The gut–liver-axis is a bidirectional coordination between the gut, including microbial residents, the gut microbiota, from one side and the liver on the other side. Any disturbance in this crosstalk may lead to a disease status that impacts the functionality of both the gut and the liver. A major cause of liver disorders is hepatitis C virus (HCV) infection that has been illustrated to be associated with gut microbiota dysbiosis at different stages of the disease progression. This dysbiosis may start a cycle of inflammation and metabolic disturbance that impacts the gut and liver health and contributes to the disease progression. This review discusses the latest literature addressing this interplay between the gut microbiota and the liver in HCV infection from both directions. Additionally, we highlight the contribution of gut microbiota to the metabolism of antivirals used in HCV treatment regimens and the impact of these medications on the microbiota composition.
- gut microbiota
- gut liver axis
- HCV
- Dysbiosis
- microbiome
1. Gut–Liver Axis
The gut–liver axis represents the link between the gut microbiota and an extraintestinal organ, the liver, where both communicate via the portal vein, systemic circulation, and biliary tract[1]. Portal circulation through the portal vein is responsible for most anatomical interactions and communications between the liver and the gut. It supplies about 70% of the blood to the liver and, therefore, is responsible for transporting nutrients and metabolites produced in the gut to the liver[2]. The gut microbiota and host cells metabolize nutritional macromolecules such as carbohydrates, lipids, and proteins. The metabolic products may be transferred to the liver through the portal vein[3]. This communication also leads to the transport of toxic byproducts of the gut microbiota to the liver such as peptidoglycans, endotoxins, or the intact bacteria, which may disrupt metabolic functions of the liver[4]. On the other side, bile acids are generated in the liver and then released into the intestine, and therefore they get involved in the regulation and communication of the gut–liver axis[5][6]. Portal circulation through the portal vein is responsible for most anatomical interactions and communications between the liver and the gut. It supplies about 70% of the blood to the liver and, therefore, is responsible for transporting nutrients and metabolites produced in the gut to the liver[7]. The gut microbiota and host cells metabolize nutritional macromolecules such as carbohydrates, lipids, and proteins. The metabolic products may be transferred to the liver through the portal vein[8]. As mentioned above, this communication also leads to the transport of harmful byproducts of the gut microbiota to the liver, which may disrupt metabolic functions of the liver[9]. On the other side, bile acids are generated in the liver and then released into the intestine, and therefore they get involved in the regulation and communication of the gut–liver axis [10].
1.1. Bile Acids and Gut-Liver Axis
Synthesis of bile acids occurs in the liver from cholesterol to produce the primary bile acids, including cholic acid and chenodeoxycholic acid. These bile acids are transported to the duodenum by the biliary tract where they are converted by 7α-dehydroxylation and deconjugation into secondary bile acids including deoxycholic acid and lithocholic acid. Such bile acid conversion is mediated by some gut microbes, mainly Clostridiales[11][12]. Bile acids have crucial roles in food digestion, keeping the integrity of gut mucosa and having antimicrobial activity against invading pathogens[12][13]. It is well reported that late complicated cases of HCV infections, especially cirrhosis, are characterized by alteration of the conversion of primary bile acids into secondary bile acids; however, the mechanism of this alteration is still unknown[14]. One scenario that may explain the bile acid reduction with the progression of HCV infection may be because of gut microbiota dysbiosis, which eventually leads to decreasing the microbial diversity and increasing the abundance certain microbial taxa such as the Proteobacteria phylum,
Enterobacteriaceae family
Staphylococcus
Bile acids have attracted considerable research interest in HCV treatment due the reported defect in the treatment with IFN therapies in patients who have high serum levels of bile acids[16][17]. On the other side, HCV treatment with direct-acting antiviral (DAA) was associated with a four-fold increase in the total bile acid levels by week 4 antiviral therapy. Moreover, ritonavir was reported to induce changes in bile acid transport. Regarding the functions of bile acids, it is well known that bile salts play a vital role in absorption and digestion of lipid components in addition to their role as ligands for nuclear receptor known as farnesoid X receptor (FXR) that controls several genes required for the metabolism of different macromolecules such as lipids, glucose, and bile acids[18] . It was reported that bile salts lead to activation of FXR that was associated with the activation of lipoprotein lipase, which induces entry of HCV to different cell types and interferes with its infection[19]. Another study reported that bile acids induce HCV replication depending on EGFR/ERK pathway that affects the efficacy of anti-viral treatment drugs[20]. There are previous studies that reported the role of bile acids in the replication of HCV genotype 1[21][22]. Another study detected the ability of bile acids to increase genotype 1 and genotype 2a replication in Huh7 and Huh6 cell lines, but with lower capacity in the case of genotype 2a that suggest the interference of bile salts with anti-viral treatment and their role to improve HCV replication in vitro in cell lines [23].
1.2. Intestinal Barrier
The intestinal barrier prevents toxic compounds such as bacteria and their byproducts from reaching extraintestinal body organs and other tissues including the liver. Thus, impaired intestinal barrier exposes the liver to harmful and toxic compounds from the intestine, which may cause liver damage and impairment of its function, such as alcoholic liver disease, primary biliary cholangitis, and liver cirrhosis [24]. For instance, the increase in intestinal permeability, associated with gut microbiota dysbiosis, exposes hepatocytes pathogen-associated molecular patterns (PAMP), and damage-associated molecular patterns that contribute to liver injury[25]. PAMPs have a direct effect either on hepatocytes or innate immune cells in the liver, such as kupffer and stellate cells [26]. Accordingly, the above discussed findings illustrate that the relationship between the liver and the gut microbiota is bidirectional, and gut microbiota dysbiosis is associated with liver disorders. However, the mechanistic understanding of the cause–effect relationship requires further studies on a case-by-case fashion.
2. Gut Microbiota Dysbiosis in HCV
2.1. Gut Microbiota During Liver Disease Manifestations
Gut microbiota have been linked to fatty liver disease, autoimmune hepatitis, alcoholic liver disease, viral hepatitis (HBV and HCV), primary biliary cholangitis, and primary sclerosing cholangitis (PSC) [26][27][28][29][30][31]. Some gut microbes such as
Lactobacillus plantarum
Saccharomyces boulardii
Bifidobacterium species may mitigate different types of hepatitis and metabolic disorders [32]. The gut mucous membranes act as the entrance doors for invading pathogens[33]. In the case of viruses causing hepatitis, the virus shatters the intestinal mucosa and breaches the permeability, resulting in gut dysbiosis and proclamation of liver cirrhosis and HCC through inducing the release of pro-inflammatory cytokines[34]. Therefore, it is not surprising that mitigation of gut dysbiosis, for example, by using probiotics or prebiotics, helps lessen the tolerogenic response and improve mucosal protection against viral pathogens[35]. For instance, the increased abundance of
Lactobacillus in the gut microbiota positively correlates with murine norovirus inhibition by vitamin A, through the upregulation of interferon (IFN-β) [36]. Moreover, a mixture of
Bifidobacterium and various probiotics with fructo-oligosaccharides and galacto-oligosaccharides has a protective effect against Rotavirus infection in a rat model [35]. This is mediated by upregulating the expression of IFN-γ, IL-4, TNF-α, and TLR2 promoting their production[35]. The major complications of liver diseases, notably cirrhosis, are characterized by gut dysbiosis depicting an increase in the families of
Enterobacteriaceae
Veillonellaceae
Lachnospiraceae family and the phylum of Bacteroidetes [37]. Consistently, the cirrhosis dysbiosis ratio is derived to describe the variations in gut microbiota in patients suffering from cirrhosis with beneficial
Ruminococcaceae
Lachnospiraceae
Enterobacteriaceae bacteria [38]. Other liver manifestations involving severe cirrhosis and hepatic encephalopathy are characterized by an elevation in
Enterobacteriaceae levels[39].
2.2. Dysbiosis of Gut Microbiota During Hepatic Viral Manifestations
Hepatitis occurs due to infection with different viruses, including hepatitis A (HAV), HBV, HCV, or hepatitis E virus (HEV). Hepatitis represents an important health concern, particularly in developing countries[34]. HAV and HEV lead to acute manifestations that may be self-cured and short-lived except in immunosuppressed individuals. Taxonomically, HAV and HEV belong to RNA viruses that can be transmitted via ingestion of contaminated food and water and may severely impact intestinal microbiota[40]. A previous study demonstrated that HEV’s manifestations in pigs can be treated using probiotics containing
Enterococcus faecium NCIMB 10415[41]. Nonetheless, there is a deficiency of related records in humans. Besides, HAV and HEV, HBV, and HCV are serious health problems globally that are responsible for numerous reported cases of chronic hepatitis and death[42][43]. The major concern regarding these two viruses is their ability to establish chronic infections (at 10% in HBV and more than 30% in HCV) that finally result in severe complications, including cirrhosis and HCC[42][43]. In order to evade the immune system, hepatic viruses have developed many mechanisms, including dysbiosis of intestinal microbiota [44]. It is well documented that chronic hepatitis infections lead to massive translocation of the intestinal microbiota [45][46]. Such translocation impairs the primary barrier causing the growth of pathogenic bacteria at high rates, and abnormal regulation of immune cells that finally results in severe intestinal inflammation [47]. Moreover, the situation may worsen due to loss of homeostasis of the intestinal microbiota, which results in the progression of hepatitis viral infection [48]. Therefore, during chronic hepatic viral infections, microbiota have a great influence on viral replication as well as the interactions between host cells and the virus. During viral hepatitis, some taxa prevail including the family of
Enterobacteriaceae
Enterococcus faecalis
Escherichia coli
Faecalibacterium prausnitzii
Leuconostoc
Lactobacillus
Weissella
Pediococcus[38][49]. Moreover, prevalence of other bacterial species are related to liver complications (cirrhosis and/or HCC) following HBV or HCV infections, including bacterial families such as
Enterobacteriaceae
Neisseria
Gemella
E. faecalis
E. coli
F. prausnitzii [42][43]. Finally, a fungal pathogen, namely
Candida, is reported in patients suffering from HBV related cirrhosis [50].
2.3. Dysbiosis of Gut Microbiota During HCV Infection
HCV is considered one of the major etiological agents of hepatitis, which results in severe liver complications, including cirrhosis, HCC, and it may even lead to liver failure and death[41]. There is little published literature regarding the impact of HCV infection on gut microbiota[9][37][51][52][53][54][55][56]. Additionally, the mechanistic understanding of how microbial dysbiosis participates in the disease’s progression is not completely addressed. It is well documented that dysbiosis can be classified into three main categories; (i) alteration of beneficial microorganisms, (ii) predominance of harmful microorganisms or pathobionts, and (iii) alteration of total microbial diversity[57]. The studies performed to reveal the effect of HCV on microbiota dysbiosis depicted lower bacterial diversity in HCV infected patients than healthy individuals. This diversity alteration is directly related to the severity and stage of the disease[28]. The previous finding may be attributed to immunoglobulin A (IgA) production by gastric B-lymphocytes infected by HCV that induces changes in the constitution of gut microbiota [58]. Interestingly, chronic liver diseases in advanced stages are associated with more alterations in gut microbiota than patients with the less developed disease[59]. This HCV-associated depletion of the gut microbiota diversity was mitigated after treatment with antiviral drugs[15]. In contrast, a recent study reported a lower microbial diversity in healthy adults as compared to treatment of naïve newly diagnosed HCV patients, which illustrates the impact of the treatment as a confounding factor in microbiome studies[54]. Additionally, this difference in HCV-associated diversity may be attributed to different cohort’s ethnicities, therapeutic factors, and different disease stages.
Lactobacillus
Streptococcus genera are significantly increased[34]. In addition, it is well documented that the phylum of Bacterioidetes, the family of
Enterobacteriaceae, and viridans streptococci are increased in the case of chronic HCV patients, while the phylum of Firmicutes is decreased[28]. It is also noteworthy that HCV is associated with an increase in the lipopolysaccharides (LPS) serum levels, which indicates a damaged intestinal barrier and microbial translocation and inflammation during the disease progression[28][54].
The stages of HCV infection can be classified into (i) persistently normal serum alanine aminotransferase stage (PNALT), (ii) chronic hepatitis, (iii) liver cirrhosis, and (iv) HCC [28]. It is reported that there is a significant reduction in
Ruminococcaceae
Lachnospiraceae
Bacteroides
Enterobacteriaceae
Streptococcus salivarius
S. salivarius may play a role in developing liver cirrhosis and progression to HCC[27]. Metabolic dysfunction of bile acids associated with HCV may be the key factor in the gut microbiota dysbiosis. This bile disturbance may result in overgrowth of proinflammatory bacteria, involving
Porphyromonadaceae
Enterobacteriaceae families along with reducing the phylum of Firmicutes, the key producers of secondary bile acid generation[55]. In addition,
Ruminococcaceae
Lachnospiraceae, the two Firmicutes families, are major SCFAs producers through fermentation of carbohydrates in human intestines[60][61]. Such SCFAs are crucial for the induction and differentiation of colonic regulatory T (Treg) cells responsible for suppressing inflammation [62][63]. SCFAs are also crucial for the nutrition of colon epithelia and adjusting its pH[64]. Thus, deprivation of SCFAs as a result of gut dysbiosis due to alteration of
Ruminococcaceae
Lachnospiraceae would result in worsening the state of chronic HCV patients. This is mainly due to the loss of pH control, leading to hyperammonemia and ammonia absorption in the gut[65].
Bacteroides
Enterobacteriaceae family[15][43][66]. These reports explain that gut dysbiosis results in decreasing bile acids that finally alter the gut microbiota diversity [67]. In addition, the increase in
Bacteroides
Enterobacteriaceae may explain the inflammation that occurs in patients suffering from hepatic encephalopathy[62][68]. Therefore, the increase in levels of
Enterobacteriaceae
Bacteroides,
S. salivarius
S. salivarius plays a pivotal role in the progression of chronic hepatitis into liver cirrhosis and even the development of HCC [55].
S. salivarius has down-regulatory impacts on innate immune responses; therefore, their presence may speed up the progression of HCC[66].
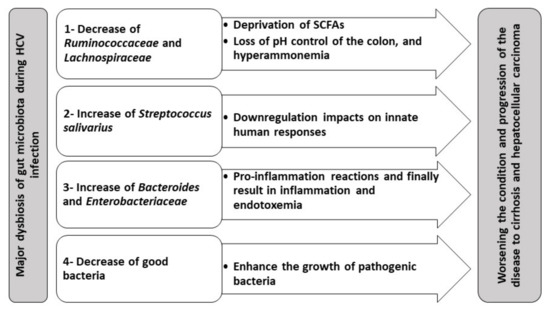
Figure 1.
Therefore, gut dysbiosis is directly linked to the progression of chronic HCV, probably via endotoxemia and hyperammonemia. Thus, gut dysbiosis can be a novel therapeutic target for alleviating the complications of chronic HCV. This can be achieved by either using fecal microbiota transplantation (FMT) or prebiotics and probiotics. Thus, it is not surprising that many reports have recommended the utilization of FMT with treatment regimens of fatty liver disease, PSC, liver cirrhosis, and HCC[69][70][71]. Other studies suggested the incorporation of a broad-spectrum antimicrobial as rifaximin to reduce endotoxemia and harmful metabolites [72][73], as well as to compensate for the reduction in the bile acid levels[14] that all attributed to
Porphyromonadaceae
Enterobacteriaceae
Bacteroidaceae[73] Thus, there are recommendations for using rifaximin followed by FMT as an augmenting therapy of HCV, stating that this would help get rid of
S. salivarius
Moreover, it is reported that there is no direct effect of HCV treatment using ribavirin (RBV) combined with the immune modulator pegylated interferon (PEG-IFN) on gut dysbiosis[50] Mechanistically, this regimen elevates bile acid production that is essential for gut homeostasis[14]. Therefore, we can postulate that direct-acting antivirals (DAAs) may correct for the reduced bile acid levels induced by some liver cirrhosis-associated microbiota, including the family of
Enterobacteriaceae
Staphylococcus
Enterococcus[14]. HCV complications treatment can also be enhanced through utilizing
Bifidobacterium
lactobacillus acidophilus, which have probiotic attributes [74]. The beneficial uses of probiotics in treatment HCV-infected patients with cirrhosis are well documented [75]. Moreover, some microbiota can enhance the immune response in HCV patients through activation of CD56+ NK cell counts and CD3+ cells [53]. Enhancement of the cytotoxic activity of NK cells can inhibit HCV replication.
Bacteroides
Enterobacteriaceae during mild liver disease or the significant increase in lactobacilli and viridans streptococci are associated with the progression of the disease[28]. Sultan and coauthors have recently identified 5 microbiota OTUs that can differentiate HCV patients from healthy individuals[54].
