Carbon materials such as carbon graphitic structures, carbon nanotubes, and graphene nanosheets are extensively used as supports for electrocatalysts in fuel cells. Alternatively, conducting polymers displayed ultrahigh electrical conductivity and high chemical stability havegenerated an intense research interest as catalysts support for polymer electrolyte membrane fuel cells (PEMFCs) as well as microbial fuel cells (MFCs). Moreover, metal or metal oxides catalysts can be immobilized on the pure polymer or the functionalized polymer surface to generate conducting polymer-based nanohybrids (CPNHs) with improved catalytic performance and stability. Metal oxides generally have large surface area and/or porous structures and showed unique synergistic effects with CPs. Therefore, a stable, environmentally friendly bio/electro-catalyst can be obtained with CPNHs along with better catalytic activity and enhanced electron-transfer rate.
- fuel cell
- conducting polymer
- nanohybrids
- catalysts support
- electrooxidation
- functionalization
- anode catalysts
- cathode catalysts
- maximum power density
1. Introduction
Fuel cells have appeared as potential energy conversion devices which operate in the presence of the hydrogen or hydrogen-rich fuels with low CO2 emissions [1][2][3][1,2,3]. Among these, proton exchange membrane fuel cells (PEMFC) have gained more attention due to their superior properties such as high open-circuit potential, high energy conversion efficiency, limited fuel crossover effects, and efficient electrooxidation of organic molecules such as ethanol (DEFC), methanol (DMFC), formic acid (DFAFC), etc. at low-temperature [4][5][6][7][4,5,6,7].
On the other hand, microbial fuel cells (MFCs) provide the possibility of transforming organic waste directly into electricity through microbially catalyzed anodic and microbial/enzymatic/abiotic cathodic electrochemical reactions with solid electrodes [8][9][8,9]. The main reasons which hinders the practical application of the MFC are the lower power output and high cost Pt-based catalysts. Therefore, various carbonaceous, metallic nanoparticles (NPs) as anode materials and carbonaceous, platinum-group metal and platinum-group-metal-free materials as cathode catalysts are used to improve the performance of the MFC along with lowering the cost [10][11][12][10,11,12].
Despite significant progress, high material cost and limited availability of Pt together with intrinsic sluggish kinetics of anodes limit the commercial use of FCs [13]. Moreover, stability and efficiency of the fuel cell has been associated with the optimized three-phase interface that is composed of an ion-conducting electrolyte membrane (ionomer) attached to the catalyst surface [14][15][16][14,15,16]. Initially, hydrogen gas molecules react with the solid phase of the anode, and protons are accessible to the electrolyte phase and then react with O2 in the solid–electrolyte interface. Thus, the development of stable, low-cost electrocatalysts and polymer membranes, and understanding the effectiveness of catalytic activity of small organic molecules are crucial to designing large-scale and cost-effective fuel cells.
Up until now, it has been demonstrated that carbon supports such as carbon black, carbon nanotubes (CNTs), carbon fibers, mesoporous carbon, graphitic carbons, graphene etc. with suitable surface properties and functional groups can be used for metal NP deposition to fabricate efficient catalysts FCs [17][18][19][20][21][22][23][24][25][26][17,18,19,20,21,22,23,24,25,26]. However, significantly lower interactions between hydrophobic surfaces of carbon supports with the catalyst and corrosion of supporting materials under experimental conditions hinder high catalytic activity and durability, which remain a significant challenge [27][28][27,28]. Moreover, the efficiency and power density of alcohol FCs may be strongly affected due to the fuel crossover through the traditionally used Nafion membrane [29]. Alternatively, conducting polymers (CP) such as polypyrrole (PPy), polyaniline (PANI), poly-3-methyl thiophene (PMT), poly(3,4-ethylenedioxythiophene) (PEDOT), etc. have been successfully used as supporting material for electrocatalysts in fuel cell applications [30][31][32][33][34][30,31,32,33,34]. Conducting polymers have exhibited promising applications as catalyst supports due to unique conjugated structures with high electrical conductivity, exceptional chemical stability, facile fabrication, and low cost. Thus, conducting polymer-based nanohybrids (CPNHs) with a series of metal or metal oxides deposited on the polymer surface have been employed for electrooxidation of small molecules such as hydrogen, methanol, and formic acid [35][36][35,36]. Recently, multidirectional efforts have been devoted to fabricate electrode materials via depositing the nanostructured metal catalysts on conducting polymer surfaces directly or on modified polymer nanostructures [37][38][37,38].
2. CPNH-Based Electrode Materials
Conducting polymer-supported nanomaterials represent a unique class of hybrid electrocatalysts for fuel cells applications that synergize the beneficial properties of both nanomaterials and conducting polymers [35]. The higher electronic conductivity of CPs compared to that of the conventional carbon-based catalyst support this higher attention during past two decades. Additionally, the higher surface area and unique synergistic effects with the metal NPs/metal oxide play an important role to improve the electrocatalytic activity of the catalysts and to stabilize fuel cell performance [39][40][39,40]. A significant effort has been made in the fabrication of Pt-free catalysts deposited on CPs, although loading of low-Pt nanomaterials on polymers is also used due to their high catalytic performance. Various synthetic strategies have been developed successfully by several research groups for conducting polymer-supported hybrid catalysts, including metal NPs, multimetallic NPs, and metal oxides which can be used as anode, cathode, and electrolyte membrane, as shown in Figure 1.
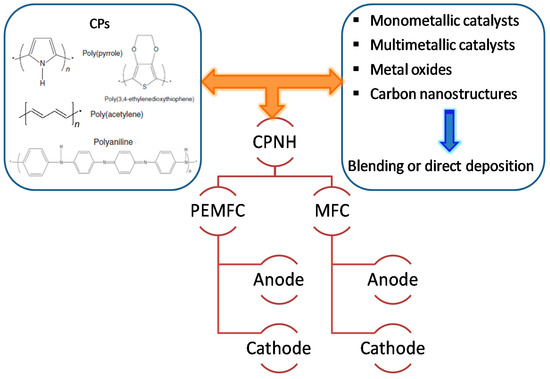
Figure 1.
The active components of conducting polymer-based nanohybrids (CPNHs) in fuel cell materials
2.1. CPNH-Based Electrode for Alcohol Fuel Cells
Conducting polymer-supported hybrid electrocatalysts have been widely used for the oxidation of small organic molecules and oxygen reduction, which are relevant to fuel cells. In principle, direct alcohol fuel cells (DAFCs) convert the chemical energy stored in the alcohol molecules into electricity through electrooxidation at the anode in the presence of catalysts (Figure 2). For example, the oxidation of methanol in alkaline or acid media is a six-electron oxidation process that forms CO2 (Equations (1) and (2)):
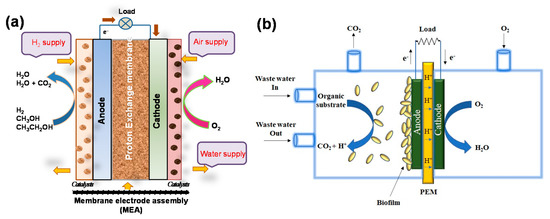
Figure 2.
Schematic illustration of the working principle in (
a
) direct alcohol fuel cells and (
b
) microbial fuel cells.
Another commonly used fuel, ethanol involves an alkaline oxidation reaction (EOR), which generates carbon intermediates as well as acetaldehyde (CH3CHO), acetic acid (CH3COOH), and 12 e− (Equations (3) and (4)):
Up to now, Pt-based catalysts have been utilized as an effective electrocatalyst for alcohol fuel cells, and a significant effort has been made to lower the cost of such catalysts through lowering Pt loadings [41]. Moreover, low-cost Pd also demonstrated efficient alcohol oxidation in alkaline media with high catalytic activity and anti-CO-poisoning [42][43][42,43]. A series of conducting polymers such as PPy, PANI, PEDOT, etc. (as shown in Figure 1) with large surface areas, high conductivity, and shortened pathways for charge/mass transport have been successfully used as catalyst supports for fuel cell applications.
On the other hand, in microbial fuel cells, microorganisms employed as catalysts to generate electric current from biodegradable organic and inorganic compounds may be useful for a wide range of applications including electricity generation, bio-hydrogen production, waste water treatment, medical devices, and other electrochemical devices [44][45][46][44,45,46]. MFC is mainly composed of an anode; a cathode (example, graphite, graphite felt, carbon paper, carbon cloth etc.); a polymer electrolyte membrane, such as nafion, ultrex, polyethylene, poly(styrene-co-divinylbenzene), etc. substrates; and electrode catalysts (Figure 2b). A wide range of metal and metal-based catalysts, like Pt, Pt black, and MnO2 have been used as catalysts for the electrodes, mainly for cathodes, while microorganisms themselves act as catalysts in the anode. Nowadays, to speed up the anode reaction, nontoxic graphene-based catalysts have also been used. Electrons flow from the anode to the cathode through an external electrical connection. Anodic oxidation of the substrate, including carbohydrate, proteins, volatile acids, cellulose, or waste water (for example, acetate) in the presence of microbes produced electrons and protons as well as carbon dioxide (Equations (5) and (6)).
3. CPNH-Based Electrode for Microbial Fuel Cells
3.1. CPNH-Based Anode for MFCs
Performance of the MFC mainly depends on the functioning of the electrode materials, reactor configuration, and seed culture. At the interface of the anode catalyst layer and the microbe layer, the generated extracellular electron transference is considered a crucial factor for MFC performance. Therefore, a variety of electrodes such as graphite plate; titanium plate; gold sheets; or carbon materials like brush, cloth, paper, mesh etc. was employed as anodes of MFCs to improve its performance. However, several barriers, for example, non-porous structure, limited surface area, and poor contact between microbes and electrodes, limit the overall performance of the MFCs [47][48][97,98]. Recently, non-precious metal oxide catalysts have attracted great interest because of their high catalytic activity, lower cost, and biocompatibility. Again, agglomeration and dissolution of this metal oxide results in deterioration of the electrocatalytic activity of catalysts, which causes a sharp drop in MFC performance. To overcome this drawback, metal oxides have been anchored on conducting polymer substrates [49][99].
Biochar (BC) modified with the nickel ferrite (NiFe2O4) nanorod/poly(3,4-ethylenedioxythiophene) (PEDOT) composite has been employed as an anode catalyst in MFC [50][100]. Biochar has been prepared from neem wood carbonization. Electrocatalytic activity, electrochemical impedance spectroscopy (EIS) (Figure 3c), MFC performance (Figure 3a,b), and stability(Figure 3d) of bare BC, Fe3O4/BC, NiFe2O4/BC, and PEDOT/NiFe2O4(1:2)/BC were studied and explained. The voltammograms using the PEDOT/NiFe2O4/BC catalyst showed superior oxidation current, which confirms the improvement of the bioelectrochemical activity of the exoelectrogens. MFC with PEDOT/NiFe2O4 (1:2)/BC exhibited the lowest charge transfer resistance (Rct) of 441.4 Ω, among all the assembled MFCs (Figure 3c). The coordination bond between the spinel oxide NiFe2O4 and the PEDOT layer enhanced the electron transfer by lowering the electrode–electrolyte interfacial resistance. Finally, the MFC showed a peak power density of 1200 mWm−2 (Figure 3b) mainly because of the unique nanostructure of the catalyst, better electron conductivity, and chemical stability [50][100]. The polyaniline (PANI)/mesoporous TiO2 composite was synthesized to be utilized as an anode in MFCs. The composite with 30 wt% PANI exhibited the best catalytic activity by showing a maximum power density of 1495 mW m−2, which is found be one of the highest power outputs among all the reported works to date. Large specific surface areas and uniform nanopore distribution of the newly developed nanohybrid could be the key factors of its improved performance [51][101].
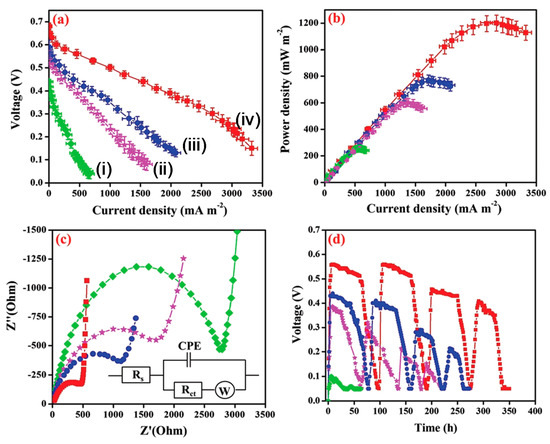
Figure 3. (a) Polarization and (b) power density curves (error bars indicate the standard deviation acquired from three microbial fuel cell (MFC) experiments), (c) electrochemical impedance spectroscopy (EIS) analysis, Inset: depicting the equivalent circuit used for fitting the EIS data, and (d) durability performances of MFCs with (green dots) bare Biochar (BC) anode (i), (violet dots) Fe3O4/BC (ii), (blue dots) NiFe2O4/BC (iii), and (red dots) PEDOT/NiFe2O4(1:2)/BC (iv) [51][101].
3.2. CPNH-Based Cathode for MFCs
One of the major barriers which hinder practical application of the MFC is the slow oxygen reduction reaction (ORR) kinetics and the high cost of the ORR catalysts based on Pt. As a result, the total cost of MFC was enhanced to very high levels along with the lower efficiency. Nowadays, nanostructured carbon such as carbon nanofibers (CNF) [52][102], carbon nanotubes [53][103], and activated carbon showed their potential to replace Pt either partially or in full. Non-platinum group metal catalysts based on Mn, Fe, Co, and Cu etc. have also been tested as cathode catalysts for MFCs [54][104].
Polyindole (PID) and iron phthalocyanine (FePc) was deposited on carbon nanotubes and on vulcan carbon separately. Among all the studied catalysts, FePc/CNTs and FePc/PID/CNTs catalysts exhibited better electrocatalytic activity in terms of more positive half-wave potential values and higher kinetic current density values compared to that of the conventional Pt/C. Finally, the MFC using FePc/PID/CNT cathodes showed a maximum power density of 799 ± 41 mW m−2 and a current density of 3480 ± 83 mA m−2, which are higher than those achieved with commercial Pt/C cathode (646 ± 25 mW m−2 and 3011 ± 84 mA m−2, respectively) (Figure 4a). Again, the MFC with FePc/PID/CNT cathodes achieved a stable performance (Figure 4b), which also confirms its potential to replace Pt as cathode catalysts [55][105].
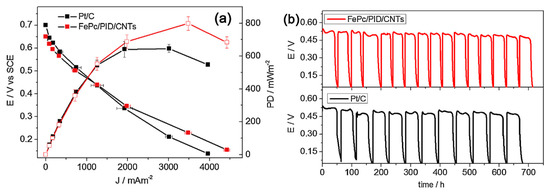
Figure 4.
a
b) voltage cycles under a 1 kV external resistance by MFCs assembled with FePc/PID/CNTs_CC and Pt/C_CC cathodes, recorded for 700 h after inoculation [55].
Iron(II) phthalocyanine (FePc) and cobalt tetramethoxyphenylporphyrin (CoTMPP)-based material had also been synthesized and tested as cathode catalysts for a two-chamber microbial fuel cell. The CoTMPP-based catalyst achieved better maximum current (16.67 mA) and maximum power output (14.32 mW L−1) compared to the FePc-based cathode (maximum current 14.31 mA and maximum power output 13.88 mW L−1).Stronger back bonding between cobalt and oxygen could be responsible for its better performance. These two inexpensive catalysts also showed comparable electrocatalytic activity to Pt black itself [56][106]. The manganese-polypyrrole-carbon nanotube (Mn-PPy-CNT) composite was synthesized and used as a cathode catalyst in an air-cathode MFC. Mn-PPy-CNT-based MFC achieved a maximum power density of 169 mW m−2 and 213 mW m−2 at loadings of 1 mg cm−2 and 2 mg cm−2, respectively, which are comparable to the MFCs with the commercial Pt/C catalyst. The presence of the Mn–N active site makes this low-cost catalyst potentially applicable as a cathode of the MFCs. Additionally, Mn-PPy-CNT-based MFCs exhibited long-term stability, which is another important factor for practical application of the MFC [57][107].
Pyrrole was polymerized on reduced graphene oxide (rGO) to use as a catalystsupport. Further, Ni–NiO NPs were deposited on the synthesized support matrix, which was tested as cathode catalysts in single-chambered MFCs. MFCs employing the Ni–NiO/PPy–rGO nanohybrid catalyst exhibited a maximum current density of 2134.56 mA m−2 and a maximum power density of ~678.79 ± 34 mW m−2, whereas the MFC using the commercial Pt/C catalyst showed lower current and power density, i.e., 1788.2 mA m−2 and ~481.02 ± 24 mW m−2, respectively. Higher electrical conductivity of the PPy–rGO support matrix provided a good platform for the deposition of the Ni–NiO NPs. The longer conjugation length of PPy–rGO helps to improve the synergistic effect between the metal NPs and the support. Thus, the resulting nanohybrid catalysts led to better electrocatalytic activity and long-term stability compared to the conventional Pt/C cathode [58][108]. Iron phthalocyanine (FePc) supported on Polyaniline/carbon black (PANI/C) was synthesized and utilized as a cathode catalyst in an air–cathode microbial fuel cell (MFC). PANI/C/FePc achieved better electrocatalytic activity in terms of the ORR peak shift toward positive potential and higher peak current. MFC with the PANI/C/FePc cathode exhibited a maximum power density of 630.5 mW m−2, which was found to be higher than the MFC with Pt cathode (575.6 mW m−2). Lower cost and higher power output make the PANI/C/FePc catalyst a promising candidate towards an alternative cathode catalyst [59][109]. The fibrousPani–MnO2 nanocomposite was synthesized to investigate its electrocatalytic activity towards ORR. Furthermore, when the PANI–MnO2 nanohybrid was used as a cathode catalyst in a MFC that exhibited an improved power density of 0.0588 W m−2, the synergistic effect of PANI and MnO2 in the nanohybrid catalyst mainly enhanced the contact between the electrode and the electrolyte. As a result, the electronic conductivity and the surface area of the catalyst were improved [60][110].



