. 2020, 7.
- Kumari, V.; Mittal, A.; Jindal, J.; Yadav, S.; Kumar, N. S-, N- and C-doped ZnO as Semiconductor Photocatalysts: A Review. Front. Mater. Sci. 2019, 13, 1–22.
- Din, M.I.; Khalid, R.; Hussain, Z. Recent Research on Development and Modification of Nontoxic Semiconductor for Environmental Application. Sep. Purif. Rev. 2020, 1–18.
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248.
- Ramandi, S.; Entezari, M.H.; Ghows, N. Sono-Synthesis of Solar Light Responsive S–N–C–Tri Doped TiO2 Photo-Catalyst under Optimized Conditions for Degradation and Mineralization of Diclofenac. Ultrason. Sonochem. 2017, 38, 234–245.
- Khedr, T.M.; El-Sheikh, S.M.; Hakki, A.; Ismail, A.A.; Badawy, W.A.; Bahnemann, D.W. Highly Active Non-metals Doped Mixed-Phase TiO2 for Photocatalytic Oxidation of Ibuprofen under Visible Light. J. Photoch. Photobiol. A. 2017, 346, 530–540.
- Nasirian, M.; Lin, Y.P.; Bustillo-Lecompte, C.F.; Mehrvar, M. Enhancement of Photocatalytic Activity of Titanium Dioxide Using Non-metal Doping Methods under Visible Light: A Review. Int. J. Environ. Sci. Technol. 2017, 15, 2009–2032.
- Islam, S.; Nagpure, S.; Kim, D.; Rankin, S. Synthesis and Catalytic Applications of Non-metal Doped Mesoporous Titania. Inorganics 2017, 5, 15.
- Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Koop-Santa, C.; López-Mena, E.R.; Orozco-Guareño, E.; García-Guaderrama, M. N-doped TiO2 Nanoparticles Obtained by a Facile Coprecipitation Method at Low Temperature. Ceram. Int. 2018, 44, 5273–5283.
- Lazar, M.; Varghese, S.; Nair, S. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601.
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent Progress in Enhancing Photocatalytic Efficiency of TiO2 -based Materials. Appl. Catal. A-Gen. 2015, 495, 131–140.
- Devi, L.G.; Kavitha, R. A Review on Non Metal Ion Doped Titania for the Photocatalytic Degradation of Organic Pollutants under UV/Solar Light: Role of Photogenerated Charge Carrier Dynamics in Enhancing the Activity. Appl. Catal. B-Environ. 2013, 140–141, 559–587.
- Al Jitan, S.; Palmisano, G.; Garlisi, C. Synthesis and Surface Modification of TiO2-based Photocatalysts for the Conversion of CO2. Catalysts 2020, 10, 227.
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent Progress in Metal-doped TiO2, Non-metal Doped/Codoped TiO2 and TiO2 Nanostructured Hybrids for Enhanced Photocatalysis. Int. J. Hydrogen Energ. 2020, 45, 7764–7778.
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852.
- Rammohan, G.; Nadagouda, M. Green Photocatalysis for Degradation of Organic Contaminants: A Review. COC 2013, 17, 2338–2348.
- Teh, C.M.; Mohamed, A.R. Roles of Titanium Dioxide and Ion-Doped Titanium Dioxide on Photocatalytic Degradation of Organic Pollutants (Phenolic Compounds and Dyes) in Aqueous Solutions: A Review. J. Alloys Compd. 2011, 509, 1648–1660.
- Ismail, A.A.; Bahnemann, D.W. Mesoporous Titania Photocatalysts: Preparation, Characterization and Reaction Mechanisms. J. Mater. Chem. 2011, 21, 11686.
- Akpan, U.G.; Hameed, B.H. The Advancements in Sol–Gel Method of Doped-TiO2 Photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11.
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and Mechanisms of Photocatalytic Dye Degradation on TiO2based Photocatalysts: A Comparative Overview. RSC Adv. 2014, 4, 37003–37026.
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Heterogeneous Photocatalytic Degradation of Phenols in Wastewater: A Review on Current Status and Developments. Desalination 2010, 261, 3–18.
- Wang, W.; Chen, M.; Huang, D.; Zeng, G.; Zhang, C.; Lai, C.; Zhou, C.; Yang, Y.; Cheng, M.; Hu, L.; et al. An Overview on Nitride and Nitrogen-doped Photocatalysts for Energy and Environmental Applications. Compos. Part B Eng. 2019, 172, 704–723.
- Kaur, N.; Shahi, S.K.; Shahi, J.S.; Sandhu, S.; Sharma, R.; Singh, V. Comprehensive Review and Future Perspectives of Efficient N-doped, Fe-doped and (N,Fe)-co-doped Titania as Visible Light Active Photocatalysts. Vacuum 2020, 178, 109429.
- Samokhvalov, A. Hydrogen by Photocatalysis with Nitrogen Codoped Titanium Dioxide. Renew. Sustain. Energ. Rev. 2017, 72, 981–1000.
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped Titanium Dioxide (N-doped TiO2) for Visible Light Photocatalysis. New J. Chem. 2016, 40, 3000–3009.
- Bakar, S.A.; Ribeiro, C. Nitrogen-doped Titanium Dioxide: An Overview of Material Design and Dimensionality Effect over Modern Applications. J. Photoch. Photobiol. C 2016, 27, 1–29.
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.; Martins, R. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373.
- Zhang, W.; Jia, B.; Wang, Q.; Dionysiou, D. Visible-light Sensitization of TiO2 Photocatalysts via Wet Chemical N-doping for the Degradation of Dissolved Organic Compounds in Wastewater Treatment: A Review. J. Nanopart. Res. 2015, 17.
- Spadavecchia, F.; Ceotto, M.; Presti, L.L.; Aieta, C.; Biraghi, I.; Meroni, D.; Ardizzone, S.; Cappelletti, G. Second Generation Nitrogen Doped Titania Nanoparticles: A Comprehensive Electronic and Microstructural Picture. Chin. J. Chem. 2014, 32, 1195–1213.
- Gomathi Devi, L.; Kavitha, R. Review on Modified N–TiO2 for Green Energy Applications under UV/Visible Light: Selected Results and Reaction Mechanisms. RSC Adv. 2014, 4, 28265–28299.
- Dunnill, C.W.; Parkin, I.P. Nitrogen-Doped TiO2 thin Films: Photocatalytic Applications for Healthcare Environments. Dalton Trans. 2011, 40, 1635–1640.
- Thompson, T.L.; Yates, J.T., Jr. TiO2-Based Photocatalysis: Surface Defects, Oxygen and Charge Transfer. Top. Catal. 2005, 35, 197–210.
- Lamo, M.P.B.; Nowotny, J. Water Purification Using Solar Energy: Effect of Sulphur on Photocatalytic Properties of TiO2. Energy Mater. 2009, 4, 150–158.
- Shi, Z.-J.; Ma, M.-G.; Zhu, J.-F. Recent Development of Photocatalysts Containing Carbon Species: A Review. Catalysts 2018, 9, 20.
- Mestre, A.S.; Carvalho, A.P. Photocatalytic Degradation of Pharmaceuticals Carbamazepine, Diclofenac, and Sulfamethoxazole by Semiconductor and Carbon Materials: A Review. Molecules 2019, 24, 3702.
- Zhao, X.; Zhang, G.; Zhang, Z. TiO2-Based Catalysts for Photocatalytic Reduction of Aqueous Oxyanions: State-of-the-Art and Future Prospects. Environ. Int. 2020, 136, 105453.
- Sushma, C.; Kumar, S.G. C–N–S Tridoping into TiO2 matrix for Photocatalytic Applications: Observations, Speculations and Contradictions in the Codoping Process. Inorg. Chem. Front. 2017, 4, 1250–1267.
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758.
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96.
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554.
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582.
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photoch. Photobiol. C 2000, 1, 1–21.
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of Parameters on the Heterogeneous Photocatalytic Degradation of Pesticides and Phenolic Contaminants in Wastewater: A Short Review. J. Environ. Manag. 2011, 92, 311–330.
- Ramanathan, R.; Bansal, V. Ionic Liquid Mediated Synthesis of Nitrogen, Carbon and Fluorine-codoped Rutile TiO2 Nanorods for Improved UV and Visible Light Photocatalysis. RSC Adv. 2015, 5, 1424–1429.
- Chen, M.; Chu, J.-W. NOx Photocatalytic Degradation on Active Concrete Road Surface—From Experiment to Real-Scale Application. J. Clean. Prod. 2011, 19, 1266–1272.
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting. ACS Catal. 2018, 8, 2253–2276.
- Razzaq, A.; Sinhamahapatra, T.-H.; Kang, C.A.; Grimes, J.-S.; Yu, S.-I. Efficient Solar Light Photoreduction of CO2 to Hydrocarbon Fuels via Magnesiothermally Reduced TiO2 Photocatalyst. Appl. Catal. B-Environ. 2017, 215, 28–35.
- Spadaro, L.; Arena, F.; Negro, P.; Palella, A. Sunfuels from CO2 Exhaust Emissions: Insights Into the Role of Photoreactor Configuration by the Study in Laboratory and Industrial Environment. J. CO2 Util. 2018, 26, 445–453.
- Tahir, B.; Tahir, M.; Amin, N.S. Gold-Indium Modified TiO2 Nanocatalysts for Photocatalytic CO2 Reduction with H2 as Reductant in a Monolith Photoreactor. Appl. Surf. Sci. 2015, 338, 1–14.
- Hu, B.; Guild, C.; Suib, S.L. Thermal, Electrochemical, and Photochemical Conversion of CO2 to Fuels and Value-Added Products. J. CO2 Util. 2013, 1, 18–27.
- Tahir, M.; Amin, N.S. Advances in Visible Light Responsive Titanium Oxide-Based Photocatalysts for CO2 Conversion to Hydrocarbon Fuels. Energ. Convers. Manag. 2013, 76, 194–214.
- Spadaro, L.; Arena, F.; Palella, A. Which Future Route in the Methanol Synthesis? Photocatalytic Reduction of CO2, the New Challenge in the Solar Energy Exploitation. In Methanol: Science and Engineering; Dalena, F., Basile, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018.
- Abdullah, H.; Khan, M.M.; Ong, H.R.; Yaakob, Z. Modified TiO2 Photocatalyst for CO2 Photocatalytic Reduction: An Overview. J. CO2 Util. 2017, 22, 15–32.
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32, 33–177.
- Kočí, K.; Obalová, L.; Matějová, L.; Plachá, D.; Lacný, Z.; Jirkovský, J.; Šolcová, O. Effect of TiO2 Particle Size on the Photocatalytic Reduction of CO2. Appl. Catal. B-Environ. 2009, 89, 494–502.
- Adekoya, D.; Tahir, M.; Amin, N.A. Recent Trends in Photocatalytic Materials for Reduction of Carbon Dioxide to Methanol. Renew. Sustain. Energy Rev. 2019, 116, 109389.
- Wang, P.; Yin, G.; Bi, Q.; Huang, X.; Du, X.; Zhao, W.; Huang, F.-Q. Efficient Photocatalytic Reduction of CO2 Using Carbon-Doped Amorphous Titanium Oxide. Chem. Cat. Chem. 2018, 10, 3854–3861.
- Akple, M.S.; Low, J.; Qin, Z.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Liu, S. Nitrogen-Doped TiO2 Microsheets with Enhanced Visible Light Photocatalytic Activity for CO2 Reduction. Chin. J. Catal. 2015, 36, 2127–2134.
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408.
- Friedmann, D.; Hakki, A.; Kim, H.; Choi, W.; Bahnemann, D. Heterogeneous Photocatalytic Organic Synthesis: State-of-the-Art and Future Perspectives. Green Chem. 2016, 18, 5391–5411.
- Qiu, H.; Fang, S.; Huang, G.; Bi, J. A Novel Application of In2S3 for Visible-Light-Driven Photocatalytic Inactivation of Bacteria: Kinetics, Stability, Toxicity and Mechanism. Environ. Res. 2020, 190, 110018.
- Pan, J.; Shen, S.; Zhou, W.; Tang, J.; Ding, H.; Wang, J.; Chen, L.; Au, C.-T.; Yin, S.-F. Recent Progress in Photocatalytic Hydrogen Evolution. Acta Phys. Chim. Sin. 2020, 36, 1–9.
- Sakar, M.; Mithun Prakash, R.; Do, T.-O. Insights into the TiO2-Based Photocatalytic Systems and Their Mechanisms. Catalysts 2019, 9, 680.
- Fang, Y.; Zheng, Y.; Fang, T.; Chen, Y.; Zhu, Y.; Liang, Q.; Sheng, H.; Li, Z.; Chen, C.; Wang, X. Photocatalysis: An Overview of Recent Developments and Technological Advancements. Sci. China Chem. 2019, 63, 149–181.
