Coronavirus Disease 19 (COVID-19), due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has become an on-going global health emergency affecting over 94 million cases with more than 2 million deaths globally. Primarily identified as atypical pneumonia, it has developed into severe acute respiratory distress syndrome (ARDS), a multi-organ dysfunction with associated fatality. Ever since its emergence, COVID-19 with its plethora of clinical presentations has signalled its dynamic nature and versatility of the disease process. Being a disease with droplet transmission has now assumed the proportion of a suspected airborne nature which, once proved, poses a Herculean task to control. Because of the wide distribution of the human angio-tensin-converting enzyme-2 (hACE2) receptors, known for its transmission, we envisage its mul-tiorgan spread and extensive disease distribution.
- SARS-CoV-2
- COVID-19
- ACE-2
- neurological
- hepatic
- dermatological
- pathogenesis
- therapeu-tics
- vaccines
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a novel emerging human infectious disease due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) first originated in Wuhan, China, in December 2019. Having been present for one year, SARS-CoV-2 had infected more than 94 million individuals with 2,031,875 deaths from 218 countries globally as of 17 January 2021 [1]. COVID-19 spread quickly across the world until a global emergency and pandemic were declared by the World Health Organization (WHO) on 30 January and 11 March 2020, respectively [2]. Untiring efforts are being invested to understand the origin, transmission, and pathogenesis of COVID-19 so that effective therapeutic agents, as well as an effective vaccine, can be developed. The R (reproductive number) for SARS-CoV-2 is estimated between 1.5–3.5 in comparison to 2.0 of SARS 2002, however, the case fatality rate (CFR) is around 2–3% in SARS-CoV-2 in comparison to 10% for SARS 2002 [3][4][3,4].
Of the seven coronaviruses (CoVs), 229E, NL63, OC43, and HKU1 are known for self-limiting upper respiratory tract infections [5], whereas Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV, and the novel SARS-CoV-2 end up with life-threatening respiratory failure and multi-organ dysfunction [6][7] [6,7]. SARS-CoV-2 through spike (S) glycoproteins recognizes and binds specifically to the human angiotensin-converting enzyme 2 (hACE2) receptors expressed on type-II alveolar epithelial cells for its entry [8]. SARS-CoV-2 has a stronger binding affinity with ACE2 along with cellular transmembrane serine protease 2 (TMPRSS2) imparting virulence and aggressive properties. Following the SARS-CoV-2 binding to alveolar epithelial cells, the innate and adaptive immune system is activated leading to cytokine-release syndrome (CRS) or macrophage activation syndrome (MAS). Increased production of interleukin (IL-1, IL-6, IL-8) cytokines in plasma resulting in dyspnea, acute respiratory distress syndrome (ARDS), and death [9]. High levels of SARS-CoV-2 shedding in the upper respiratory tract, even among presymptomatic patients, is a key factor in the transmissibility of COVID-19.
2. Clinical Presentation and Transmission of Coronavirus Disease 2019 (COVID-19)
Because of the novel nature of the virus and lack of immunity, the presentations are dynamic and frequently changing. Being an unknown cause of atypical pneumonia, the infection gradually progressed affecting the population in Hubai province, China and thereafter, spread to different countries, the presentations varied from mild respiratory tract infection to severe pneumonia and ARDS or multi-organ dysfunction with increased mortality [10]. Other typical presentations like fever, cough, diarrhea, hemoptysis, rhinorrhea, shortness of breath, myalgia, fatigue and severe dyspnoea, lymphopenia, chest radiographic findings like ground-glass opacity are observed in COVID-19 [11]; 20% of COVID-19 patients in an older age group with pre-existing morbidities present with severe respiratory illness and ARDS whereas children and young adults have a milder illness and better prognosis [12].
The asymptomatic cases contributed to a major source of the virus and resulted in a high rate of community transmission, and thus extensive screening was required [13]. It has been estimated that up to 86% of cases with unusual presentations might have been missed in China [14]. In the beginning, COVID-19 was suspected with unusual respiratory symptoms, whereas over the progression of the pandemic involving different countries the extra-pulmonary symptoms like the neurological, cardiac, renal, gastrointestinal tract, ocular, vascular, olfactory including anosmia and ageusia were reported. The multiorgan manifestations may be correlated due to the abundancy of the ACE2 receptors in various organs [15][16][17][18] [15–18] and observed with symptoms including diarrhea, poor appetite, nausea, vomiting (digestive); headache, and confusion (nervous), palmus, chest distress (cardiovascular) [19]. SARS-CoV-2 is commonly transmitted from person-to-person mainly by respiratory droplets and fomites through cough, sneeze, or by droplet inhalation and contact transmission with oral, nasal and eye, mucous membrane, including saliva.
COVID-19 diagnosis is mainly made on radiological settings like X-ray, chest computed tomography (CT) scan, and laboratory findings like lymphopenia and elevated Lactate Dehydrogenase (LDH) [12]. Nasopharyngeal and oropharyngeal swabs help in virus identification through nucleic acid detection by real-time polymerase chain reaction (RT-PCR) which is the method of choice for lab diagnosis as isolation in cell lines requires BSL-3/4 facilities [20]. Such labs are highly specialized to deal with potentially deadly infectious and exotic agents requiring the most stringent containment. The classical characteristics of BSL-4 is a full-body, air-supplied, positive pressure suit, class III biological safety cabinet, and rooms with negative pressure facility.
3. Cutaneous Manifestations of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)
Skin rashes and purpuric plaques are an interesting clinical presentation of classical coronavirus infections. The first report of the cutaneous manifestations was reported from Italy, where 20.4% (18/88) hospitalized COVID-19 patients developed an erythematous rash (14), widespread urticaria (3), and chickenpox-like vesicles (1) distributed in the trunk area [21]. In severe cases, erythematous rash, and localized or widespread urticarial rashes seem to be the most common cutaneous manifestation whereas in China only 0.2% (2/1099) confirmed COVID-19 cases had skin rashes [22].
Fernandez et al. [23] [23] had reported a skin biopsy of a 32-year-old woman from France with COVID-19 having urticariform rash with perivascular infiltration of lymphocytes, eosinophils, and upper dermal edema on histopathology. Urticaria (1.4%) is also reported as cutaneous symptoms. A rare COVID-19 associated varicella-like papulovesicular exanthem was first observed in Italian patients by Marzano et al. [24]. Lesions were varied from scattered to diffuse with vesicular predominance in 12 (54.5%) patients with trunk and limbs involvement generally appearing 3 days after systemic symptoms. Fever, cough, headache, weakness, coryza, dyspnea, hyposmia, and hypogeusia were common systemic symptoms reported. However, SARS-CoV-2 detection in skin lesional was not performed but still represents a useful clue to suspect COVID-19 in asymptomatic patients. A dengue-like petechial rash with thrombocytopenia was reported by Joob and Wiwanitkit [25] in a COVID-19 patient from Thailand. Unusual skin manifestations like confluent erythematous-yellowish papules on both heels of a 28-year-old COVID-19 infected woman with symptoms of diarrhea, ageusia, and anosmia has been reported [26].
In a few COVID-19 patients, atopic dermatitis, and psoriasis has been aggravated as pre-existing skin disease. Joob and Wiwanitkit [27] presented an 84-year-old woman with arterial hypertension history having COVID-19 related bilateral pneumonia, later developed mild pruriginous rashes in the peri-axillary area and coalescing macules in flexural regions. Thus, various studies have reflected the possibilities of potential skin lesions of COVID-19.
4. Hepatic Manifestations of SARS-CoV-2
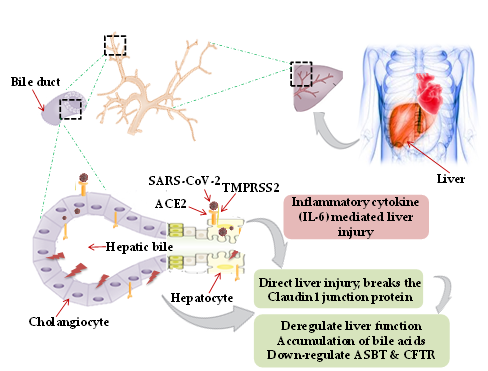
Though primarily a respiratory pathogen, shreds of evidence indicate the liver as an extra-pulmonary site for SARS-CoV-2 infection causing liver injury ranging between 14.8% to 78% [28][29] [28,29]. The possible mechanism of hepatic injury in COVID-19 could either be virus related cytopathic effect or infection-induced cytokine storm. Two independent studies on healthy cohorts by single RNA sequencing data demonstrated significant ACE2 expression (59.7%) in cholangiocytes. SARS-CoV-2 binds to the ACE2 expressed cholangiocytes and being facilitated its entry by TMPRSS2 [30]. Higher coexpression of ACE-2 and TMPRSS2 in human trophoblast cell surface antigen 2 (TROP2high) cholangiocyte progenitor cells of the liver has been reported [31]. The human liver ductal organoid model revealed that cholangiocyte permissiveness for SARS-CoV-2 causes direct liver injury leading to the accumulation of bile acids [32]. In 54% of COVID-19 patients, the ACE2 expression was found to be high in bile duct cells as evidenced by elevated gamma-glutamyl transferase (GGT) levels [33]. Ablation of tight junction protein claudin 1 and down-regulation of apical sodium-dependent bile acid transporter (ASBT) and cystic fibrosis transmembrane conductance regulator (CFTR) might be the contributing factors towards liver injury in COVID-19 [32] (Figure 1).
A liver biopsy of a COVID-19 positive deceased patient revealed portal inflammation with microvesicular steatosis [34]. Virus-mediated persistent activation of lymphocytes and macrophages secretes inflammatory IL-6, IL-10, IL-2, and IFN-c causing CRS and hepatic injury [35].
Figure 1. Mechanism of hepatic injury: in coronavirus disease 2019 (COVID-19) patients, hepatic injury attributed by (i) direct virus-induced cytopathic effect; (ii) virus-mediated infection-induced cytokine storm. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) binds to the angiotensin-converting enzyme 2 (ACE2), expressed on hepatocytes and cholangiocytes in the bile duct cells causing ablation of tight junction protein claudin 1 and down-regulation of apical sodium-dependent bile acid transporter (ASBT) and cystic fibrosis transmembrane conductance regulator (CFTR), leading to the accumulation of bile acids and contributing towards liver injury. Inflammatory cytokines (interleukin-6, IL-10, and IL-2) secretion by lymphocytes and macrophages aggravate inflammatory responses causing hepatic injury. Black dotted square frame in the figures denotes the selected area for the magnified portion.