One of the main constraints in aquaculture production is farmed fish vulnerability to diseases due to husbandry practices or external factors like pollution, climate changes, or even the alterations in the dynamic of product transactions in this industry. It is though important to better understand and characterize the intervenients in the process of a disease outbreak as these lead to huge economical losses in aquaculture industries. High-throughput technologies like proteomics can be an important characterization tool especially in pathogen identification and the virulence mechanisms related to host-pathogen interactions on disease research and diagnostics that will help to control, prevent, and treat diseases in farmed fish. Proteomics' important role is also maximized by its holistic approach to understanding pathogenesis processes and fish responses to external factors like stress or temperature making it one of the most promising tools for fish pathology research.
- proteomics
- fish diseases
- aquaculture
- fish welfare
- fish pathologies
1. Introduction
The demand for animal protein for human consumption is rising as a result of an exponential increase in the world population. Aquaculture is becoming an increasingly important source of protein available for human consumption since is an industry capable of providing solutions to feed a rapidly growing human population and reduce poverty in many countries [1,2,3][1][2][3]. To achieve that, the scale of aquaculture production and the range of farmed species has increased dramatically over the last two decades [4]. Live production always comprises a risk for loss due to infectious diseases [5], with farmed fish, due to husbandry practices in aquaculture, being more vulnerable than wild fish to diseases from a wide range of bacterial, viral, parasitic, and fungal infections [6]. Also, the tendency to higher density production systems, the perturbations in ecological systems balance related to pollution and climatic changes, and the expected increase in international transactions of aquaculture products and their derivatives contributed to alterations in the dynamics of interaction between organisms, infectious agents, and people. This influences pathogen rates of replication and proliferation, leading to a broader geographic distribution of pathogenic agents and an increase in species affected by disease outbreaks [7,8][7][8]. This makes disease outbreaks an important constraint to this industry, with a significant impact on the quality, safety, and volume of the fish produced throughout the world [9[9][10][11][12],10,11,12], that can lead to market access exclusion and major economic loss or costs to the producer [8,13,14][8][13][14].
For several authors, disease outbreaks in aquaculture are the result of a complex network of interactions on aquatic systems between the produced organism, several environmental and zootechnical aspects, and possible pathogenic agents, that present a series of unique challenges in aquatic organism’s health [15[15][16][17][18][19],16,17,18,19], as represented in Figure 1.
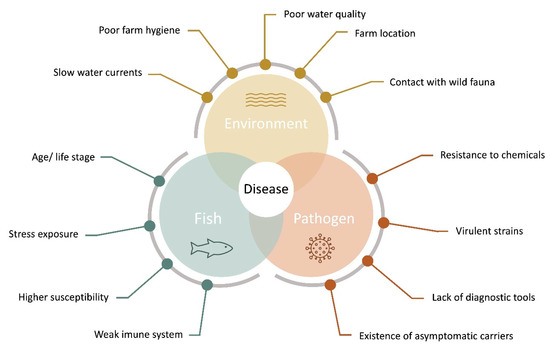
Aquaculture disease diagram, indicating the main factors for the evaluation of pathogen, and host-pathogen interactions intervening in fish disease outbreaks (adapted from [19][20]).
To address infectious pathologies in farmed fish approaches like epidemiological studies on main areas of aquatic animal health as transboundary and emerging aquatic animal diseases, animal health surveillance and biosecurity program development should be performed. These are crucial to disease prevalence monitorization, early detection of emerging exotic and new diseases, and quality management improvement of aquaculture operations [15,18,19,21][15][18][19][21].
Nevertheless, to obtain proper epidemiological models, animal health surveillance and biosecurity programs must integrate environmental information and information from different areas like pathogenesis, disease diagnosis, disease resistance, physiological response to pathogens, pathogen characterization, host immune system responses characterization, disease biomarkers, and organism response to disease treatment products [22,23][22][23].
The amount of data from different origins and an increase in the reported frequency and severity of marine diseases demands that new diagnostic tools should be implemented for a more rapid and effective diagnosis [24,25,26][24][25][26]. Thus, several scientific advances in aquatic health continue to close the gap to veterinary medicine, and new optical, analytical chemistry, molecular biology [27], and Omics techniques are becoming a reality that offers a vast array of benefits to the aquaculture industry [12,28][12][28]. Proteomics techniques are one of those new tools, and one of the most interesting approaches for health management, epidemiology, and fish disease research [3,22,23,29,30][3][22][23][29][30]. Proteomics refers to the methodology that addresses the study of the entire complement of proteins expressed in a specific state of an organism or a cell population [31,32][31][32]. The proteome, or the full protein complement of the genome, is a highly structured entity, where proteins exert their cellular functions with specificity in time and location, in physical or functional association with other proteins or biomolecules [33,34][33][34]. High-throughput proteomics methods based on mass spectrometry (MS) allow the measurement of multiple properties for thousands of proteins, including their abundance, tissue distribution, subcellular localization, post-translational modifications, and protein-protein interactions [34]. Proteomics-based approaches can therefore offer unique insights into fish cellular regulation in response to pathogens and during disease progression, besides enabling fast and sensitive pathogen detection and identification.
2. Fish Health, Stress, and Welfare
Despite being the most consumed animal, fish are seldom afforded the same level of concern regarding their welfare as other vertebrates. The scientific research around fish welfare is at an early stage compared with other land animals produced for human consumption [35]. In part, this lack of consideration is due to the gap between public perception of their intelligence and the scientific evidence [36], along with the absence of a unified definition of the concept [37]. Nevertheless, most definitions consider mainly a feelings-based and a function-based approach [38]. The first gives regard to the emotional-like state of the animal, while good welfare is defined as the absence of negative feelings and the presence of positive feelings [39]. The second definition is more focused on the biological, physiological, and health perspective of the animal, while good welfare is defined as the fish’s ability to cope and adapt to its environment while maintaining homeostasis [40]. Although the fish’s health state offers objective criteria as part of a welfare assessment, it does not provide the complete picture. Good health is essential to ensure good welfare, however, it does not necessarily indicate that the fish is in a good welfare state [37]. On the other hand, poor health i.e., the reduced ability of the animal to normal functioning, to cope with stressful conditions, and to prevent disease, generally implies/leads to a bad welfare status in a variety of contexts. For example, deceased fish, as a consequence of disease, constitute a source of infection and compromise water quality [41]. Additionally, chemical treatments for specific outbreaks can also trigger some level of disturbance on the fish [42,43][42][43]. Importantly, a healthy animal in an optimal welfare environment can also be suddenly struck by an acute infection reducing its welfare. For instance, in the case of fish produced in cages, pathogens are naturally embedded in the environment [44]. In most cases, it is often the lousy welfare status itself, due to poor husbandry conditions, which translates into impaired health. Thus, in summary, health and welfare are intimately linked, and poor welfare can be interpreted both as a cause and a consequence of poor health. This section focuses on health as a cornerstone for fish welfare assessment and the effects of stressors on disease resistance, reviewing the most recent approaches employed to study the relationship between certain diseases/pathologies and welfare.
In aquaculture, inappropriate husbandry conditions, or even standard farming practices, are everyday stressors in culture systems [45]. The allostatic load imposed on the animals can reduce functioning immune mechanisms, consequently favoring diseases and threatening fish welfare (Figure 2). For instance, drastic changes in water temperature (from 27 °C to either 19–23 °C or 31–35 °C) decreased the immune response and resistance to pathogens in Mozambique tilapia (Oreochromis mossambicus) [46]. More recently, using a transcriptomics approach, the rearing density in Nile tilapia (Oreochromis niloticus) was shown to significantly impact the susceptibility to the oomycete Saprolegnia parasitica [47]. However, the association between husbandry-induced stress and disease is not that straightforward. For example, acute stressors have been reported to enhance [48][49][50][51] [48,49,50,51] or decrease [52][53] [52,53] some innate immune responses in fish. On the contrary, chronic stressors have mainly been indicated as immunosuppressors [54,55,56,57,58][54][55][56][57][58]. From a productivity perspective, the health of the fish is often interpreted as “absence of disease”, since, from either an ethical or an economic point of view, any disease state is unacceptable for the industry [44]. Therefore, disease prevention and eradication are crucial aspects of a fish farm to ensure the product’s sustainability. Providing optimal welfare conditions, monitoring the health parameters routinely, and alleviating stress are necessary steps towards this goal.
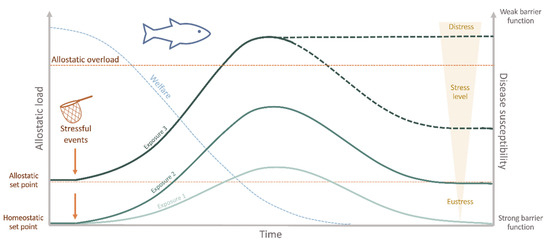
Interaction between welfare, allostatic load, disease susceptibility, and the repetitive/chronic stressful experiences appraised by the fish. Stressful stimuli may induce either adaptive (eustress) or maladaptive allostasis (distress). If the stressor persists, recovery to the original homeostatic state (homeostatic set point) may be incomplete. In this case, a newly defined set point for future adaptation is settled (allostatic setpoint). As a result, the welfare status is decreased with time and stress experienced. The cumulative burden of adaptation (allostatic load) is thus constituted by the beneficial stressful events that the fish can cope with, while the allostatic overload represents the state when stress overcomes the organism’s natural regulatory capacity, which may induce a state of no-recovery. At this step, primary barrier function is severely impaired increasing disease susceptibility, which may cause illness and ultimately death.
Stress is considered a state of threatened homeostasis [59], which is re-established by a complex network of changes in the physiological systems (allostasis) [60]. As in all other vertebrates, in the face of a perceived stressor, fish launch a widespread reaction, the so-called physiological stress response, which allows the individual to adjust and cope with the predictable and unpredictable changes in its surroundings (eustress) [61]. As a primary response, cortisol and catecholamines are released into the bloodstream, which will induce a series of downstream reactions [62]. In fact, stress is not necessarily detrimental nor immediately equates to compromised welfare. Instead, in the short term, it is an essential adaptation to ensure the best chances of survival [37]. However, when reaching an allostatic overload, usually as a result of a prolonged, repeated, and/or unavoidable stressor, maladaptive effects such as impaired growth and/or reproductive and immune functions, arise (Figure 2) [63,64][63][64]. In this case, these are largely associated with diminished welfare and may jeopardize fish health and survival (distress) [65]. The questions raised here are the cost of this acclimation and why stress increases diseases’ susceptibility in fish. First, in terms of energetic costs, the adaptive physiological response needed to counteract the disrupted homeostasis requires a significant amount of energy. This means that if part of the fish’s energy is allocated to face the challenge, then fewer resources will be available for other energy-demanding biological functions, such as some mechanisms of the defense repertoire: the epithelial barriers and the immune system [44]. In terms of immune responses, several mechanisms are immediately activated to respond directly to the challenge. These include an increase of inflammatory markers, the release of hormones, and the expression of acute-phase proteins [66]. Even if a fish has managed to adapt to the stressor for a certain period, these energy stores will eventually be depleted if the stressor persists. Consequently, the total consumption of energy reserves gives rise to the allostatic overload, and the fish may no longer be able to adapt, which can lead to immunosuppression, disease, and in the case of more severe disturbances, even death (Figure 2) [63]. Moreover, several studies also demonstrated the impact of stressful husbandry conditions on the functioning of the epithelial barriers, i.e., the mucus and the epidermal surfaces of the skin, gills, and intestine, which constitute the primary lines of defense against pathogens and harmful substances, showing that injury of these barriers, inevitably leads to impaired disease resistance [67]. Changes in these barriers have been reported in Atlantic salmon (Salmo salar), Atlantic cod (Gadus morhua), and rainbow trout (Oncorhynchus mykiss) subjected to different acute stressors [68,69][68][69]. Moreover, in Atlantic salmon reared under low dissolved oxygen levels, impaired intestinal barrier function was also observed [70]. These disturbances have mainly been associated with high cortisol levels, though various other hormones, such as catecholamines, endogenous opioids, pituitary hormones, and serotonin, intervene here [71]. Indeed, it is known that cortisol plays an immunomodulatory role, inhibiting specific constituents of the immune system and enhancing others, such as induction of apoptosis, change of differentiation patterns, inhibition of cytokine release, and inhibition of immunocyte migration [72,73,74,75][72][73][74][75]. Nevertheless, the cortisol response may vary among different species and even among individuals (coping styles) [76] [76] and be affected by several other parameters (e.g., domestication level, age, nutritional state, stressor severity, among others) [53,77,78,79][53][77][78][79], which may obscure the relationship between stress and immune status. A detailed description of how the endocrine-immune response is mounted and the mechanisms behind these immunoregulatory changes is out of the scope of this review, for this, the authors refer to recent publications [66,80][66][80].
Deepening our scientific knowledge on the mechanisms relating to stress, fish health, and welfare, is paramount for the sustainable aquaculture industry. In recent years, more advanced high-throughput technologies, as the case of proteomics, started to be successfully employed in aquaculture research, including for the study of fish diseases and welfare, providing a holistic understanding of the molecular events underlying the physiological stress response and valuable insights on the differential proteins involved in inflammatory processes and immune responses [30,58,81][30][58][81]. Proteomic studies on fish target mainly the liver, however, blood plasma and mucus are taking crescent importance, mainly from an immunological point of view, as skin mucus is one of the primary barriers of defense in fish [82,83,84,85,86][82][83][84][85][86] and plasma acts as a mirror/reporter of physiological or pathological conditions [87,88][87][88]. Important applications of proteomics in this field concern the study of the effects of certain diseases and parasites on the proteins’ abundance and modifications and the investigation of the host-pathogen interactions [88,89,90,91,92,93,94,95][88][89][90][91][92][93][94][95].
References
- Bank, T.W. Fish to 2030—Prospects for Fisheries and Aquaculture; International Bank for Reconstruction and Development/International Development Association or The World Bank: Washington, DC, USA, 2013; p. 77.
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020.
- Rodrigues, P.M.; Schrama, D.; Campos, A.; Osório, H.; Freitas, M. Applications of Proteomics in Aquaculture. In Agricultural Proteomics Volume 1: Crops, Horticulture, Farm Animals, Food, Insect and Microorganisms; Salekdeh, G.H., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 165–199.
- Murray, A.G.; Peeler, E.J. A framework for understanding the potential for emerging diseases in aquaculture. Prev. Vet. Med. 2005, 67, 223–235.
- Waagbø, R. Chapter 13 Feeding and disease resistance in fish. In Biology of Growing Animals; Mosenthin, R., Zentek, J., Żebrowska, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 4, pp. 387–415.
- Barber, I. Parasites, behaviour and welfare in fish. Appl. Anim. Behav. Sci. 2007, 104, 251–264.
- Brugere, C.; Onuigbo, D.M.; Morgan, K.L. People matter in animal disease surveillance: Challenges and opportunities for the aquaculture sector. Aquaculture 2017, 467, 158–169.
- Chintagari, S.; Hazard, N.; Edwards, G.; Jadeja, R.; Janes, M. Risks associated with fish and seafood. Preharvest Food Saf. 2018, 123–142.
- Hill, B.J. The need for effective disease control in international aquaculture. Dev. Biol. 2005, 121, 3–12.
- Shinn, A.P.; Pratoomyot, J.; Bron, J.E.; Paladini, G.; Brooker, E.E.; Brooker, A.J. Economic costs of protistan and metazoan parasites to global mariculture. Parasitology 2015, 142, 196–270.
- Iwama, G.K. The welfare of fish. Dis. Aquat. Org. 2007, 75, 155–158.
- Adams, A.; Thompson, K.D. Biotechnology offers revolution to fish health management. Trends Biotechnol. 2006, 24, 201–205.
- Aung, M.M.; Chang, Y.S. Traceability in a food supply chain: Safety and quality perspectives. Food Control 2014, 39, 172–184.
- Trienekens, J.; Zuurbier, P. Quality and safety standards in the food industry, developments and challenges. Int. J. Prod. Econ. 2008, 113, 107–122.
- Peeler, E.J.; Taylor, N.G.H. The application of epidemiology in aquatic animal health -opportunities and challenges. Vet. Res. 2011, 42, 94.
- Hedrick, R.P. Movement of pathogens with the international trade of live fish: Problems and solutions. Rev. Sci. Tech. 1996, 15, 523–531.
- Cameron, A. Survey Toolbox for Aquatic Animal Diseases: A Practical Manual and Software Package; ACIAR Monograph No. 94; Australian Centre for International Agricultural Research: Canberra, Australia, 2002; 375p.
- Oidtmann, B.C.; Peeler, E.J.; Thrush, M.A.; Cameron, A.R.; Reese, R.A.; Pearce, F.M.; Dunn, P.; Lyngstad, T.M.; Tavornpanich, S.; Brun, E.; et al. Expert consultation on risk factors for introduction of infectious pathogens into fish farms. Prev. Vet. Med. 2014, 115, 238–254.
- Sitjà-Bobadilla, A.; Oidtmann, B. Chapter 5—Integrated Pathogen Management Strategies in Fish Farming. In Fish Diseases; Jeney, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 119–144.
- Freitas, J.; Vaz-Pires, P.; Câmara, J.S. From aquaculture production to consumption: Freshness, safety, traceability and authentication, the four pillars of quality. Aquaculture 2020, 518, 734857.
- Scarfe, A.D.; Palić, D. Aquaculture biosecurity: Practical approach to prevent, control, and eradicate diseases. In Aquaculture Health Management; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–116.
- Rodrigues, P.M.; Martin, S.A.M.; Silva, T.S.; Boonanuntanasarn, S.; Schrama, D.; Moreira, M.; Raposo, C. Proteomics in Fish and Aquaculture Research. In Proteomics in Domestic Animals: From Farm to Systems Biology; de Almeida, A.M., Eckersall, D., Miller, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 311–338.
- Cash, P. Proteomics in the study of the molecular taxonomy and epidemiology of bacterial pathogens. Electrophoresis 2009, 30, S133–S141.
- Lafferty, K.D. The ecology of climate change and infectious diseases. Ecology 2009, 90, 888–900.
- Parrington, J.; Coward, K. Use of emerging genomic and proteomic technologies in fish physiology. Aquat. Living Resour. 2002, 15, 193–196.
- Burge, C.A.; Friedman, C.S.; Getchell, R.; House, M.; Lafferty, K.D.; Mydlarz, L.D.; Prager, K.C.; Sutherland, K.P.; Renault, T.; Kiryu, I.; et al. Complementary approaches to diagnosing marine diseases: A union of the modern and the classic. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371.
- Gotesman, M.; Menanteau-Ledouble, S.; Saleh, M.; Bergmann, S.M.; El-Matbouli, M. A new age in AquaMedicine: Unconventional approach in studying aquatic diseases. BMC Vet. Res. 2018, 14, 178.
- Oskoueian, E.; Eckersall, P.D.; Bencurova, E.; Dandekar, T. Application of Proteomic Biomarkers in Livestock Disease Management. In Agricultural Proteomics Volume 2: Environmental Stresses; Salekdeh, G.H., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 299–310.
- Alves, R.N.; Cordeiro, O.; Silva, T.S.; Richard, N.; de Vareilles, M.; Marino, G.; Di Marco, P.; Rodrigues, P.M.; Conceição, L.E.C. Metabolic molecular indicators of chronic stress in gilthead seabream (Sparus aurata) using comparative proteomics. Aquaculture 2010, 299, 57–66.
- Rodrigues, P.M.; Silva, T.S.; Dias, J.; Jessen, F. PROTEOMICS in aquaculture: Applications and trends. J. Proteom. 2012, 75, 4325–4345.
- Wilkins, M.R.; Pasquali, C.; Appel, R.D.; Ou, K.; Golaz, O.; Sanchez, J.-C.; Yan, J.X.; Gooley, A.A.; Hughes, G.; Humphery-Smith, I.; et al. From Proteins to Proteomes: Large Scale Protein Identification by Two-Dimensional Electrophoresis and Arnino Acid Analysis. Bio/Technology 1996, 14, 61–65.
- Cox, J.; Mann, M. Quantitative, high-resolution proteomics for data-driven systems biology. Annu. Rev. Biochem. 2011, 80, 273–299.
- Aebersold, R.; Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 2016, 537, 347–355.
- Parker, C.G.; Pratt, M.R. Click Chemistry in Proteomic Investigations. Cell 2020, 180, 605–632.
- Huntingford, F.A.; Adams, C.; Braithwaite, V.A.; Kadri, S.; Pottinger, T.G.; Sandøe, P.; Turnbull, J.F. Current issues in fish welfare. J. Fish Biol. 2006, 68, 332–372.
- Brown, C. Fish intelligence, sentience and ethics. Anim. Cogn. 2015, 18, 1–17.
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235.
- Martins, C.I.M.; Galhardo, L.; Noble, C.; Damsgård, B.; Spedicato, M.T.; Zupa, W.; Beauchaud, M.; Kulczykowska, E.; Massabuau, J.-C.; Carter, T.; et al. Behavioural indicators of welfare in farmed fish. Fish Physiol. Biochem. 2012, 38, 17–41.
- Dawkins, M.S. Evolution and Animal Welfare. Q. Rev. Biol. 1998, 73, 305–328.
- Saraiva, J.L.; Castanheira, M.F.; Arechavala-López, P.; Volstorf, J.; Studer, B.H. Domestication and welfare in farmed fish. In Animal Domestication; IntechOpen: London, UK, 2018.
- Wall, T. Disease and Medicines—The Welfare Implications. In Fish Welfare; Blackwell Publishing Ltd.: Oxford, Uk, 2008; pp. 195–201.
- Sørum, U.; Damsgård, B. Effects of anaesthetisation and vaccination on feed intake and growth in Atlantic salmon (Salmo salar L.). Aquaculture 2004, 232, 333–341.
- Huntingford, F.A.; Kadri, S. Defining, assessing and promoting the welfare of farmed fish. Rev. Sci. Tech. 2014, 33, 233–244.
- Segner, H.; Sundh, H.; Buchmann, K.; Douxfils, J.; Sundell, K.S.; Mathieu, C.; Ruane, N.; Jutfelt, F.; Toften, H.; Vaughan, L. Health of farmed fish: Its relation to fish welfare and its utility as welfare indicator. Fish Physiol. Biochem. 2012, 38, 85–105.
- Conte, F.S. Stress and the welfare of cultured fish. Appl. Anim. Behav. Sci. 2004, 86, 205–223.
- Ndong, D.; Chen, Y.-Y.; Lin, Y.-H.; Vaseeharan, B.; Chen, J.-C. The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol. 2007, 22, 686–694.
- Ellison, A.R.; Uren Webster, T.M.; Rey, O.; Garcia de Leaniz, C.; Consuegra, S.; Orozco-terWengel, P.; Cable, J. Transcriptomic response to parasite infection in Nile tilapia (Oreochromis niloticus) depends on rearing density. BMC Genom. 2018, 19, 723.
- Jiang, I.-F.; Bharath Kumar, V.; Lee, D.-N.; Weng, C.-F. Acute osmotic stress affects Tilapia (Oreochromis mossambicus) innate immune responses. Fish Shellfish Immunol. 2008, 25, 841–846.
- Caipang, C.M.A.; Brinchmann, M.F.; Berg, I.; Iversen, M.; Eliassen, R.; Kiron, V. Changes in selected stress and immune-related genes in Atlantic cod, Gadus morhua, following overcrowding. Aquac. Res. 2008, 39, 1533–1540.
- Korytář, T.; Nipkow, M.; Altmann, S.; Goldammer, T.; Köllner, B.; Rebl, A. Adverse Husbandry of Maraena Whitefish Directs the Immune System to Increase Mobilization of Myeloid Cells and Proinflammatory Responses. Front. Immunol. 2016, 7, 631.
- Caipang, C.M.A.; Berg, I.; Brinchmann, M.F.; Kiron, V. Short-term crowding stress in Atlantic cod, Gadus morhua L. modulates the humoral immune response. Aquaculture 2009, 295, 110–115.
- Costas, B.; Conceição, L.E.C.; Aragão, C.; Martos, J.A.; Ruiz-Jarabo, I.; Mancera, J.M.; Afonso, A. Physiological responses of Senegalese sole (Solea senegalensis Kaup, 1858) after stress challenge: Effects on non-specific immune parameters, plasma free amino acids and energy metabolism. Aquaculture 2011, 316, 68–76.
- Fast, M.D.; Hosoya, S.; Johnson, S.C.; Afonso, L.O.B. Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long-term stress. Fish Shellfish Immunol. 2008, 24, 194–204.
- Vazzana, M.; Cammarata, M.; Cooper, E.L.; Parrinello, N. Confinement stress in sea bass (Dicentrarchus labrax) depresses peritoneal leukocyte cytotoxicity. Aquaculture 2002, 210, 231–243.
- Mauri, I.; Romero, A.; Acerete, L.; MacKenzie, S.; Roher, N.; Callol, A.; Cano, I.; Alvarez, M.C.; Tort, L. Changes in complement responses in Gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) under crowding stress, plus viral and bacterial challenges. Fish Shellfish Immunol. 2011, 30, 182–188.
- MacKenzie, S.; Iliev, D.; Liarte, C.; Koskinen, H.; Planas, J.V.; Goetz, F.W.; Mölsä, H.; Krasnov, A.; Tort, L. Transcriptional analysis of LPS-stimulated activation of trout (Oncorhynchus mykiss) monocyte/macrophage cells in primary culture treated with cortisol. Mol. Immunol. 2006, 43, 1340–1348.
- Douxfils, J.; Lambert, S.; Mathieu, C.; Milla, S.; Mandiki, S.N.M.; Henrotte, E.; Wang, N.; Dieu, M.; Raes, M.; Rougeot, C.; et al. Influence of domestication process on immune response to repeated emersion stressors in Eurasian perch (Perca fluviatilis L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 173, 52–60.
- de Magalhães, C.R.; Schrama, D.; Farinha, A.P.; Revets, D.; Kuehn, A.; Planchon, S.; Rodrigues, P.M.; Cerqueira, M. Protein changes as robust signatures of fish chronic stress: A proteomics approach to fish welfare research. BMC Genom. 2020, 21, 309.
- Moberg, G.P. Biological response to stress: Implications for animal welfare. Biol. Anim. Stress Basic Princ. Implic. Anim. Welf. 2000, 1, 21.
- McEwen, B.S.; Wingfield, J.C. What is in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2010, 57, 105–111.
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625.
- Pankhurst, N.W. The endocrinology of stress in fish: An environmental perspective. Gen. Comp. Endocrinol. 2011, 170, 265–275.
- Korte, S.M.; Olivier, B.; Koolhaas, J.M. A new animal welfare concept based on allostasis. Physiol. Behav. 2007, 92, 422–428.
- Boonstra, R. Reality as the leading cause of stress: Rethinking the impact of chronic stress in nature. Funct. Ecol. 2013, 27, 11–23.
- Holden, C. Researchers Pained by Effort to Define Distress Precisely. Science 2000, 290, 1474–1475.
- Yada, T.; Tort, L. Interactions. In Fish Physiology; Schreck, C.B., Tort, L., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 365–403.
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739.
- Olsen, R.E.; Sundell, K.; Hansen, T.; Hemre, G.-I.; Myklebust, R.; Mayhew, T.M.; Ringø, E. Acute stress alters the intestinal lining of Atlantic salmon, Salmo salar L.: An electron microscopical study. Fish Physiol. Biochem. 2002, 26, 211–221.
- Olsen, R.E.; Sundell, K.; Mayhew, T.M.; Myklebust, R.; Ringø, E. Acute stress alters intestinal function of rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture 2005, 250, 480–495.
- Sundh, H.; Kvamme, B.O.; Fridell, F.; Olsen, R.E.; Ellis, T.; Taranger, G.L.; Sundell, K. Intestinal barrier function of Atlantic salmon (Salmo salar L.) post smolts is reduced by common sea cage environments and suggested as a possible physiological welfare indicator. BMC Physiol. 2010, 10, 22.
- Douxfils, J.; Mathieu, C.; Mandiki, S.N.M.; Milla, S.; Henrotte, E.; Wang, N.; Vandecan, M.; Dieu, M.; Dauchot, N.; Pigneur, L.M.; et al. Physiological and proteomic evidences that domestication process differentially modulates the immune status of juvenile Eurasian perch (Perca fluviatilis) under chronic confinement stress. Fish Shellfish Immunol. 2011, 31, 1113–1121.
- Saeij, J.P.; Verburg-van Kemenade, L.B.; van Muiswinkel, W.B.; Wiegertjes, G.F. Daily handling stress reduces resistance of carp to Trypanoplasma borreli: In vitro modulatory effects of cortisol on leukocyte function and apoptosis. Dev. Comp. Immunol. 2003, 27, 233–245.
- Pruett, S.B. Stress and the immune system. Pathophysiology 2003, 9, 133–153.
- Esteban, M.A.; Rodríguez, A.; Ayala, A.G.; Meseguer, J. Effects of high doses of cortisol on innate cellular immune response of seabream (Sparus aurata L.). Gen. Comp. Endocrinol. 2004, 137, 89–98.
- Baschant, U.; Tuckermann, J. The role of the glucocorticoid receptor in inflammation and immunity. J. Steroid Biochem. Mol. Biol. 2010, 120, 69–75.
- Castanheira, M.F.; Conceição, L.E.C.; Millot, S.; Rey, S.; Bégout, M.-L.; Damsgård, B.; Kristiansen, T.; Höglund, E.; Øverli, Ø.; Martins, C.I.M. Coping styles in farmed fish: Consequences for aquaculture. Rev. Aquac. 2017, 9, 23–41.
- Koakoski, G.; Oliveira, T.A.; da Rosa, J.G.; Fagundes, M.; Kreutz, L.C.; Barcellos, L.J. Divergent time course of cortisol response to stress in fish of different ages. Physiol. Behav. 2012, 106, 129–132.
- Madaro, A.; Fernö, A.; Kristiansen, T.S.; Olsen, R.E.; Gorissen, M.; Flik, G.; Nilsson, J. Effect of predictability on the stress response to chasing in Atlantic salmon (Salmo salar L.) parr. Physiol. Behav. 2016, 153, 1–6.
- Martinez-Porchas, M.; Martinez-Cordova, L.R.; Ramos-Enriquez, R. Cortisol and glucose: Reliable indicators of fish stress. Pan-Am. J. Aquat. Sci. 2009, 4, 158–178.
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375.
- Peng, X.-X. Proteomics and its applications to aquaculture in China: Infection, immunity, and interaction of aquaculture hosts with pathogens. Dev. Comp. Immunol. 2013, 39, 63–71.
- Provan, F.; Jensen, L.B.; Uleberg, K.E.; Larssen, E.; Rajalahti, T.; Mullins, J.; Obach, A. Proteomic analysis of epidermal mucus from sea lice–infected Atlantic salmon, Salmo salar L. J. Fish Dis. 2013, 36, 311–321.
- Rajan, B.; Lokesh, J.; Kiron, V.; Brinchmann, M.F. Differentially expressed proteins in the skin mucus of Atlantic cod (Gadus morhua) upon natural infection with Vibrio anguillarum. BMC Vet. Res. 2013, 9, 1–11.
- Easy, R.H.; Ross, N.W. Changes in Atlantic salmon Salmo salar mucus components following short- and long-term handling stress. J. Fish Biol. 2010, 77, 1616–1631.
- Cordero, H.; Morcillo, P.; Cuesta, A.; Brinchmann, M.F.; Esteban, M.A. Differential proteome profile of skin mucus of gilthead seabream (Sparus aurata) after probiotic intake and/or overcrowding stress. J. Proteom. 2016, 132, 41–50.
- Guardiola, F.A.; Cuartero, M.; Del Mar Collado-González, M.; Díaz Baños, F.G.; Cuesta, A.; Moriñigo, M.; Esteban, M. Terminal carbohydrates abundance, immune related enzymes, bactericidal activity and physico-chemical parameters of the Senegalese sole (Solea senegalensis, Kaup) skin mucus. Fish Shellfish Immunol. 2017, 60, 483–491.
- Isani, G.; Andreani, G.; Carpenè, E.; Di Molfetta, S.; Eletto, D.; Spisni, E. Effects of waterborne Cu exposure in gilthead sea bream (Sparus aurata): A proteomic approach. Fish Shellfish Immunol. 2011, 31, 1051–1058.
- Moreira, M.; Schrama, D.; Soares, F.; Wulff, T.; Pousão-Ferreira, P.; Rodrigues, P. Physiological responses of reared sea bream (Sparus aurata Linnaeus, 1758) to an Amyloodinium ocellatum outbreak. J. Fish Dis. 2017, 40, 1545–1560.
- Xiong, X.-P.; Dong, C.-F.; Xu, X.; Weng, S.-P.; Liu, Z.-Y.; He, J.-G. Proteomic analysis of zebrafish (Danio rerio) infected with infectious spleen and kidney necrosis virus. Dev. Comp. Immunol. 2011, 35, 431–440.
- Ji, C.; Wu, H.; Wei, L.; Zhao, J.; Wang, Q.; Lu, H. Responses of Mytilus galloprovincialis to bacterial challenges by metabolomics and proteomics. Fish Shellfish Immunol. 2013, 35, 489–498.
- Lü, A.; Hu, X.; Wang, Y.; Shen, X.; Li, X.; Zhu, A.; Tian, J.; Ming, Q.; Feng, Z. iTRAQ analysis of gill proteins from the zebrafish (Danio rerio) infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2014, 36, 229–239.
- Buján, N.; Hernández-Haro, C.; Monteoliva, L.; Gil, C.; Magariños, B. Comparative proteomic study of Edwardsiella tarda strains with different degrees of virulence. J. Proteom. 2015, 127 Part B, 310–320.
- Xu, D.; Song, L.; Wang, H.; Xu, X.; Wang, T.; Lu, L. Proteomic analysis of cellular protein expression profiles in response to grass carp reovirus infection. Fish Shellfish Immunol. 2015, 44, 515–524.
- Braceland, M.; Bickerdike, R.; Tinsley, J.; Cockerill, D.; McLoughlin, M.F.; Graham, D.A.; Burchmore, R.J.; Weir, W.; Wallace, C.; Eckersall, P.D. The serum proteome of Atlantic salmon, Salmo salar, during pancreas disease (PD) following infection with salmonid alphavirus subtype 3 (SAV3). J. Proteom. 2013, 94, 423–436.
- Kumar, G.; Hummel, K.; Noebauer, K.; Welch, T.J.; Razzazi-Fazeli, E.; El-Matbouli, M. Proteome analysis reveals a role of rainbow trout lymphoid organs during Yersinia ruckeri infection process. Sci. Rep. 2018, 8, 13998.
