Green propellants are usually defined as low-hazard, low-toxicity, environmentally friendly propellants that are considered safe during various phases of spacecraft development, launch, and operations. Such propellants provide safe handling and storability when compared to conventional toxic propellants such as hydrazine and its derivatives that require special handling protocols and adhering to strict safety measurements that, in addition to others, include using Self-Contained Atmospheric Protective Ensemble (SCAPE) suits. Due to their favorable characteristics, green propellants demonstrate higher commercial value by being able to cut costs related to transportation, storage, handling, and further reduces ground operations time. Recently, a more specified definition has been noted by Mayer et al. (2018) [3], based on the Acute Toxicity Classification (ATC) by the Global Harmonized System of classification and labeling of chemicals (GHS) [6], which denotes that propellants possessing ATC levels of three and safer are considered as green propellants. ATC levels are typically categorized on a 1:5 scale where level one denotes the most toxic class and level five is considered the least toxic class.
Moreover, a controversial topic arises when referring to some modern green propellants, whether to address them by the term “monopropellants” or by more specific terms including (premixed propellants, fuel blends, or mixtures). Monopropellants are fundamentally defined as propellants consisting of chemical compounds (for example N2H4), which release energy through exothermic chemical decomposition. Since the evolution of liquid gun propellants based on HAN compound and other nitrate salts aqueous solutions, the term “monopropellants” was used to describe such premixed formulations. As widely used in literature and industry, some modern green propellants, for instance the Energetic Ionic Liquids (EILs), are undoubtedly classified and described as “monopropellants.”
Basically, it can be interpreted from the previous that a "green monopropellant" may be defined as: "A low-hazard, low-toxicity, and safe-to handle propellant that is stored in a single tank and is able to decompose from its storage state by the help of a catalyst or other ignition method, such as thermal or electric ignition, can be considered a “green monopropellant” as long as it does not require another separately stored propellant for decomposition."
- green propellant
- monopropellant
- chemical rocket propulsion
- small satellites
- CubeSat
- in-space propulsion
- liquid propulsion system
1. Introduction
The current trend in the rocket propulsion field is directed towards greenifying the use of propellants. Monopropellant hydrazine was classically widely used and favored for thrusters and gas generators due to its high performance, system’s simpler design, and “clean” relatively cool exhaust products as compared to bipropellant systems at that time [1]. European CHemicals Agency (ECHA) in (REACH) Registration, Evaluation, Authorization and restriction of Chemicals has included hydrazine on the list of Substances of Very High Concern (SVHC) for authorization, thus opening a process that will eventually lead to a ban on the use of hydrazine and its derivatives as space propellant in European countries [2]. Moreover, transportability and handling of hydrazine and similar hazardous propellants extend an economic burden on the space industry. Accordingly, greener alternatives that would compensate for these drawbacks are being studied and developed rapidly nowadays [3][4]. Different global entities were involved in accelerating such research activities through various projects and missions such as Green Advanced Space Propulsion (GRASP), Pulsed Chemical Rocket with Green High Performance Propellants (PulCheR), and Replacement of hydrazine for orbital and launcher propulsion systems (RHEFORM) European projects and Green Propellant Infusion Mission (GPIM) technology demonstrator project by NASA. Since the beginning of their development, modern green propellants have shown high favorability not only in terms of operability, cost efficiency, and environmental safety but also in performance, and physicochemical properties [5].
As widely interpreted, green propellants are usually defined as low-hazard, low-toxicity, environmentally friendly propellants that are considered safe during various phases of spacecraft development, launch, and operations. Such propellants provide safe handling and storability when compared to conventional toxic propellants such as hydrazine and its derivatives that require special handling protocols and adhering to strict safety measurements that, in addition to others, include using Self-Contained Atmospheric Protective Ensemble (SCAPE) suits. Due to their favorable characteristics, green propellants demonstrate higher commercial value by being able to cut costs related to transportation, storage, handling, and further reduces ground operations time. Recently, a more specified definition has been noted by Mayer et al. [3], based on the Acute Toxicity Classification (ATC) by the Global Harmonized System of classification and labeling of chemicals (GHS) [6], which denotes that propellants possessing ATC levels of three and safer are considered as green propellants. ATC levels are typically categorized on a 1:5 scale where level one denotes the most toxic class and level five is considered the least toxic class.
Before discussing each green monopropellant family in detail, relatively different classifications in literature were proposed for green alternatives of hydrazine monopropellant, one of them considered mainly three types: Energetic Ionic Liquids, Hydrogen Peroxide, and Nitrous Oxide by Batonneau et al. [7] as cited in [8]. However, Mayer et al. [3] further classified propellants including nitrogen compounds with oxygen into two groups: Oxides of Nitrogen subcategory and Nitro Compounds subcategory. The former included mono and dinitrogen oxides (NO, NO2, N2O, N2O3, N2O4, N2O5), which were evaluated as potential oxidizers for bipropellant systems; among which the only compound that was considered as potential green propellant was the nitrous oxide (N2O) due to its relative nontoxicity (GHS [6] class 5) and being liquid within a wide part of the typically requested temperature-pressure envelope of [−30, +80] °C and [0.1, 3] MPa, respectively. The latter, Nitro Compounds group, was described as organic substances containing dinitrogen monoxide (i.e., hydrocarbons and nitrous oxide) group, such as (mono)nitromethane (CH3NO2 or NM), which was also considered as a promising candidate green propellant being relatively nontoxic (GHS class 4) [3]. It was found that the current available green monopropellants that are suitable for space propulsion, either in primary or auxiliary (secondary) propulsion systems, can be classified into three more collective major categories:
-
Energetic Ionic Liquids (EILs) green monopropellants (or premixed oxidizer/fuel ionic aqueous solutions).
-
Liquid NOx Monopropellants (either in binary compound, nitro compound, or premixed/blend form).
-
Hydrogen Peroxide Aqueous Solutions (HPAS).
2. Green Monopropellants Classification
A controversial topic arises when referring to some modern green propellants, whether to address them by the term “monopropellants” or by more specific terms including (premixed propellants, fuel blends, or mixtures). Monopropellants are commonly defined as propellants consisting of chemical compounds (for example N2H4), which release energy through exothermic chemical decomposition. Since the evolution of liquid gun propellants based on HAN compound and other nitrate salts aqueous solutions—discussed in the next section, the term “monopropellants” was used to describe such premixed formulations. As widely used in literature and industry, some modern green propellants, for instance the EILs, are undoubtedly classified and described as “monopropellants.” Basically, it can be interpreted from the previous example that a propellant that is stored in a single tank and is able to decompose from its storage form by the help of a catalyst or other ignition method, such as thermal or electric ignition, can be considered a “monopropellant” as long as it does not require another separately stored propellant for decomposition. Nitrous Oxide Fuel Blends (NOFBs)—discussed in Section 2.2—were mostly described as green monopropellants as well, maintaining the above-mentioned unique conditions for storage and decomposition of monopropellants.
This section handles each of the three classes proposed for the state-of-the-art green monopropellants. History and origins of development are entitled along with technical data and characteristics including the chemical formulations and constituents of each monopropellant. Thermodynamic and thermochemical properties are gathered from various literature sources—especially resources solely focused on studying propellants thermochemistry. Flight heritage of mature propellants is mentioned, and promising lab test results of other promising propellants are highlighted when possible. Ignition techniques are another important property sought by propulsion systems designers that assist in giving insights about spacecraft mass and volume preliminary requirements as well as electric power needs, thus is noted and discussed deliberately whenever reliable data were available. Finally, physical properties and performance parameters of each propellant are tabulated, and relevant group comparisons are made for the reader’s convenience.
2.1. Energetic Ionic Liquids (EILs)
Energetic Ionic Liquids (or premixed oxidizer/fuel ionic propellant blends) consist of oxidizer salts dissolved in aqueous solutions, called Ionic Liquids (ILs), mixed with Ionic Fuel (IF) or Molecular Fuel (MF), refer to Table 1, forming a premixed propellant (i.e., Energetic Ionic Liquid monopropellant as widely referred to among the rocket propulsion community [8]). Addition of the fuel component increases the performance of the propellant blend by reducing the high adiabatic temperature of the ionic liquid binary aqueous solution and further stabilizing the combustion process. Typically, methanol is used to control the burning rate of the monopropellant while the ammonium nitrate (AN) is used as a stabilizer [9] beside other stabilizing additives; a new article by M. Claßen et al. [10] introduced novel additive promoters of new azido esters and suggested it would improve the total energy and performance of ionic liquid propellants. As an example, the maximum specific impulse of 78 wt% ADN in water mixture (Ionic Liquid) is 192 s when used as monopropellant, while the specific impulse rises to 252 s when methanol (molecular fuel) is added to the mixture, as in the case of LMP-103S (63.4 wt% ADN, 25.4 wt% water and 11.2 wt% methanol) at a nozzle area expansion ratio of 50 [11] as cited in [3]. In the next paragraphs different EILs will be reviewed (i.e., HAN, HAN/HN, HNF, ADN based green monopropellants) emphasizing on their composition, physical properties, performance, stability of storage and handling, toxicity, material compatibility, ignition methods, and in-flight heritage or proposed missions.
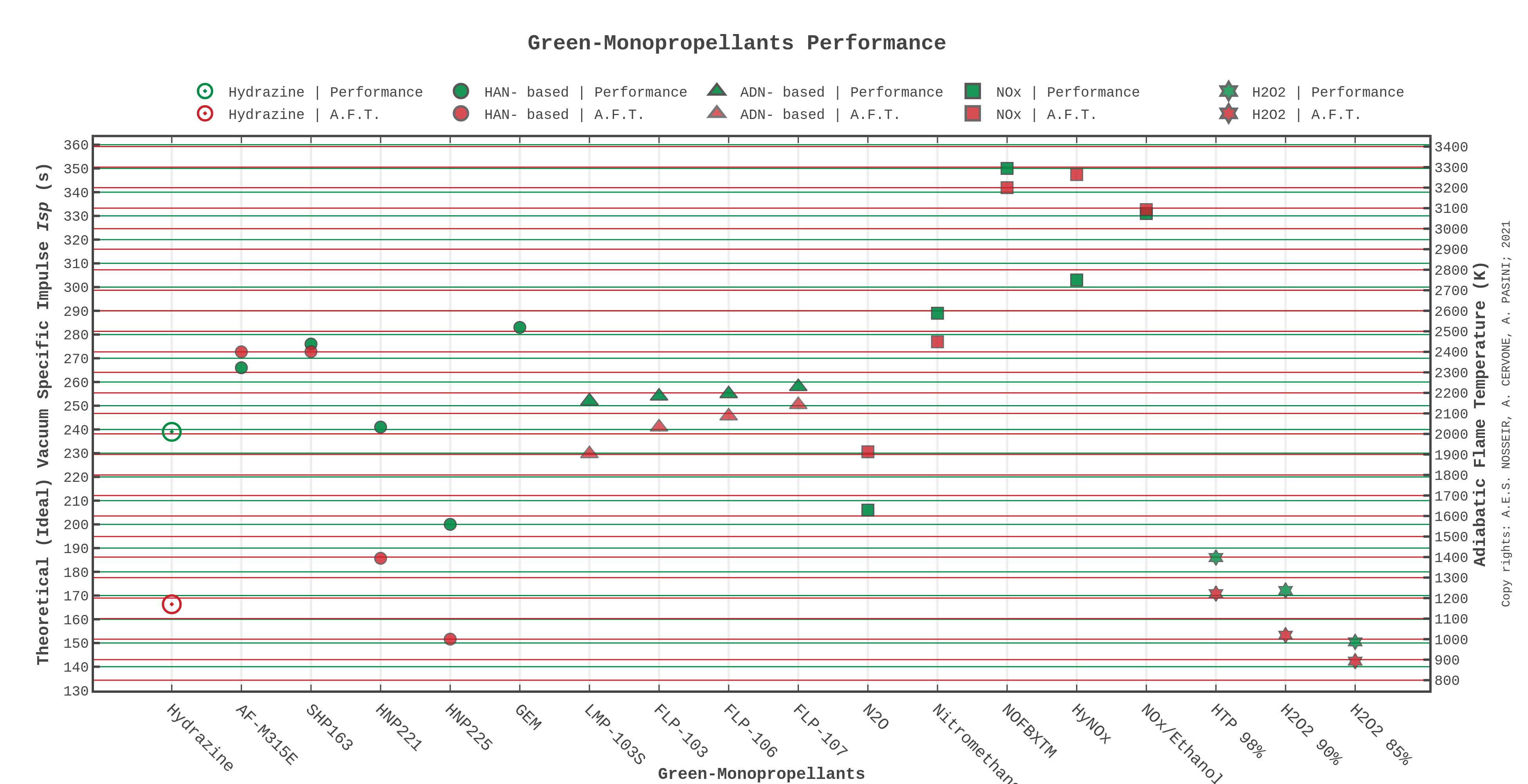
HAN-based monopropellants origins can be traced back to the development of liquid gun propellants (LGPs) in the U.S. Army [12]. Three formulations of LGPs were addressed, namely LP1846, LP1845, and LP1898 [5] and their properties are listed in Table 2. The first two of these aqueous solutions are HAN/TEAN-based (tri-ethanol-ammonium nitrate) while the third is HAN/DEHAN-based (di-ethyl hydroxyl ammonium nitrate). The unsuitability of these propellants for rocket’s relatively low combustion pressure [13], as well as the high combustion temperature (2500 K [5]) eventually lead to the development of the state-of-the-art AF-M315E (Air Force Monopropellant 315E) HAN-based green monopropellant for space propulsion formulated by the U.S. Air Force Research Laboratory (AFRL) [14].
Table 1. Energetic ionic liquids: oxidizers and fuels thermochemical properties [8][15][16][17][18].
| Ionic Oxidizer | Molecular Weight (g mol | −1 | ) | Standard Heat of Formation (kJ mol | −1 | ) | |
|---|---|---|---|---|---|---|---|
. An advantage AF-M315E possesses over current state-of-the-art green propellants is its maturity. Thorough development has taken place to reach this product and be able to test in space on 1 N and 22 N thrusters through the Green Propellant Infusion Mission (GPIM) launched in 2019 [30]. However, a disadvantage over the latest state-of-the-art green propellants rises from the relatively high flame temperature, which makes it difficult to rapidly manufacture an economic and simpler design of thrusters especially for the micro/nano satellites industry. It is worthy of mentioning that current advancements, especially related to rapid prototyping, in low-cost thrusters of small spacecrafts would benefit greatly from using additive manufacturing techniques such as metal 3D printing. Such techniques facilitate the design process and reduce the build time, they typically use metal alloys such as Ti-6Al-4V (Ti64) and Inconel®-625 (nickel-chromium superalloy) with melting points of approximately 1900 and 1570 K, respectively [31][32]. Catalytic decomposition of AF-M315E requires higher preheating temperature, compared to hydrazine, where it typically consumes up to 15 kJ of energy [33] and the catalyst bed preheating nominal start temperature is 315 °C [34], while Busek Co. Inc researchers reported successful ignition at 400 °C preheating temperature [12].
SHP163 is another very interesting HAN-based green propellant, which was being developed since the year 2000 at the Institute of Space and Astronautical Science (ISAS)/(JAXA). SHP163 is composed of 73.6 wt% HAN, 3.9 wt% AN, 16.3 wt% methanol, and 6.2 wt% water [35]. This propellant has density of 1.4 g cm−3 yet achieves high volumetric specific impulse ρIsp of 396 g s cm−3, which is higher than AF-315E (at 0.7 MPa chamber pressure and 50:1 nozzle expansion ratio at frozen conditions) [35]. The flame temperature is considered very high, as it records about 2400 K [35][36]. As SHP163 shows to be one of the most energetic propellants for use in a thruster, it demonstrates operational stability and shows enough safety levels to be accepted as a green and safe liquid propellant [37]. SHP163 is only ignitable using a preheated catalyst-bed under 1.0 MPa [38][39]. Finally, SHP163 was tested in space in the Green Propellant Reaction Control System (GPRCS) utilizing a 1 N class thruster in the RAPIS-1 satellite launched in 2019 by JAXA.
HNP-xxx family (High-performance Non-detonating Propellant) are HAN/HN-based green propellants that have been under development for over 10 years by IHI Aerospace co. in Japan. This green monopropellant family include HNP209, HNP221, and HNP225, and they are formulated from HAN, HN, methanol, and water [32]. They all possess volume specific impulse (ρIsp) superior to hydrazine, but what characterizes them most is their relatively low adiabatic flame temperature compared to other energetic ionic liquid monopropellants such as AF-M315E and SHP163. HNP209 typically has a theoretical specific impulse around 260 s with the highest combustion temperature (~1900 K), while HNP221 and HNP225 have specific impulse of 241 and 213 s, respectively (at chamber pressure of 1.0 MPa and expansion ratio of 100:1) [40][41][42], as shown in Table 3.
Table 3. Physical properties and performance (@ 1 MPa chamber pressure, 100:1 expansion ratio, and vacuum conditions, using NASA Chemical Equilibrium Analysis (CEA) [31] and verified from [4][29][32][35][36][43][44][45]).
| Properties | Hydrazine | AF-M315E | SHP163 | HAN/HN-Based | ||||
|---|---|---|---|---|---|---|---|---|
| HNP221 | HNP225 | |||||||
| 266 | 283 | |||||||
| Density | ρ | (g cm | −3 | ) (@ 20 °C) | 1.0 | 1.24 | 1.47 | 1.51 |
| Volumetric Specific Impulse | ρIsp | (g s cm | −3 | ) | 236 | 312.48 | 391 | 427 |
| Vapor Pressure | PV | (kPa) (@ 25 °C) | 1.91 | 15.1 | 1.4 | < | 1 | |
| Toxicity | High | Moderate | Low | Low | ||||
ADN (ammonium dinitramide)-based green propellants development started at the Swedish Defense Research Agency (FOI) in Europe in 1997 [21][49][50]. The ADN-based monopropellants family mainly consists of FLP-103, 105, 106, 107 and LMP-103S, where the latter was developed by Bradford ECAPS Co. LMP-103S and FLP-106 are the most mature, and the former was qualified by the European Space Agency (ESA) and in-space demonstrated through the High Performance Green Propulsion system (HPGP) on the Mango-PRISMA satellite launched in June 2010 [51][52][53]. Different fuels were used within this energetic ionic liquid mixture such as methanol, monomethyl-formamide MMF and dimethyl-formamide DMF. However, methanol was found incompatible with ADN unless by addition of ammonia (NH3) in order to increase the pH of the mixture [21][54]. Composition of some ADN-based monopropellants are shown in Table 5 where the performance of the FLP-family is shown to be higher than LMP-103S. However, all ADN-based monopropellants mentioned in this study possess volumetric specific impulse lower than that of AF-M315E (391 g s cm−3). ADN-based green monopropellants are not only ignited by preheated catalytic beds, same as all monopropellants, but can also be ignited electrically or using thermal ignition. Larsson et al. [55] found that ADN-based propellants can be ignited using resistive heating by conducting electrical current through the propellants, and very rapid ignition was obtained (less than 2 ms); moreover, the least amount of electric energy utilized for successful ignition was in terms of (20 J). While Wilhelm et al. [52] found that glow-plug ignition was successful for LMP-103S and FLP-106 with satisfying ignition behavior and decomposition. Advantages of the LMP-103S and FLP-family over AF-M315E include, but are not limited to, lower combustion temperature, which allows using materials with lower melting point, and simpler designs for thruster development. Moreover, flexibility in using different ignition techniques and not just being restricted to catalytic decomposition of ADN-based green monopropellants would allow for development of novel designs of monopropellant thrusters.
Table 5. ADN-based monopropellants properties [21][56][57] (ideal vacuum Isp by [56] using NASA CEA @ 2.0 MPa chamber pressure, 50:1 expansion ratio assuming frozen condition [52]).
| Propellant. | Formulation | Theoretical | Isp | (s) | Density | ρ | (g cm | −3 | ) * | ρIsp | (g s cm | −3 | ) | Tc | (°C) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Theoretical Specific Impulse | Isp | (s) | 239 | 260–27020.0% | 276 | 241 | ||||||||||||||||||||
| LMP-103S | (1) | 63.0% | (2) | 213 | ||||||||||||||||||||||
| 18.4% | (6) | 18.6% | 252 | 1.24 | 312.48 | 1630 | ADN, ammonium dinitramide | Density | ρ | (g cm | −3 | ) (@ 20 °C)[NH | 4 | ] | + | [N(NO | 2 | ) | 2 | ] | − | 124.06 | −134.6 | [20] | as cited in | [ |
| LP1845 | 21 | ] | ||||||||||||||||||||||||
| FLP-103 | 63.2%1.0 | (1) | 63.4% 1.47 | (2) | 11.2% | (5) | 1.4 | 25.4% | 2541.22 | 1.16 | HNF, hydrazinium nitroformate | [N | 2 | H | 5 | ] | + | [C(NO | 2 | ) | 3 | ] | − | 183.08 | −72.104 | [19] |
| 20.0% | 0.0% | 16.8% | AN, ammonium nitrate | [NH | 4 | ] | + | [NO | 3 | ] | − | 80.043 | ||||||||||||||
| AA, ammonium azide | [NH | |||||||||||||||||||||||||
| LP1898 | 60.7% | 0.0% | 19.3% | −365.28 | [19] | |||||||||||||||||||||
| HN, hydrazinium nitrate | [N | 2 | ||||||||||||||||||||||||
| 1.31 | 332.74 | 1760 | Volumetric Specific Impulse | ρIsp | (g s cm | −3 | ) | 239 | ~ | 390 | 386 | 294 | H | 5 | ||||||||||||
| FLP-106 | (1) | 64.6% | (3) | 11.5% | 247 | |||||||||||||||||||||
| (5) | 23.9% | 255 | 1.357 | 344.6 | 1814 | Adiabatic Flame Temperature (K) | 1170 | 2166 | 2401 | 1394 | 990 | ] | + | [NO | 3 | ] | − | 95.06 | −211.36 | [19] | ||||||
| Freezing Point (°C) | ||||||||||||||||||||||||||
| FLP-107 | (1) | 65.4% | (4) | 9.3% | (5) | 25.3% | 258 | 1.351 | Ionic Fuel | 4 | ] | + | [N | 3 | ] | − | 60.06 | 113.66 | [19] | |||||||
| 348.5 | 1869 | 1.5 | < | −80 | ≤ | −30 | ≤0 | ≤ | HA, hydrazinium azide | [N | 2 | H | 5 | ] | + | [N | 3 | ] | − | 75.07 | 228.53 | [19] | ||||
| −10 | HEHN,2-hydroxyethyl-hydrazinium nitrate | [HO-C | 2 | H | 4 | -N | 2 | H | 4 | ] | + | [NO | 3 | ] | − | 139.11 | [22] | −388.69 | [23] | |||||||
| Molecular Fuel | ||||||||||||||||||||||||||
| MMF, mono-methylformamide | CH | 3 | HNCHO | 59.067 | −247.4 | [21] | ||||||||||||||||||||
| DMF, di-methylformamide | (CH | 3 | ) | 2 | NCHO | 73.094 | −239.3 | [24] | as cited in | [21] | ||||||||||||||||
| Methanol | ||||||||||||||||||||||||||
HNP225 is the one among the family with the least adiabatic flame temperature around 1000 K (even less than hydrazine ~1200 K), while HNP221 is approximately 1400 K [32][42]. The low temperature combustion gasses allowed IHI Aerospace co. to develop low-cost thrusters since the need for high heat resistant materials or complex cooling for the thruster’s combustion chamber is no longer required. The HNP2xx family of propellants are ignited using catalytic decomposition. Igarashi et al. 2017 [32] performed tests for HNP221 and HNP225 with newly developed catalysts showing excellent response and combustion pressure stability compared to hydrazine, either in continuous mode or pulsed mode operation, with preheating temperatures starting from 200 and 300 °C for HNP221 and HNP225, respectively.
GEM or the Green Electrical Monopropellant is a novel HAN-based energetic ionic liquid composed of HAN, AN, (2,2′-dipyridyl), (1,2,4-triazole), 1H-pyrozol, and water [46]. GEM is a proprietary of Digital Solid-State Propulsion company (DSSP) [46] and is developed as a superior replacement for AF-M315E [45]. This propellant is demonstrated on a lab-scale to be capable of taking place in a multi-mode propulsion system. A “multi-mode” system is where a propulsion system in a satellite can operate as two or more separate modes (e.g., chemical high-thrust mode and electric high-specific impulse mode) under a condition of using a shared propellant tank [47] as cited in [48]. The most appealing properties in GEM is that it can also be electrically ignited without the use of any heavy catalytic beds, and it possesses a significantly large volumetric specific impulse as compared to AF-M315E and the ADN-based LMP-103S green monopropellants [45], refer to Table 4.
Table 4. Performance and physical properties of Green Electric Monopropellant (GEM) compared to state-of-the-art Green Monopropellants (@ 2.0 MPa chamber pressure, 50:1 expansion ratio, and vacuum conditions) [45].
| Properties | Hydrazine | LMP-103S | AF-M315E | GEM |
|---|---|---|---|---|
| Theoretical Specific Impulse | Isp | (s) | 236 | 252 |
6]. Nitrous oxide N2O is the only compound in this group that falls under green propellant umbrella since it is considered GHS class 5 relatively nontoxic, moreover, the critical point stands at 36.4 °C and 7.24 MPa [60] and is liquid in a wide part of the pressure–temperature range mentioned above. N2O possesses good storability characteristics at room temperature especially for long term storage since it does not have decomposition or boiling problems when compared to H2O2 or cryogenic LOX as examples. At 20 °C the saturated vapor pressure of nitrous oxide is ~5.2 MPa, which is high compared to EILs and HPAS, but still considered favorable when considering this propellant for self-pressurizing feed systems.
N2O (liquid) storage density is ~0.745 g cm−3 at 20 °C and ~5.2 MPa vapor pressure [61]. Thus, with suitable pressure vessels N2O can be kept under stable and readily-operating conditions, adding to that, its material compatibility with common tank materials including metals, plastics and composite-materials [59]. Although N2O as monopropellant has lower performance than most EILs, it has an experimental result Isp = 206 s (Tc = 1913.15 K, @ pc = 0.3 MPa, nozzle expansion 200:1 [62]), which is higher than high-test peroxide HTP ~180 s [61]. The most compelling about N2O for modern propulsion system design is that it can be used in the so called “multi-mode” propulsion system [47], where it can act as a propellant for cold-gas, monopropellant propulsion, and/or bipropellant systems while sharing the same propellant tank.
Nitromethane (NM, CH3NO2) is a promising nitro compound green monopropellant candidate for modern in-space propulsion systems for relatively low- to high-thrust range. NM is a relatively nontoxic (GHS class 4 toxicity), viscous, flammable liquid with density of 1.1371 g cm−3, and has a freezing temperature of −28.4 °C [63]. It shows good storability for in-space applications and by adding stabilizer additives (such as, ditertiarybutyl peroxide or chloral, and diacetyl [64]) it could be a highly-attractive liquid monopropellant [65]. Nitromethane can be considered in “multi-mode” systems since it can be used in both mono- and bipropellant propulsion systems, thus increase the overall system optimization. NM as a green monopropellant, possesses high performance (Isp = 289 s) [3], high volumetric specific impulse, and combustion temperature around 2449 K.
NOFB nitrous oxide fuel blends were studied as monopropellants since World War II by the Germans [66]; however, their utilization and development were ceased due to the availability of hydrazine since the 1960s until recently. Because of the current economic and political reforms to greenify the use of propellants, nitrous oxide fuel blends research has been revived in Europe by the European Fuel Blend Development (EUFBD) program carried between 2015–2018 [67].
NOFBXTM monopropellant was invented in the U.S. between 2005 and 2007 under the NASA Mars Advanced Technology program, and later a proprietary of Firestar company [68]. NOFBXTM was demonstrated in the 0.4 N–445 N thrust range with measured specific impulse performance around 325 s, while the theoretical value was ~345 s and chamber temperature ~3200 K at 0.7 MPa with stoichiometric O/F = 3 (or 25%fuel). It is storable as saturated liquid under a wide range of temperatures, with a critical point of 39.48 °C and 7.19 MPa [69].
HyNOx or Hydrocarbon NOx as denoted by German scientists in the German Aerospace Center (DLR) are premixed monopropellants where oxidizer and fuel are premixed in a single tank [70]. HyNOx fuel blend used nitrous oxide with ethylene (i.e., ethene IUPAC name) (N2O/C2H4) due to the similar vapor pressure of the two compounds [71] and has density of 0.879 g cm−3. The recorded theoretical vacuum specific impulse is 303 s obtained with O/F ratio = 6 (~14.29%fuel) under 230 K and 2.5 MPa temperature and pressure [43]. A high combustion temperature up to 3264 K is also recorded as a drawback besides the important need for a flashback-arrestor, by Werling et al. [72].
Nitrous oxide/ethanol (N2O/C2H5OH), another hydrocarbon NOx blend, was the mixture of choice after a tradeoff study carried by Mayer et al. [73] in the EUFBD program. Among hydrocarbons, ethanol showed better ignitibility and moderate flame temperature, and further demonstrates stability and miscibility with nitrous oxide. Physical properties of the selected mixture resulted in saturated liquid density of 0.892 g cm−3 with a stoichiometric O/F ratio of 5.73 (~14.86%fuel), the critical point stood at 36.45 °C and 6.3 MPa and the vapor pressure at bubble point was 2.6 MPa, while a theoretical specific impulse Isp = 331 s and combustion temperature 3093 K were reported [73]. A test campaign was carried out with a 600 N thruster and specific impulse of 259 s was achievable [74]. Drawbacks reported during this study are: high combustion temperature, which requires complex engine design and active cooling system, incompatibility of the nitrous oxide fuel blend with titanium was expected to exist, possibility of flammable vapors in the propellant tank, and low density at practical storage temperatures [71][73][74]. Advantages of nitrous oxide fuel blends, as most green monopropellants, are nontoxic and noncarcinogenic nature, low freezing point, higher specific impulse than hydrazine, and the most prominent advantage is self-pressurization capabilities, which allows for simple feed-system and tank-pressurization system design. Performance and properties of liquid NOx monopropellants are shown in Table 6.
Table 6. Ideal (vacuum) performance and physical properties of the Liquid NOx Monopropellants class (compounds and premixed fuel blends).
| Propellant | Theoretical | Isp | (s) | ρ | (g cm | −3 | ) | a | ρIsp | (g s cm | −3 | ) | Tc | (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 2 | O (liquid) * | 206 | 0.745 | 153.5 | 1640 | ||||||||
| Nitromethane ** | 289 | 1.1371 | 328.6 | 2175.85 | ||||||||||
| NOFBX | TM | *** | 350 | 0.700 | 245 | 2926.85 | ||||||||
| HyNOx (Ethene) | † | 303 | 0.879 | 266.3 | 2990.85 | |||||||||
| CH | ||||||||||||||
| 3 | ||||||||||||||
| OH | ||||||||||||||
| NOx/Ethanol | ‡ | 331 | 0.892 | 295.3 | 2819.85 | |||||||||
| 32.04 | ||||||||||||||
a @ 20 °C. * @ pc = 0.3 MPa. ** @ pc =1 MPa. *** @ pc = 0.7 MPa and stoichiometric O/F = 3. † @ pc = 2.5 MPa and stoichiometric O/F = 6. ‡ @ pc =1 MPa and stoichiometric O/F = 5.73.
2.3. Hydrogen Peroxide Aqueous Solutions (HPAS)
Hydrogen peroxide (H2O2) has been used as monopropellant in different aerospace applications since 1938 [75]. H2O2 is type-classified according to its concentration in aqueous solution, and grade-classified according to the concentration of stabilizers and impurities [76], as shown in Table 7. HTP (High-Test Peroxide) is a highly concentrated H2O2, greater than >85% weight concentration. Rocket grade HTP is used in space propulsion for low and medium thrust applications and is typically of 98% concentration [77]. The high density of 98% HTP (~1.43 g cm−3), and the nontoxic nature, makes it an interesting candidate for storage in propulsion systems in general. In monopropellant systems it can catalytically decompose reaching temperatures in terms of 1222 K [77]. Performance of HTP 98% in monopropellant systems is ~20% less than hydrazine [78], with Isp ~186 s (at 1 MPa and 50:1 expansion conditions). However, in bipropellant systems, especially with hydrocarbons such as ethanol, it can reach Isp > 325 s (combustion temperature 2752 K) [3], which makes it a very competitive propellant for this kind of propulsion system. Another concentration of hydrogen peroxide that is widely used is the HTP 87.5% with a density of ~1.38 g cm−3 at 20 °C and possesses a theoretical specific impulse of ~144 s when evaluated at a chamber pressure of 1 MPa and Ae/At of 7.841 at sea level [79]. H2O2 at concentrations of 90% and 85% were simulated on RPA to give, respectively, theoretical vacuum specific impulse of 172.13 and 150.47 s, chamber temperature 1019.3 and 892.65 K at 1 MPa, and expansion ratios of 40:1 and 10:1 applying the shifting equilibrium model for the whole nozzle.
Table 7. Physical and chemical properties of H2O2 propellant with different concentrations [76][80].
| Properties | H | 2 | O | 2 | Propellant Classification | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 70 | Type 85 | Type 90 | Type 98 | ||||||||||||||||||||||
| Grade ES | Grade ES | Grade ES | Grade HP | Grade HP | |||||||||||||||||||||
| Concentration % | 71.0–73.0 | 85.0–87.0 | 90.0–91.5 | 98.0–99.0 | |||||||||||||||||||||
| Stability * | ≤ | 2% | |||||||||||||||||||||||
| Density | ρ | (g cm | −3 | ) ** | ~1.29 | ~1.34 | ~1.40 | ~1.43 | |||||||||||||||||
| Freezing point (°C) | −40 | −17 | −12 | −2 | |||||||||||||||||||||
| −238.77 | |||||||||||||||||||||||||
| [ | |||||||||||||||||||||||||
| 19 | |||||||||||||||||||||||||
| [ | |||||||||||||||||||||||||
| 25 | |||||||||||||||||||||||||
| ] | |||||||||||||||||||||||||
| 20.0% | |||||||||||||||||||||||||
| ] | |||||||||||||||||||||||||
| Ethanol | CH | 3 | CH | 2 | OH | 46.07 | −277.755 | [19] | |||||||||||||||||
| Glycerol | (CH | 2 | OH) | 2 | CHOH | 92.094 | −669.6 | ||||||||||||||||||
| Glycine | NH | 2 | CH | 2 | COOH | 75.07 | −528.0 | [26] | |||||||||||||||||
| Urea | CO(NH | 2 | ) | 2 | 60.06 | −333.43 | [19] | ||||||||||||||||||
AF-M315E when decomposed produces an adiabatic flame temperature around 2100 K, which is much higher than that of hydrazine (nearly 1200 K). AF-M315E offers 13% increase of specific impulse and 63% increase in density over hydrazine [12], which makes it superior in the miniaturization of propulsion systems over the latter. The propellant possesses high solubility and negligible vapor-pressure of all its solution constituents, thus promoting low toxicity hazards and high mixture stability at various temperature levels, which makes exposure in open environments have no safety issues [29]
(1) ADN. (2) Methanol. (3) MMF. (4) DMF. (5) Water. (6) Ammonia (aq. 25% concentration). * @ 20 °C.
2.2. Liquid NOx Monopropellants
In this section green monopropellants of N2O, nitro compounds, and premixed N2O with hydrocarbons (i.e., nitrous oxide fuel blends NOFB [43][58]) will be introduced, emphasizing on relevant properties needed for propulsion system design either for small satellites or high-thrust in-space propulsion up to nearly 600 N.
Generally, the binary compounds of nitrogen and oxygen (e.g., NO, NO2, N2O, N2O3, N2O4) have been considered as oxidizers in hybrid rocket engines and bipropellant systems [59]. Nitric oxide NO is gaseous in the typically requested temperature-pressure range of [−30, +80] °C and [0.1, 3] MPa, which does not suit the propulsion applications of concern, since the sought propellant is required to be in liquid phase and easily storable in lighter weight tanks within the given operation conditions. Although NO2, N2O3, and N2O4 form an equilibrium mixture within the mentioned temperature–pressure range, they are still highly toxic and considered as GHS class 1 acute-toxicity [
| Boiling Point (°C) | |||
| 125 | |||
| 137 | 140 | 147 | |
ES: Extra Stabilizers; HP: High Purity; * (24 h/100 °C) %Loss of active O2; ** @ 20 °C.
The Hydrogen Peroxide Aqueous Solutions (HPAS) class possesses the lowest performance values among green monopropellants; however, a unique characteristic of this family of propellants can make it of high interest from the point of view of rocket propulsion designers in terms of increasing the overall system performance and size optimization. This unique characteristic is the hypergolic ignition of hydrogen peroxide with various propellants making it a distinguishable candidate for in-space propulsion systems. Hydrogen peroxide has been experimented thoroughly for hypergolic ignition with hydrocarbons such as ethanol [81] and propyne [82]. Further, hypergolic ignition with ionic-liquid fuels such as 1-ethyl-3-methyl imidazolium cyanoborohydride ([EMIM][BH3CN]) [83] and 1-allyl-3-ethyl imidazolium cyanoborohydride ([AEIM][BH3CN]) [84] would allow for developing new generations of green propellants for effective in-space bipropellant propulsion systems, namely green hypergolic ionic liquids (HILs) [85].
Undoubtedly, the utilization of hydrogen peroxide as a well-studied and experimented propellant in modern green propulsion systems can be of great benefits to the overall system optimization and overall increase in performance, since it would allow for implementation of multi-mode [48] systems where two or more propulsion systems can rely on the same propellant tank. In a multi-mode system, hydrogen peroxide can decompose catalytically in monopropellant auxiliary propulsion (e.g., reaction control system RCS or attitude control system ACS), as well as being used as an oxidizer in bipropellant primary propulsion system as it distinguishably can ignite through a hypergolic reaction without the need for separate ignition power source. A recent research work by Rhodes and Ronney (2019, 2020) theorized [86], investigated, and experimented [87] a first prototype of a Hydrogen Peroxide Vapor Propulsion, which can provide attitude control capability for small satellites with thrust range of millinewtons. Although hydrogen peroxide in vapor phase is extremely unstable and prone to detonation as widely known, in this novel concept it is claimed that the reactive vapor phase was utilized within a low-thrust propulsion system where the vapor was used as a propellant. Hydrogen peroxide reactive vapor was vacuum evaporated from the surface of the stored propellant in liquid phase and then passed over a catalytic bed where a chemical reaction occurs and temperature is increased; this eventually leads to a theoretical specific impulse in vacuum (>200 s), which is very high when compared to conventional H2O2 systems. This proposed novel system would add to the scope of applications of hydrogen peroxide aqueous solution propellants.
Figure 1. State-of-the-art green monopropellants performance chart.
References
- Price, T.W.; Evans, D.D. The Status of Monopropellant Hydrazine Technology—Technical Report 32-1227; Jet Propulsion Laboratory, NASA: Pasadena, CA, USA, 1968.
- ECHA European Chemicals Agency. Candidate List of Substances of Very High Concern for Authorisation. 20 June 2011. Available online: https://echa.europa.eu/documents/10162/c5b972a9-f57f-4fd5-8177-04b4e46c5e93 (accessed on 1 April 2020).
- Mayer, A.; Wieling, W. Green Propulsion Research at TNO the Netherlands. Trans. Inst. Aviation. 2018, 4, 1–24.
- Uramachi, H.; Shiraiwa, D.; Takai, T.; Tanaka, N.; Kaneko, T.; Furukawa, K. Green Propulsion Systems for Satellites—Development of Thrusters and Propulsion Systems using Low-toxicity Propellants. Mitsubishi Heavy Ind. Tech. Rev. 2019, 56, 1–7.
- Jankovsky, R.S. HAN-Based Monopropellant Assessment for Spacecraft—NASA Technical Memorandum 107287. In Proceedings of the 32nd Joint Propulsion Conference AIAA/ASME/SAE/ASEE, Lake Buena Vista, FL, USA, 1–3 July 1996.
- United Nations. Globally Harmonized System of Classification and Labeling of Chemicals (GHS), 4th ed.; United Nations: New York, NY, USA, 2011.
- Batonneau, Y.; Kappenstein, C.; Keim, W. Catalytic decomposition of energetic compounds: Gas generator, propulsion. In Handbook of Heterogeneous Catalysis, 2nd ed.; VCh-Wiley: Weinheim, Germany, 2008; pp. 2647–2680.
- Batonneau, Y.; Brahmi, R. Application of Ionic Liquids to Space Propulsion. In Applications of Ionic Liquids in Science and Technology; InTech: Poitiers, France, 2011; pp. 447–466.
- Amrousse, R.; Katsumi, T.; Itouyama, N.; Azuma, N.; Kagawa, H.; Hatai, K.; Ikeda, H.; Hori, K. New HAN-based mixtures for reaction control system and low toxic spacecraft propulsion subsystem: Therman decomposition and possible thruster applications. Combust. Flame 2015, 162, 2686–2692.
- Claßen, M.; Heimsch, S.B.; Klapötke, T.M. Synthesis and Characterization of New Azido Esters Derived from Malonic Acid, Propellants, Explosives. Pyrotechnics 2019, 44, 1515–1520.
- Larson, A.; Wingborg, N. Green Propellants Based on Ammonium Dinitramide (ADN). In Advances in Spacecraft Technologies; InTech: Poitiers, France, 2011; pp. 139–156.
- Tsay, M.; Lafko, D.; Zwahlen, J.; William, C. Development of Busek 0.5N Monopropellant Thruster. In Proceedings of the 27th Annual AIAA/USU Conference on Small Satellites, Logan, UT, USA, 10–15 August 2013.
- Meinhardt, D.; Brewster, G.; Christofferson, S.; Wucherer, E. Development and Testing of New HAN-based Monopropellants in Small Rocket Thrusters. In Proceedings of the 34th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Cleveland, OH, USA, 13–15 July 1998.
- Masse, R.K.; Overly, J.A.; Allen, M.Y.; Spores, R.A. A New State-of-the-Art in AF-315E Thruster Technologies. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–1 August 2012.
- Ertl, G.; Knözinger, H.; Schüth, F.; Weitkamp, J. Handbook of Heterogeneous Catalysis, 2nd ed.; Wiley-VCH Verlag GmbH& Co.: Weinheim, Germany, 2008; Volume 1.
- Wade, L.G. Encyclopædia Britannica, Encyclopædia Britannica, Inc. 13 December 2019. Available online: https://www.britannica.com/science/alcohol (accessed on 24 April 2020).
- Lide, D.R. Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2006.
- National Center for Biotechnology Information. PubChem Database. 18 April 2020. Available online: https://pubchem-ncbi-nlm-nih-gov.tudelft.idm.oclc.org (accessed on 25 April 2020).
- Purdue School of Aeronautics and Astronautics. Propulsion Web Page—Heats of Formation and Chemical Compositions. 1998. Available online: https://engineering.purdue.edu/~propulsi/propulsion/comb/propellants.html (accessed on 24 April 2020).
- Kon’kova, T.S.; Matyushin, Y.N.; Miroshnichenko, E.A.; Vorob’ev, A.B. Thermochemical properties of dinitramidic acid salts. Russ. Chem. Bull. 2009, 58, 2020–2027.
- Wingborg, N. Heat of Formation of ADN-Based Liquid Monopropellants, Propellants, Explosives. Pyrotechnics 2019, 44, 1090–1095.
- Swami, U.; Senapathi, K.; Srinivasulu, K.M. Energetic ionic liquid hydroxyethylhydrazinium nitrate as an alternative monopropellant. Combust. Flame 2020, 215, 93–102.
- Swami, U.; Senapathi, K.; Srinivasulu, K.; Desingu, J.; Chowdhury, A. Ignition Delays of Mixtures of the Non-Hypergolic Energetic Ionic Liquid Hydroxyethylhydrazinium Nitrate Blended with Unsymmetrical Dimethylhydrazine, Propellants, Explosives. Pyrotechnics 2019, 44, 1139–1146.
- CRC Handbook of Chemistry and Physics, 83rd ed.; CRC Press: Boca Raton, FL, USA, 2003.
- National Institute of Standards and Technology—NIST Chemistry WebBook SRD 69, Glycerine. 2018. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C56815&Units=SI&Mask=1#ref-2 (accessed on 24 April 2020).
- National Institute of Standards and Technology—NIST Chemistry WebBook SRD69, Glycine. 2018. Available online: https://webbook.nist.gov/cgi/cbook.cgi?Source=1959TAK%2FCHI84-88&Units=SI&Mask=1E9F (accessed on 24 April 2020).
- Decker, M.M.; Klein, N.; Freedman, E.; Leveritt, C.S.; Wojciechowski, J.Q. HAN- Based Liquid Gun Propellants: Physical Properties—BRL-TR-2864; US Army Ballistic Research Laboratories: Aberdeen Proving Ground, MD, USA, 1987.
- Jet Propulsion Laboratory. Liquid Propellant 1846 Handbook; U.S. Department of the Army, ARDEC: Picatinny Arsenal, NJ, USA, 1994.
- Masse, R.K.; Allen, M.; Driscoll, E.; Spores, R.A. AF-M315E Propulsion System Advances & Improvements. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016.
- NASA. Green Propellant Infusion Mission (GPIM) Overview, NASA. 5 December 2019. Available online: https://www.nasa.gov/mission_pages/tdm/green/overview.html (accessed on 24 April 2020).
- Igarashi, S.; Yamamoto, K.; Fukuchi, A.B. Development Status of a 0.5N-Class Low-Cost Thruster for Small Satellites. In Proceedings of the AIAA Propulsion and Energy Forum Joint Propulsion Conference, Cincinnati, OH, USA, 9–11 July 2018.
- Igarashi, S.; Matsuura, Y. Development Status of a Hydrazine Alternative and Low-cost Thruster Using HAN/HN-Based Green Propellant. In Proceedings of the 53rd AIAA/SAE/ASEE Joint Propulsion Conference, Atlanta, GA, USA, 10–12 July 2017.
- Tummala, A.R.; Dutta, A. An Overview of Cube-Satellite Propulsion Technologies and Trends. Aerospace 2017, 58, 1–30.
- Masse, R.; Spores, R.A.; Kimbrel, S.; Allen, M.; Lorimor, E.; Myers, P. GPIM AF-M315E Propulsion System. In Proceedings of the 51st AIAA/SAE/ASEE Joint Propulsion Conference, Orlando, FL, USA, 27–29 July 2015.
- Hori, K.; Katsumi, T.; Sawai, S.; Azuma, N.; Hatai, K.; Nakatsuka, J. HAN-Based Green Propellant, SHP163—Its R&D and Test in Space, Propellants, Explosives. Pyrotechnics 2019, 44, 1080–1083.
- Amrousse, R.; Katsumi, T.; Azuma, N.; Hori, K. Hydroxylammonium nitrate (HAN)-based green propellant as alternative energy resource for potential hydrazine substitution: From lab scale to pilot plant scale-up. Combust. Flame 2017, 176, 334–348.
- Azuma, N.; Hori, K.; Katsumi, T.; Amrousse, R.; Nagata, T.; Hatai, K. Research and Development on Thrusters with HAN (Hydroxyl Ammonium Nitrate) Based Monopropellant. In Proceedings of the 5th EUCASS, Munich, Germany, 1–5 July 2013.
- Togo, S.; Hori, K.; Shibamoto, H. Improvement of HAN-based Liquid Monopropellant Combustion Characteristics. In Proceedings of the HEMS, Belokurikha, Russia, 5–9 September 2004.
- Katsumi, T.; Kodama, H.; Ogawa, H.; Tsuboi, N.; Sawai, S.; Hori, K. Combustion Characteristics of HAN-Based Liquid Monopropellant. Sci. Tech. Energetic Mater. 2009, 70, 27–32.
- Fukuchi, A.; Inamoto, T.; Miyazaki, S.; Maruizumi, H.; Kohono, H. HAN/HN-based Monopropellant Thrusters. In Proceedings of the 26th International Symposium on Space Technology and Science, Hamamatsu, Japan, 1–8 June 2008.
- Igarashi, S.; Fukuchi, A.; Azuma, N.; Hatai, K.; Kagawa, H.; Ikeda, H. Development of a high-performance HAN/HN-based low-toxicity Monopropellant. Trans. JSASS Aerosp. Tech. Jpn. 2016, 14, 101–105.
- Igarashi, S.; Matsuura, Y.; Hatai, K.; Ikeda, H. Safe 0.5N Green Monopropellant Thruster for Small Satellite Propulsion Systems. In Proceedings of the AIAA Propulsion and Energy Forum, Indianapolis, IN, USA, 19–22 August 2019.
- Werling, L.; Haßler, M.; Bätz, P.; Helmut, C.; Schlechtriem, S. Experimental Performance Analysis (c* & c* efficiency) of a Premixed Green Propellant consisting of N2O and C2H4. In Proceedings of the 53rd AIAA/SAE/ASEE Joint Propulsion Conference, Atlanta, GA, USA, 10–12 July 2017.
- Spores, R.A.; Masse, R.; Kimbrel, S. GPIM AF-M315E Propulsion System. In Proceedings of the 49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Jose, CL, USA, 15–17 July 2013.
- Thrasher, J.; Williams, S.; Takahashi, P.; Sousa, J. Pulsed Plasma Thruster Development Using A Novel HAN- Based Green Electric Monopropellant. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016.
- DSSP Digital Solid State Propulsion. Safety Data Sheet—Green Electrical Monopropellant (GEM Mod 3). 1 December 2015. Available online: https://dssptech.com/propellant-products (accessed on 25 April 2020).
- Berg, S.P.; Rovey, J.L. Assessment of Multi-Mode Spacecraft Micropropulsion Systems. In Proceedings of the 50th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, Cleveland, OH, USA, 28–30 July 2014.
- Rovey, J.L.; Lyne, C.T.; Mundahl, A.J.; Rasmont, N. Review of Chemical-Electric Multimode Space Propulsion. In Proceedings of the AIAA Propulsion and Energy Forum, Indianapolis, IN, USA, 19–22 August 2019.
- Anflo, K.; Grönland, T.; Wingborg, N. Development and Testing of ADN-Based Monopropellants in Small Rocket Engines. In Proceedings of the 36th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Las Vegas, NV, USA, 24–28 July 2000.
- Anflo, K.; Wingborg, N. Dinitramide Based Liquid Mono-Propellants. Sweden Patent WO0050363, 31 August 2000.
- Persson, M.; Anflo, K.; Friedhoff, P. Flight Heritage of Ammonium Dinitramide (ADN) Based High Performance Green Propulsion (HPGP) Systems, Propellants, Explosives. Pyrotechnics 2019, 44, 1073–1079.
- Wilhelm, M.; Negri, M.; Ciezki, H.; Schlechtriem, S. Preliminary tests on thermal ignition of ADN-based liquid monopropellants. Acta Astronaut. 2019, 158, 388–396.
- Anflo, K.; Crowe, B. In-Space Demonstration of an ADN-based Propulsion System. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Diego, CA, USA, 31 July–3 August 2011.
- Anflo, K.; Wingborg, N. Ammonium Dinitramide Based Liquid Monopropellants Exhibiting Improved Combustion Stability and Storage Life. Svenska Rymdaktiebolaget. Sweden Patent WO02096832, 5 December 2002.
- Larsson, A.; Wingborg, N.; Elfsberg, M.; Appelgren, P. Characterization and Electrical Ignition of ADN-Based Liquid Monopropellants—FOI-R--1639—SE; Weapons and Protection—FOI: Tumba, Sweden, 2005.
- Wingborg, N.; Eldsäter, C.; Skifs, H. Formulation and Characterization of ADN-based Liquid Monopropellants. In Proceedings of the 2nd International Conference on Green Propellants for Space Propulsion, Cagliari, Italy, 7–8 June 2004.
- Wingborg, N.; Johansson, M.; Bodin, L. Initial development of a Laboratory Rocket Thruster for ADN-Based Liquid Monopropellants—FOI-R--2123—SE; Weapons and Protection—FOI: Tumba, Sweden, 2006.
- Werling, L.; Perakis, N.; Muller, S.; Hauck, A.; Ciezki, H.; Schlechtriem, S. Hot firing of a N2O/C2H4 premixed green propellant: First combustion tests and results. In Proceedings of the Space Propulsion Conference, Rome, Italy, 2–6 May 2016.
- Palacz, T. Nitrous Oxide Application for Low-Thrust and Low-Cost Liquid Rocket Engine. In Proceedings of the 7th EUCASS, Milano, Italy, 3−6 July 2017.
- National Institute of Standards and Technology—NIST Chemistry WebBook SRD 69, Nitrous Oxide. 2018. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=10024-97-2 (accessed on 26 April 2020).
- Wallbank, J.; Sermon, P.; Baker, A.; Coutney, L.; Sambrook, R. Nitrous Oxide as a Green Monopropellant for Small Satellites. In Proceedings of the 2nd International Conference on Green Propellants for Space Propulsion, Sardinia, Italy, 7–8 June 2004.
- Zakirov, V.; Sweeting, M.; Goeman, V.; Lawrence, T. Surrey Research on Nitrous Oxide Catalytic Decomposition for Space Applications. In Proceedings of the 14th AIAA/USU Conference on Small Satellites, Logan, UT, USA, 21–24 August 2000.
- Haynes, W. CRC Handbook of Chemistry and Physics, 94th ed.; CRC Press LLC: Boca Raton, FL, USA, 2013.
- Kindsvater, H.M.; Kendall, K.K.; Müller, K.H.; Datner, P.P. Research on Nitromethane; Navy Department Bureau of Aeronautics: Washington, DC, USA, 1951.
- Boyer, E.; Kuo, K.K. Characteristics of Nitromethane for Propulsion Applications. In Proceedings of the 44th AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 9–12 January 2006.
- Clark, J.D. Ignition! An Informal History of Liquid Rocket Propellants; Rutgers University: New Jersey, NY, USA, 1972.
- ESA European Space Agency. European Fuel Blend Development, Estec—ESA. 14 March 2016. Available online: https://artes.esa.int/projects/european-fuel-blend-development (accessed on 4 May 2020).
- FIRESTAR Tech. LLC. Technology Updates, Firestar. Available online: http://www.firestar-engineering.com/NOFBX-MP.html (accessed on 4 May 2020).
- Mungas, G.; Vozoff, M.; Rishikof, B. NOFBX: A new non-toxic, Green Propulsion Technology with high performance and low cost. In Proceedings of the 63 International Astronautical Congress, Naples, Italy, 1–5 October 2012.
- Werling, L.; Freudenmann, D.; Ciezki, H.; Schlechtriem, S. Premixed green propellants: DLR research and test activities on nitrous oxide/hydrocarbon mixtures. In Proceedings of the New Energetics Workshop (NEW), Stockholm, Sweden, 29–30 May 2018.
- Waugh, L.; Moore, E.; Macfarlane, J.; Watts, A.; Mayer, A. Testing of a Novel Nitrous-oxide and Ethanol Fuel Blend. In Proceedings of the Space Propulsion Conference, Seville, Spain, 14–18 May 2018.
- Werling, L.; Perakis, N. Experimental Investigations based on a Demonstrator unit to analyze the Combustion Process of a Nitrous Oxide/Ethene Premixed Green (Bipropellant). In Proceedings of the 5th CEAS Air & Space Conference, Delft, The Netherlands, 7–11 September 2015.
- Mayer, A.; Waugh, I.; Poucet, M. European Fuel Blend Development Final Report—TNO 2018 R10640; TNO—Netherlands Organization for Applied Scientific Research: Rijswijk, The Netherlands, 2018.
- Mayer, A.; Wieling, W.; Watts, A.; Poucet, M.; Waugh, I.; Macfarlane, J.; Bel, F.V. European Fuel Blend Development for In-space propulsion. In Proceedings of the Space propulsion Conference, Seville, Spain, 14–18 May 2018.
- Pasini, A.; Torre, L.; Romeo, L.; Cervone, A.; d’Agostino, L. Performance Characterization of Pellet Catalytic Beds for Hydrogen Peroxide Monopropellant Rockets. J. Propuls. Power 2011, 27, 428–436.
- Department of Defense Index of Specifications and Standards. MIL-PRF-16005F Performance Specification: Propellant, Hydrogen Peroxide; Department of Defense: Philadelphia, PA, USA, 2003.
- Pasini, A.; Pace, G.; Torre, L. Propulsive Performance of 1N 98% Hydrogen Peroxide Thruster. In Proceedings of the 51st AIAA/SAE/ASEE Joint Propulsion Conference, Orlando, FL, USA, 27–29 July 2015.
- Krejci, D.; Woschnak, A.; Scharlemann, C.; Ponweiser, K. Structural Impact of Honeycomb Catalysts on Hydrogen Peroxide Decomposition for Micro Propulsion. Chem. Eng. Res. Des. 2012, 90, 2302–2315.
- Cervone, A.; Torre, L.; d’Agostino, L. Development of Hydrogen Peroxide Monopropellant Rockets. In Proceedings of the 42nd AIAA/ASMESAE/ASEE Joint Propulsion Conference & Exhibit, Sacramento, CA, USA, 9–12 July 2006.
- USP Technologies. H2O2 Physical Properties. 2020. Available online: http://www.h2o2.com/technical-library/physical-chemical-properties/physical-properties/default.aspx?pid=20&name=Physical-Properties (accessed on 7 May 2020).
- Naseem, M.S.; Jyoti, B.; Baek, S.W.; Lee, H.J.; Cho, S.J. Hypergolic Studies of Ethanol Based Gelled Bi-Propellant System for Propulsion Application, Propellants, Explosives. Pyrotechnics 2017, 42, 676–682.
- Pasini, A.; Pace, G.; Torre, L. A Light Unsaturated Hydrocarbon and Hydrogen Peroxide as Future Green Propellants for Bipropellant Thrusters. In Proceedings of the 51st AIAA/SAE/ASEE Joint Propulsion Conference, Orlando, FL, USA, 27–29 July 2015.
- Bhosale, V.K.; Kulkarni, S.G.; Kukarni, P.S. Ionic Liquid and Biofuel Blend: A Low–cost and High Performance Hypergolic Fuel for Propulsion Application. Chem. Sel. 2016, 1, 1921–1925.
- Bhosale, V.K.; Kulkarni, P.S. Ultrafast igniting, imidazolium based hypergolic ionic liquids with enhanced hydrophobicity. New J. Chem. 2017, 41, 1250–1258.
- Bhosale, V.K.; Jeong, J.; Choi, J.; Churchill, D.G.; Lee, Y.; Kwon, S. Additive-promoted hypergolic ignition of ionic liquid with hydrogen peroxide. Combust. Flame 2020, 214, 426–436.
- Rhodes, B.L.; Ronney, P.D. Dynamics of a Small-Scale Hydrogen Peroxide Vapor Propulsion System. J. Propuls. Power 2019, 35, 595–600.
- Rhodes, B.L.; Ulrich, E.R.; Hsu, A.G.; Ronney, P.D. Thrust Measurement of a Hydrogen Peroxide Vapor Propulsion System. In Proceedings of the AIAA Propulsion and Energy 2020 Forum, Virtual Event. 24–28 August 2020.