In response to exercise, articular chondrocytes increase their production of glycosaminoglycans, bone morphogenic proteins, and anti-inflammatory cytokines and decrease their production of proinflammatory cytokines and matrix-degrading metalloproteinases. These changes are associated with improvements in cartilage organization and reductions in cartilage degeneration. Studies in humans indicate that exercise enhances joint recruitment of bone marrow-derived mesenchymal stem cells and upregulates their expression of osteogenic and chondrogenic genes, osteogenic microRNAs, and osteogenic growth factors. Rodent experiments demonstrate that exercise enhances the osteogenic potential of bone marrow-derived mesenchymal stem cells while diminishing their adipogenic potential, and that exercise done after stem cell implantation may benefit stem cell transplant viability. Physical exercise also exerts a beneficial effect on the skeletal system by decreasing immune cell production of osteoclastogenic cytokines interleukin-1β, tumor necrosis factor-α, and interferon-γ, while increasing their production of antiosteoclastogenic cytokines interleukin-10 and transforming growth factor-β.
- exercise
- osteoarthritis
- osteoporosis
- mesenchyma
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
In the global burden of disease 2010 study, osteoarthritis accounted for 17,135 years of life lived with disability (YLDs), an increase of 64% when compared to YLDs of 1990. Overall, musculoskeletal disorders (which included inflammatory causes of arthritis) accounted for 6.8% of total YLDs [1], with osteoarthritis ranked as the 11th leading cause of disability worldwide [2]. The global prevalence of osteoarthritis is expected to increase as the average age and weight of the world’s population increases.
Physical exercise has long been recognized as an essential factor in the maintenance of skeletal health, particularly during adolescence, when ~50% of bone mass accretion occurs [3]. The 2019 American College of Rheumatology/Arthritis Foundation guidelines for the management of osteoarthritis of the hip and knee emphasized the importance of regularly performed physical exercise [4]. Both traditional (resistance, aerobic, and flexibility) and non-traditional (tai chi, yoga, and aquatic) exercises have been shown to be effective in the management of knee and hip osteoarthritis [5]. In their systematic review of 44 clinical trials involving patients with knee osteoarthritis, Fransen and associates found that land-based therapeutic exercises reduced pain and improved physical function and the quality of life for at least 2–6 months after the cessation of formal treatment [6]. In this regard, the World Health Organization recommends that adult men and women should accumulate at least 150 min of moderate intensity physical exercise per week and young people aged 5–17 years should accumulate at least 60 min of physical exercise of moderate to vigorous intensity daily [7].
There is increasing interest in treating articular cartilage and subchondral bone defects and osteoarthritis with autologous chondrocyte implants (ACIs), matrix autologous chondrocyte implants (MACIs), and bone marrow-derived mesenchymal cell implants or injections [8][9][10][11][12][13][14]. ACI and MACI procedures have been shown to produce durable long-term outcomes in the treatment of partial and full-thickness articular cartilage defects in tibiofemoral joints [15][16][17][18][19]. In addition, mesenchymal stem cells mobilized to joints from the peripheral blood or placed on implantation matrices have the potential to repair cartilage by differentiating into chondrocytes [20].
2. Exercise and Osteoarthritis
Although regularly performed moderate-intensity exercise is recognized as the mainstay treatment of OA [4][5], there are a limited number of studies sampling constituents of the OA joint before and after supervised exercise training of men and women. One of these was published by Roos and Dahlberg and involved 45 subjects who had undergone medial meniscus resection 3–5 years prior to the study and were at risk of developing OA. Subjects underwent supervised exercise training three times weekly for 4 months or were assigned to a noninterventional group. All subjects had the glycosaminoglycan content of their knee cartilage assessed by delayed gadolinium-enhanced magnetic resonance imaging. Exercise increased cartilage levels of glycosaminoglycan in proportion to the level of physical activity [21].
In a similar study, Munukka and associates assessed the effects of 12 months of leisure time physical activity on the glycosaminoglycan content of femoral cartilages in 76 post-menopausal women with knee OA using delayed gadolinium-enhanced magnetic resonance imaging. They also found that exercise increased the amount of cartilage glycosaminoglycan [22].
Iijima and associates studied the effects of 2–4 weeks of treadmill walking in 24 male Wistar rats with induced damage to their knee joints using micro-computed tomography, histology, and immunohistochemistry analysis. They found that exercise prevented the progression of post-traumatic bone and cartilage lesions and increased BMP-2 and BMP-6 expression in the joint superficial zone chondrocytes [23].
Assis and associates studied the effects of aerobic exercise training on an experimental model of knee osteoarthritis in 50 male Wistar rats. Twenty of the rats were trained on treadmills 3 days/week at 16 m/min for 50 min/day for 8 weeks. The exercising and control rats were sacrificed, and their knee joints assessed by histologic, morphometric, and immunohistochemical analysis. Compared to the controls, exercising animals had a better pattern of cartilage organization and less cartilage degeneration. Exercising animals also had lower chondrocyte nuclear or nucleolar expression of IL-1β, caspase-3, and MMP-13, confirming the ability of aerobic exercise to downregulate proinflammatory and proteolytic pathways in this model of OA [24].
3. Exercise and Mesenchymal Stem Cells
The author is using the International Society for Cell and Gene Therapy committee’s recommendation that the acronym “MSC” be used for both mesenchymal stem cells and mesenchymal stromal cells and that the MSC acronym be preceded by “BM” for bone marrow origin and “AD” for adipose tissue origin [25].
3.1. Exercise Studies in Rodents
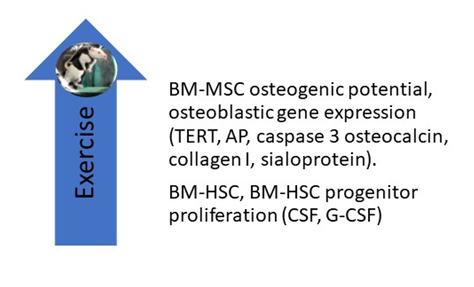
Liu et al. reported the effects of 8 weeks of treadmill exercise (60 min per day at 19.3 m/min, 5-degree incline) on the proliferative, differential, and apoptotic abilities of cultured femoral bone marrow-derived mesenchymal stem cells (BM-MSCs). They found that exercise enhanced their osteogenic potential and decreased their adipogenic potential (Figure 1) and posited that “BMSC derived from exercised rats on early passage may be a good cell source for bone tissue engineering” [26]. Emmons et al. report that 15 and 60 min of treadmill exercise done by C57 BI/6 mice increased the proliferative capacity of their bone marrow hematopoietic stem cells (BM-HSCs) and multipotential HSC progenitors by 40–61%. They attribute these findings to a change in the BM-HSC secretome, which included an upregulation of granulocyte colony-stimulating factor (G-CSF) and stem cell factor (SCF) [27]. Bourzac et al. reviewed literature reports on the effects of physical exercise on MSC proliferation, differentiation, and homing and found that the effects of exercise varied depending on the exercise protocol and the tissue from which MSCs were obtained; they concluded that “the combination of physical exercise and MSC engraftment improves neural, cartilage, and muscular tissue recovery, but it is not clear whether the effects of MSCs and exercise are additive or synergistic” [28]. Ocarino et al. studied the effects of exercise on BM-MSCs in osteopenic female Wistar rats with and without nitric oxidase inhibition. BM-MSCs were isolated from their femurs and cultured in osteogenic medium for 7, 14, and 21 days, phenotyped, and analyzed for alkaline phosphatase, collagen synthesis, and the formation of mineralized nodules. They found that exercise increased BM-MSC osteogenesis and that inhibition of nitric oxide diminished their osteogenic response. They concluded that “nitric oxide mediates the beneficial effects of physical activity upon MSCs osteogenic differentiation” [29]. Hell and associates measured the effects of treadmill exercise on the osteogenic potential of BM-MSCs in young and adult female Wistar rats by measuring cell viability, percentage of cells per field, mineralized nodular number, and gene expression for telomerase reverse transcriptase (TERT), alkaline phosphatase (AP), caspase 3, osteocalcin, collagen I, and sialoprotein. They found that exercise increased the differentiation of BM-MSCs in both study groups, but the effect was greater in young animals than in adults [30]. Using mice, Wallace and associates measured the effects of 5 days of treadmill exercise (30 min/day) on BM-MSCs and found that exercise increased their osteogenic potential [31]. Yamaguchi measured the effects of exercise on the ability of BM-MSCs obtained from male Wistar rats to repair experimentally induced femoral groove osteochondral defects in female Wistar rats. Two weeks after BM-MSCs were injected into the defective joints, rats were either sedentary or subjected to 2, 4, or 8 weeks of treadmill exercises performed 5 days/week at 12 m/min for 30 min; the animals were then sacrificed, and their joints subjected to immuno-histochemical staining. Compared to the sedentary group, they found that exercise enhanced cartilage repair and concluded that their study “highlights the importance of exercise following cell transplantation therapy” [32].
Figure 1. Studies in rodents have shown that treadmill exercise upregulates the osteogenic potential of BM-MSCs, including their expression of osteogenic genes. Exercise also increases the proliferative capacity of BM-HSC and BM-HSC progenitors by upregulating G-CSF and CSF levels in their secretome. AP, alkaline phosphatase; BM-HSC, bone marrow-derived hematopoietic stem cell; CSF, colony-stimulating factor; G-CSF, granulocyte colony stimulating factor; TERT, telomerase reverse transcriptase.
3.2. Exercise Studies in Humans
There are a limited number of studies on the effect of exercise on human BM-MSCs. Schmidt and associates studied the effects of short-term high-intensity exercise on the ability of post-exercise sera to influence the proliferation, migration, and apoptosis activity of cultured BM-MSCs. They found that post-exercise sera enhanced the migratory capacity of BM-MSCs, a finding they attributed to the generation of IL-6 by contracting skeletal muscles. They posited that “there is a direct relationship between exercise, IL-6 release and stem cell recruitment” [33]. Carbonare et al. studied the effects of running one-half of a marathon on the differentiation potential of mesenchymal circulating progenitor cells (M-CPCs) and on the effects of sera on a human bone marrow-derived mesenchymal stem cell (hBM-MSC) line in 22 athletes. They found that exercise upregulated the expression of osteogenic genes Runx2, muscle segment homeobox gene 1 (MSx1), secreted phosphoprotein 1 (SPP-1), and chondrogenic genes SRY-Box 9 (SOX9) and collagen type II alpha-1 gene (COL2A1), and apoptosis-related genes autophagy-related gene 3 (ATG3) and Unc-like kinase gene (Ulk1) in M-CPCs. BMP2 and BMP6 were also upregulated. The authors concluded that exercise upregulated the differentiation and apoptosis of BM-MSCs [34]. In a study involving 20 amateur runners, Valenti et al. assessed the effects of running one-half of a marathon on the expression of microRNAs (miRNAs) in human BM-MSCs incubated with pre- and post-exercise sera. They found that exercise upregulated the expression of miRNAs promoting osteoblast differentiation, including miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p, while downregulating the expression of a miRNA that promotes adipocyte differentiation (miRNA-188-5p). They also found that exercise upregulated the expression of the osteogenic gene Runx2 [35]. Niemiro et al. studied the kinetics of progenitor cell mobilization during 60 min of treadmill exercises (70% Vo2peak) performed by seven men. They found that exercise increased circulating levels of cysteine x cysteine (CXC) chemokine ligand (CXCL)-12 and SCF in hematopoietic stem cells but not in BM-MSCs. They concluded that exercise may serve as a valuable adjunct in the context of HSC transplants [36].
In summary, experiments in humans, while limited in number, have shown that exercise upregulates BM-MSC and BM-HSC recruitment, enhances BM-MSC osteogenic, chondrogenic, and apoptotic gene expression, and upregulates BM-MSC expression of osteogenic miRNAs and the secretion of growth factors (Figure 2).
Figure 2. Studies in humans indicate that exercise increases the recruitment of BM-MSCs and BM-HSCs and upregulates BM-MSC expression of osteogenic, chondrogenic, and apoptotic genes, osteogenic microRNAs, and osteogenic growth factors. ATG3, autophagy-related gene 3; COL2A1, collagen type II alpha-1 gene; MSx1, muscle segment homeobox gene 1; SPP-1, secreted phosphoprotein 1; SOX-9; Ulk1, Ul kinase gene 1; miR, microRNA.
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330.
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138.
- Troy, K.L.; Mancuso, M.E.; Butler, T.A.; Johnson, J.E. Exercise early and often: Effects of physical activity and exercise on women’s bone health. Int. J. Environ. Res. Public Health 2018, 15, 878, doi:10.3390/ijerph15050878.
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020, 72, 149–162.
- Wellsandt, E.; Golightly, Y. Exercise in the management of knee and hip osteoarthritis. Curr. Opin. Rheumatol. 2018, 30, 151–159.
- Fransen, M.; McConnell, S.; Harmer, A.R.; Van der Esch, M.; Simic, M.; Bennell, K.L. Exercise for osteoarthritis of the knee: A Cochrane systematic review. Br. J. Sports Med. 2015, 49, 1554–1557.
- Cavanaugh, A.R.; Schwartz, G.J.; Blouet, C. Global Health Estimates (2015). Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015; World Health Organization: Geneva, Switzerland, 2015.
- Freitag, J.; Bates, D.; Boyd, R.; Shah, R.; Barnard, A.; Huguenin, L.; Tenen, A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: Reparative pathways, safety and efficacy—A review. Musculoskelet. Disord. 2016, 17, 230, doi:10.1186/s12891-016-1085-9.
- Shariatzadeh, M.; Song, J.; Wilson, S.L. The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019, 378, 399–410.
- Song, J.-S.; Hong, K.-T.; Kim, N.-M.; Jung, J.-Y.; Park, H.-S.; Chun, Y.S.; Kim, S.J. Cartilage regeneration in osteoarthritic knees treated with distal femoral osteotomy and intra-lesional implantation of allogenic umbilical cord blood-derived mesenchymal stem cells: A report of two cases. Knee 2019, 26, 1445–1450.
- Richardson, S.M.; Gauthaman, K.; Pushparaj, P.N.; Matta, C.; Richardson, S.M.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Memic, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and interverbal disc regeneration. Methods 2016, 99, 69–80, doi:10.1016/j.ymeth.2015.09.015.
- Häfner, S.J. The body’s integrated repair kit: Studying mesenchymal stem cells for better ligament repair. Biomed. J. 2019, 42, 365–370.
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Ben, P.; Hung, B.P.; Gimble, J.M. Stromal cells and stem cells in clinical bone. Nat. Rev. Endocrinol. 2015, 11, 140–150.
- Mitxitorena, I.; Infante, A.; Gener, B.; Rodriguez, C.I. Suitability and limitations of mesenchymal stem cells to elucidate human bone illness. World J. Stem Cells 2019, 11, 578–593.
- Boehm, E.; Minkus, M.; Scheibel, M. Autologous chondrocyte implantation for treatment of focal articular cartilage defects of the humeral head. J. Shoulder Elb. Surg. 2020, 29, 2–11.
- Minas, T.; Ogura, T.; Bryant, T. Autologous chondrocyte implantation. JBJS Essent. Surg. Tech. 2016, 6, e24, doi:10.2106/JBJS.ST.16.00018.
- Marlovits, S.; Striessnig, G.; Kutscha-Lissberg, F.; Resinger, C.; Aldrian, S.M.; Vécsei, V.; Trattnig, S. Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 451–457.
- Ebert, J.R.; Fallon, M.; Ackland, T.R.; Wood, D.J.; Janes, G.C. Arthroscopic matrix-induced autologous chondrocyte implantation: 2-year outcomes. Arthroscopy 2012, 28, 952–964.
- Ebert, J.R.; Fallon, M.; Wood, D.J.; Janes, G.C. A prospective clinical and radiological evaluation at 5 years after arthroscopic matrix-induced autologous chondrocyte implantation. Am. J. Sports Med. 2017, 45, 59–69.
- Ukon, Y.; Makino, T.; Kodama, J.; Tsukazaki, H.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. Molecular-based treatment strategies for osteoporosis: A literature review. Int. J. Mol. Sci. 2019, 20, 2557, doi:10.3390/ijms20102557.
- Roos, E.M.; Dahlberg, L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage. Arthr. Rheum. 2005, 52, 3507–3514.
- Munukka, M.; Waller, B.; Häkkinen, A.; Nieminen, M.T.; Lammentausta, E.; Kujala, U.M.; Paloneva, J.; Kautiainen, H.; Kiviranta, I.; Heinonen, A. Physical activity is related to cartilage quality in women with knee osteoarthritis. Med. Sci. Sports Exerc. 2017, 49, 1323–1330.
- Iijima, H.; Aoyama, T.; Ito, A.; Tajino, J.; Yamaguchi, S.; Nagai, M.; Kiyan, W.; Zhang, X.; Kuroki, H. Exercise intervention increases expression of bone morphogenic proteins and prevents the progression of cartilage-subchondral bone lesions in a post-traumatic rat knee model. Osteoarthr. Cartil. 2016, 24, 1092–1102.
- Assis, L.; Milares, L.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.; Medalha, C.; Renno, A.M. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cart. 2016, 24, 169–177.
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (TSCT) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024.
- Liu, S.-Y.; He, Y.-B.; Deng, S.-Y.; Zhu, W.-T.; Xu, S.-Y.; Ni, G.-X. Exercise affects biological characteristics of mesenchymal stromal cells derived from bone marrow and adipose tissue. Int. Orthop. 2017, 41, 1199–1209.
- Emmons, R.; Niemiro, G.M.; Owolabi, O.; De Lisio, M. Acute exercise mobilizes hematopoietic stem and progenitor cells and alters the mesenchymal stromal cell secretome. J. Appl. Physiol. 2016, 120, 624–632.
- Bourzac, C.; Bensidhoum, M.; Pallu, S.; Portier, H. Use of adult mesenchymal stromal cells in tissue repair: Impact of physical exercise. Am. J. Physiol. Cell Physiol. 2019, 317, C642–C654.
- Ocarino, N.M.; Boeloni, J.N.; Goes, A.M.; Silva, J.F.; Marubayashi, U.; Serakides, R. Osteogenic differentiation of mesenchymal stem cells from osteopenic rats subjected to physical activity with and without nitric oxide synthase inhibition. Nitric Oxide 2008, 19, 320–325.
- Hell, R.C.R.; Ocarino, N.M.; Boeloni, J.N.; Silva, J.F.; Goes, A.M.; Santos, R.L.; Serakides, R. Physical activity improves age-related decline in the osteogenic potential of rats’ bone marrow-derived mesenchymal stem cells. Acta Physiol. 2012, 205, 292–301.
- Wallace, I.J.; Pagnotti, G.M.; Rubin-Sigler, J.; Naeher, M.; Copes, L.E.; Judex, S.; Rubin, C.T.; Demes, B. Focal enhancement of the skeleton to exercise correlates with responsivity of bone marrow mesenchymal stem cells rather than peak exertional forces. J. Exp. Biol. 2015, 218 Pt 19, 3002–3009, doi:10.1242/jeb.118729.
- Yamaguchi, S.; Aoyama, T.; Ito, A.; Nagai, M.; Iijima, H.; Tajino, J.; Zhang, X.; Kiyan, W.; Kuroki, H. The effect of exercise on the early stages of mesenchymal stromal cell-induced cartilage repair in a rat osteochondral defect model. PLoS ONE 2016, 11, e0151580, doi:10.1371/journal.pone.0151580.
- Schmidt, A.; Bierwirth, S.; Weber, S.; Platen, P.; Schinköthe, T.; Bloch, W. Short intensive exercise increases the migratory activity of mesenchymal stem cells. Br. J. Sports Med. 2009, 43, 195–198.
- Carbonare, L.D.; Mottes, M.; Cheri, S.; Deiana, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Salvagno, G.L.; Lippi, G.; Valenti, M.T. Increased gene expression of RUNX2 and SOX9 in mesenchymal circulating progenitors is associated with autophagy during physical activity. Oxidative Med. Cell. Longev. 2019, doi:10.1155/2019/8426259.
- Valenti, M.T.; Deiana, M.; Cheri, S.; Dotta, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Carbonare, L.D.; Mottes, M. Physical exercise modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p expression in progenitor cells promoting osteogenesis. Cells 2019, 8, 742, doi:10.3390/cells8070742.
- Niemiro, G.M.; Parel, J.; Beals, J.; Van Vliet, S.; Paluska, S.A.; Moore, D.R.; Burd, N.A.; De Lisio, M. Kinetics of circulating progenitor cell mobilization during submaximal exercise. J. Appl. Physiol. 2017, 122, 675–682.