Chitosan is one of the polymers containing acetyl glucosamine and glucosamine.
- meta-analysis
- chitosan
- lifestyle-related disease
- cholesterol lowering
1. Introduction
Lifestyle-related diseases, including obesity, hyperlipidemia, atherosclerosis, type II diabetes, and hypertension, are widespread in industrialized countries, and are major threats to cardiovascular health. The syndrome is related to a combination of metabolic disorders, including abdominal obesity, hypertriglyceridemia, high-density lipoprotein (HDL) cholesterol decrease, hypertension, and high blood glucose, which lead to increased cardiovascular morbidity and mortality [1][1]. Unnatural blood lipid levels such as high levels of total cholesterol (TC) or triglyceride (TG), high low-density lipoprotein (LDL) level, or low HDL-cholesterol level are correlated with heart disease and stroke. Hypertension is one of the harmful risk factors for stroke and is a key factor in heart attacks. Moreover, obesity acts as a significant risk factor for cardiovascular disease and susceptibility to diabetes [2][2]. Thus, there has been an urgent need for effective methods of controlling these health-related parameters, including food additives.
Chitosan is one of the polymers containing acetyl glucosamine and glucosamine. It may be obtained by hydrolyzing and converting chitin with alkali from crabs, shrimps, insects, mushrooms, and the cell walls of microorganisms. Chitosan manufacture by deacetylation of chitin has been utilized in wastewater treatment and the agricultural sector. As the safety of chitin or chitosan has become increasingly recognized, it has recently-been used in a variety of fields, including medical supplies, food additives, and cosmetics [3,4][3][4]. Chitosan is also known among food additives of which the effects include lowering blood or liver cholesterol and triglyceride by combining with lipids [5][5]. It even shows an anti-inflammatory effect by TNF-α inhibition [6,7,8,9][6][7][8][9]. Nauss et al. [10] assume that chitosan binds lipid micelle in the small intestine after the ingestion of a fatty meal, while Kanauchi et al. [11] [11] propose a more specific mechanism by which chitosan inhibits fat digestion in the gastrointestinal tract. In the stomach, chitosan is dissolved in acidic gastric juice. In this aqueous phase, it acts as an emulsifier on fat globules. It also mixes with fat to form an emulsion. Once transferred into the intestine, the chitosan in the emulsion turns into an insoluble gel-like form trapped fat, which cannot be decomposed by enzymes such as pancreatin or other intestinal enzymes. As a result, fat excretion in feces is increased (Figure 1). In this connection, [12] [12] have confirmed that in one animal study chitosan administration led to fecal fat excretion approximately 7.5 times higher compared to that of a cellulose-fed group.
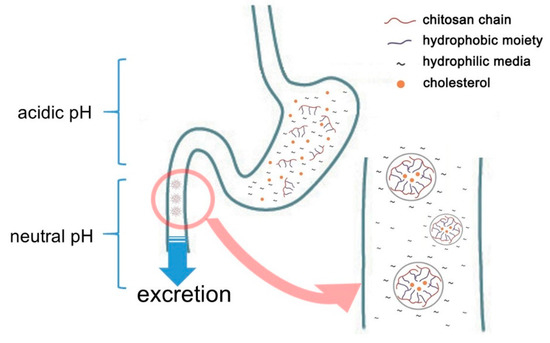
Figure 1. Schematic representation of cholesterol adsorption of chitosan in gastrointestinal tracts.
Meta-analysis is a method of statistical analysis that combines results from various scientific studies to obtain a quantified synthesis [13][13]. Meta-analysis increases the power of statistical analysis by pooling the results from multiple available studies. Therefore, this study summarizes the results of various animal experiments and provides integrated technical data for clinical trials so that clinical trials can proceed more accurately.
2. Role of Chitosan in Lowering Cholesterol
The bioavailability of dietary fat in the intestine decreased after chitosan administration. After this, reverse cholesterol transport, which is delivered from peripheral tissues to the liver, is accelerated by excretion of surplus dietary fat, resulting in an increase in the ratio of HDL-cholesterol [49][14]. Similarly,[15] [50] have reported that the addition of chitosan to an animal diet caused a decrease in LDL-cholesterol content. Generally, HDL-cholesterol may decrease cardiovascular disease by converting cholesterol condensed on peripheral tissues or blood vessel walls into an ester compound. The ester compound is then transferred to the liver, excreted by bile-salt, and cholesterol content in blood is lowered. By contrast, LDL-cholesterol, which is the most general delivery type of blood cholesterol, accumulates easily on artery walls, causing arteriosclerosis. For this reason, it is known as the leading risk factor for arteriosclerosis and cardiovascular [51][16]. In this result, increased HDL-cholesterol, fecal total cholesterol, and triglyceride after chitosan administration are related to the factors mentioned above. According to Jeon and Kim [52][17], when chitosan is cationized (–NH3+), its viscosity is increased by the formation of poly cations and gels. In high viscosity of the intestine, dietary fiber lower blood cholesterol by delaying cholesterol diffusion from micelle to mucosa, inhibiting bile acid metabolism, delaying micelle forming, and reducing cholesterol absorption rate in the intestine [19,53][18]. Based on this result, chitosan exhibits an excellent anti-hypercholesterolemic effect and is thought to be effective in mitigating cardiovascular disease caused by excessive fat intake.
Cytokines are secreted by activated lymphocytes and macrophages, and regulate the function of the cells related to immune response. They are also recognized as playing an essential role in the inflammatory response [54][19]. Yemak et al. [8] [8]report that TNF-α generation was lower in lipopolysaccharide (LPS) and chitosan-injected mice than in LPS-injected mice. Similarly, Seo et al. [7] [7]observed that TNF-α was increased by the application of special stimulants in a human mast cell line (HMC−1), but decreased by the use of chitosan. TNF-α is one of the pro-inflammatory cytokines synthesized by adipose tissue [55,56][20][21], and high TNF-α levels are one of the critical risk factors for diabetes [57][22]. In a similar vein, Yoon et al.[23] [58] state that chitosan is associated with an anti-inflammatory response to TNF-α gene expression. According to Zhu et al. [59][24], chitosan has an anti-inflammatory effect on active molecules, for example TNF-α and IL-1β via the NF-κB pathway. Activated macrophages secrete numerous pro-inflammatory cytokines, including IL-1β and TNF-α, to intermediate the inflammatory response [60][25]. However, overproduction of these pro-inflammatory mediators causes excessive inflammation [61][26]; thus, regulation of the release of pro-inflammatory mediators may be important in mitigating the inflammatory response.
According to Prabu and Naturajan [62][27], blood glucose levels decreased in streptozotocin-induced diabetic rats that were fed chitosan for 30 days. Other researchers suggest that the effectiveness of chitosan in lowering blood glucose may be due in part to the effect of total glyceride in lowering free fatty acids. Jo et al. [63][28] report that in an animal study, chitosan that was enzymatically treated and of low molecular weight (<1000 Da) was more effective in managing prandial glucose. Kim et al. [64] [29]also report that chitosan that is low in molecular weight acted similarly to acarbose, a known anti-diabetic medication, in a murine model. They also note that chitosan administration inhibited sucrase and glucoamylase activities. It is recognized that chitosan binds with glucosidase in the intestinal brush border in a manner similar to acarbose (Hanefeld, [65][30]; Puls et al. [66][31]; Krentz and Bailey [67][32]). The inference of these reports is that body weight may be decreased by chitosan administration.
In the course of this process, heterogeneity is introduced as a result of methodological differences between studies. In general, a heterogeneity test is used to decide on methods for combining studies and to evaluate the consistency or inconsistency of findings (Petitti [68][33]; Higgins et al. [69][34]). To evaluate heterogeneity in relation to effect size in the present study, Q statistics and I2 values were computed. The highest among Q statistics was TG in blood, with high significance (p < 0.0001). The significance of the Q statistic implies that the studies used to calculate the overall effect (the effect size of fixed and random effect models) do not share the same effect size with one another (Cho et al. [70][35]). In this study, the Q statistics for all items were found to be significant (p < 0.0001). However, one limitation of this method is its dependence on the number of studies (Fleiss [71][36]). I2 and τ2 values are commonly used to overcome this limitation of Q statistics by providing a concrete indication of heterogeneity. The I2 value is used most frequently in meta-analysis to compare different numbers of studies and data types. Consequently, it offers a solution to the issue of the Q statistic when analyzing heterogeneity (Higgins et al. [72][37]). All items of I2 value in the present study were above 70%, which means that they all showed significant levels of heterogeneity [73][38]. The τ2 value indicates the absolute value of heterogeneity, representing variance in true effect sizes [74][39]. In addition, liver TG showed the highest τ2 value, which means that variance in the effectiveness of chitosan administration is great (Cho et al. [70][35]).
Cholestyramine (trade name: Questran, Questran Light, Cholybar or Olestyr) and cholestipol (trade name: Colestid or Cholestabyl) as an anion-exchanger are these days used mainly for reducing cholesterol [75][40]. These medications contain amino groups, are water-insoluble, and unlike chitosan are not absorbed in the intestine. Specifically, they form insoluble complexes with bile acids in the intestines, which are then excreted in the feces. As a result, more plasma cholesterol is converted into bile acids in the liver to normalize its levels. When cholesterol is converted into bile acids, plasma cholesterol levels are lowered (National Institute of Diabetes and Digestive and Kidney Diseases [76][41]). Consequently, they are known to inhibit cholesterol absorption in the gut and to promote bile salt excretion. However, they are also known to involve a number of issues, including gastrointestinal disturbance, constipation, and colon cancer [77,78][42][43]. Valhouny et al. [79][44] report that chitosan supplementation showed a similar inhibition effect to cholestyramine in cholesterol adsorption. Similarly, an animal study by Jennings et al. [78][43] showed that chitosan was similar to cholestyramine in lowering lipids without other harmful changes in intestinal mucosa. Currently, a total of 1832 patents related to chitosan are being searched in the field of hyperlipidemia and associated cardiovascular diseases. It can thus be concluded that chitosan supplementation may be useful in lowering cholesterol and offers a promising alternative treatment for lifestyle-related diseases.
References
- Hanssen, S.V.; Daioglou, V.; Steinmann, Z.J.N.; Doelman, J.C.; van Vuuren, D.P.; Huijbregts, M.A.J. The climate change mitigation potential of bioenergy with carbon capture and storage. Nat. Clim. Chang. 2020.
- Arneth, A.; Barbosa, H.; Benton, T.; Calvin, K.; Calvo, E.; Connors, S. Summary For Policymakers. In Climate Change And Land: An IPCC Special Report on Climate Change, Desertification Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Swizerland, 2019.
- Goldemberg, J. Ethanol for a sustainable energy future. Science 2007, 315, 808–810.
- Bordonal, R.O.; Carvalho, J.L.N.; Lal, R.; de Figueiredo, E.B.; de Oliveira, B.G.; La Scala, N. Sustainability of sugarcane production in Brazil. A. review. Agron. Sustain. Dev. 2018, 38.
- OECD/FAO. OECD‑FAO Agricultural Outlook 2019–2028; OECD: Rome, Italy, 2019.
- FAO. Food and Agriculture Organization of the United Nations—FAOStat, Land Use Data 2020. Available online: http://www.fao.org/faostat/en/#data/RL (accessed on 19 November 2020).
- Companhia Nacional de Abastecimento—CONAB. Acompanhamento de Safra Brasileira de Cana-de-açúcar. v. 7—Safra 2020/2021, no. 1—Primeiro Levantamento—Maio 2020. 2020. Available online: https://www.conab.gov.br/info-agro/safras/cana/boletim-da-safra-de-cana-de-acucar (accessed on 20 June 2020).
- SINDIPEÇAS/ABIPEÇAS. Relatório da Frota Circulante 2018. Sindicato Nacional Indústria Componentes Veículos Automotores SINDIPEÇAS Associação Brasileira Indústria Autopeças ABIPEÇAS. 2019. Available online: https://www.sindipecas.org.br/sindinews/Economia/2019/RelatorioFrotaCirculante_Maio_2019.pdf (accessed on 19 November 2020).
- EPE—Empresa de Pesquisa Energética. Brazilian Energy Balance 2020. Brasilia—DF. 2020. Available online: https://www.epe.gov.br/sites-en/publicacoes-dados-abertos/publicacoes/Paginas/Brazilian-Energy-Balance-2020.aspx (accessed on 19 November 2020).
- Brazil, “Lei No. 13576, de 26 de Dezembro de 2017—Dispõe Sobre a Política Nacional de Biocombustíveis (RenovaBio) e dá Outras Providências. 2017. Available online: https://www.planalto.gov.br/ccivil_03/_ato2015-2018/2017/lei/l13576.htm (accessed on 19 November 2020).
- Brazil, “Intended Nationally Determined Contributions (iNDC)—Brazil. 2015. Available online: http://www.mma.gov.br/images/arquivos/clima/convencao/indc/BRAZIL_iNDC_english.pdf (accessed on 19 November 2020).
- Carvalho, J.L.N.; Cerri, C.E.P.; Karlen, D.L. Sustainable Sugarcane Straw Special Issue: Considerations for Brazilian Bioenergy Production. Bioenergy Res. 2019, 12, 746–748.
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574.
- Cherubin, M.R.; Karlen, D.L.; Cerri, C.E.P.; Franco, A.L.C.; Tormena, C.A.; Davies, C.A.; Cerri, C.C. Soil quality indexing strategies for evaluating sugarcane expansion in Brazil. PLoS ONE 2016, 11, e0150860.
- Cherubin, M.R.; Karlen, D.L.; Franco, A.L.C.; Cerri, C.E.P.; Tormena, C.A.; Cerri, C.C. A Soil Management Assessment Framework (SMAF) Evaluation of Brazilian Sugarcane Expansion on Soil Quality. Soil Sci. Soc. Am. J. 2016, 80, 215–226.
- Cherubin, M.R.; Karlen, D.L.; Franco, A.L.C.; Tormena, C.A.; Cerri, C.E.P.; Davies, C.A.; Cerri, C.C. Soil physical quality response to sugarcane expansion in Brazil. Geoderma 2016, 267, 156–168.
- Luz, F.B.; Carvalho, M.L.; de Borba, D.A.; Schiebelbein, B.E.; de Lima, R.P.; Cherubin, M.R. Linking Soil Water Changes to Soil Physical Quality in Sugarcane Expansion Areas in Brazil. Water 2020, 12, 3156.
- Franco, A.L.C.; Bartz, M.L.C.; Cherubin, M.R.; Baretta, D.; Cerri, C.E.P.; Feigl, B.J.; Wall, D.H.; Davis, C.A.; Cerri, C.C. Loss of soil (macro)fauna due to the expansion of Brazilian sugarcane acreage. Sci. Total Environ. 2016, 563, 160–168.
- Franco, A.L.C.; Cherubin, M.R.; Cerri, C.E.P.; Six, J.; Wall, D.H.; Cerri, C.C. Linking soil engineers, structural stability, and organic matter allocation to unravel soil carbon responses to land-use change. Soil Biol. Biochem. 2020, 150.
- Barbosa, L.C.; Magalhães, P.S.G.; Bordonal, R.O.; Cherubin, M.R.; Castioni, G.A.F.; Tenelli, S.; Franco, H.C.J.; Carvalho, J.L.N. Soil physical quality associated with tillage practices during sugarcane planting in south-central Brazil. Soil Tillage Res. 2019, 195, 104383.
- Lehmann, J.; Bossio, D.A.; Knabner, I.K.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020.
- Vera, I.; Wicke, B.; van der Hilst, F. Spatial variation in environmental impacts of sugarcane expansion in Brazil. Land 2020, 9, 397.
- Filoso, S.; Carmo, J.B.D.; Mardegan, S.F.; Lins, S.R.M.; Gomes, T.F.; Martinelli, L.A. Reassessing the environmental impacts of sugarcane ethanol production in Brazil to help meet sustainability goals. Renew. Sustain. Energy Rev. 2015, 52, 1847–1856.
- Silva-Olaya, A.M.; Cerri, C.E.P.; La Scala, N.; Dias, C.T.S.; Cerri, C.C. Carbon dioxide emissions under different soil tillage systems in mechanically harvested sugarcane. Environ. Res. Lett. 2013, 8.
- La Scala, N.; Bolonhezi, D.; Pereira, G.T. Short-term soil CO2 emission after conventional and reduced tillage of a no-till sugar cane area in southern Brazil. Soil Tillage Res. 2006, 91, 244–248.
- Bento, C.B.; Filoso, S.; Pitombo, L.M.; Cantarella, H.; Rossetto, R.; Martinelli, L.A.; Carmo, J.B. Impacts of sugarcane agriculture expansion over low-intensity cattle ranch pasture in Brazil on greenhouse gases. J. Environ. Manag. 2018, 206, 980–988.
- Gunkel, G.; Kosmol, J.; Sobral, M.; Rohn, H.; Montenegro, S.; Aureliano, J. Sugar cane industry as a source of water pollution—Case study on the situation in Ipojuca river, Pernambuco, Brazil. Water Air Soil Pollut. 2007, 180, 261–269.
- Taniwaki, R.H.; Cassiano, C.C.; Filoso, S.; Ferraz, S.F.d.; de Camargo, P.B.; Martinelli, L.A. Impacts of converting low-intensity pastureland to high-intensity bioenergy cropland on the water quality of tropical streams in Brazil. Sci. Total Environ. 2017, 584, 339–347.
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270.
- de Weill, M.A.M.; Sparovek, G. Erosion study in the ceveiro watershed (Piracicaba, SP). II—Interpreting soil loss tolerance using the Soil Useful Life Index methodology. Rev. Bras. Cienc. do Solo 2008, 32, 815–824.
- Youlton, C.; Wendland, E.; Anache, J.A.A.; Poblete-Echeverría, C.; Dabney, S. Changes in erosion and runoff due to replacement of pasture land with sugarcane crops. Sustainability 2016, 8, 685.
- Gomes, T.F.; Van de Broek, M.; Govers, G.; Silva, R.W.C.; Moraes, J.M.; Camargo, P.B.; Mazzi, E.A.; Martinelli, L.A. Runoff, soil loss, and sources of particulate organic carbon delivered to streams by sugarcane and riparian areas: An isotopic approach. Catena 2018, 181, 104083.
- Carvalho, J.L.N.; Nogueirol, R.C.; Menandro, L.M.S.; de Oliveira Bordonal, R.; Borges, C.D.; Cantarella, H.; Franco, H.C.J. Agronomic and environmental implications of sugarcane straw removal:A major review. GCB Bioenergy 2017, 9, 1181–1195.
- Blanco-Canqui, H.; Ruis, S.J. No-tillage and soil physical environment. Geoderma 2018, 326, 164–200.
- Luo, Z.; Wang, E.; Sun, O.J. Can no-tillage stimulate carbon sequestration in agricultural soils? A meta-analysis of paired experiments. Agric. Ecosyst. Environ. 2010, 139, 224–231.
- Segnini, A.; Carvalho, J.L.N.; Bolonhezi, D.; Milori, D.M.B.P.; da Silva, W.T.L.; Simões, M.L.; Cantarella, H.; de Maria, I.C.; Martin-Neto, L. Carbon stock and humification index of organic matter affected by sugarcane straw and soil management. Sci. Agric. 2013, 70, 321–326.
- Tenelli, S.; Bordonal, R.O.; Barbosa, L.C.; Carvalho, J.L.N. Can reduced tillage sustain sugarcane yield and soil carbon if straw is removed? Bioenergy Res. 2019, 12, 764–777.
- Bai, X.; Huang, Y.; ren, W.; Coyne, M.; Jacinthe, P.A.; Tao, B.; Hui, D.; Yang, J.; Matocha, C. Responses of soil carbon sequestration to climate-smart agriculture practices: A meta-analysis. Glob. Chang. Biol. 2019, 25, 2591–2606.
- Sun, W.; Canadell, J.G.; Yu, L.; Yu, L.; Zhang, W.; Smith, P.; Fischer, T.; Huang, Y. Climate drives global soil carbon sequestration and crop yield changes under conservation agriculture. Glob. Chang. Biol. 2020, 26, 3325–3335.
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129.
- Zotarelli, L.; Zatorre, N.P.; Boddey, R.M.; Urquiaga, S.; Jantalia, C.P.; Franchini, J.C.; Alves, B.J.R. Influence of no-tillage and frequency of a green manure legume in crop rotations for balancing N outputs and preserving soil organic C stocks. F. Crop. Res. 2012, 132, 185–195.
- Cerri, C.C.; Galdos, M.V.; Maia, S.M.F.; Bernoux, M.; Feigl, B.J.; Powlson, D.; Cerri, C.E.P. Effect of sugarcane harvesting systems on soil carbon stocks in Brazil: An examination of existing data. Eur. J. Soil Sci. 2011, 62, 23–28.
- Chagas, M.F.; Bordonal, R.O.; Cavalett, O.; Carvalho, J.L.N.; Bonomi, A.; La Scala, N. Environmental and economic impacts of different sugarcane production systems in the ethanol biorefinery. Biofuels Bioprod. Biorefining 2016, 10, 89–106.
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover crops and ecosystem services: Insights from studies in temperate soils. Agron. J. 2015, 107, 2449–2474.
