Hypoxia is the most common microenvironment feature of lung cancer tumors, which affects cancer progression, metastasis and metabolism. Oxygen induces both proteomic and genomic changes within tumor cells, which cause many alternations in the tumor microenvironment (TME). This study defines current knowledge in the field of tumor hypoxia in non-small cell lung cancer (NSCLC), including biology, biomarkers, in vitro and in vivo studies and also hypoxia imaging and detection. While classic two-dimensional (2D) in vitro research models reveal some hypoxia dependent manifestations, three-dimensional (3D) cell culture models more accurately replicate the hypoxic TME.
- lung cancer
- hypoxia
- tumor microenvironment
- three-dimensional
- in vitro models
1. Introduction
Worldwide, lung cancer remains the most commonly diagnosed cancer and the greatest cause of cancer-related death. Globally, according to the latest GLOBOCAN 2018 estimates, lung cancer is the most often diagnosed malignancy (2.1 million new cases) with an age-standardized incidence rate of 22.5 per 100,000 person years worldwide in 2018. In both sexes combined, lung cancer is the most commonly diagnosed cancer (11.6% of the total cases) and the leading cause of cancer death (18.4% of the total cancer deaths) [1]. The low survival rate in lung cancer patients is related to the disease at diagnosis [2]. Although, nowadays, there are many new approaches for lung cancer therapies, the 5-year survival rate is still as low as 5–15% [3]. Adenocarcinoma is one of the three major subtypes of non-small cell lung cancer (NSCLC) and is the most common histologic subtype of lung cancer in men and women [4]. The anticancer treatments are based on chemotherapy, radiation therapy and targeted therapy. The major problem is clinical resistance, in which hypoxia is one of the key components. Oxygen deprivation results in gene expression changes and subsequent proteomic changes that have many important effects on various cellular and physiological functions and lead to therapy resistance [5]. Moreover, oxygen deprivation observed among other respiratory diseases such as severe obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD) may play a role in the initiation and progression of lung cancer [6].
At the cellular level, hypoxia is a significant tumor feature, which induces both proteomic and genomic changes within tumor cells, which cause many changes in the tumor microenvironment (TME). The TME is composed of different cells that are programmed to promote initiation, progression and metastasis of lung cancer and various secreted factors and extracellular matrix (ECM), which provides support to the surrounding cells (
).
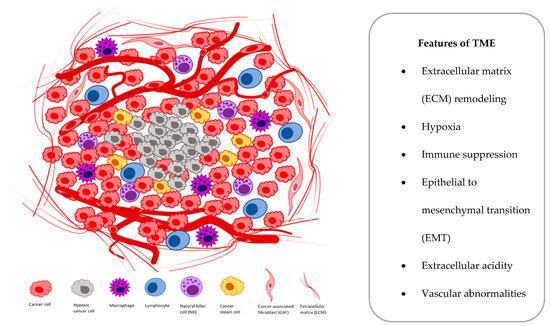
Figure 1.
Main components and features of the tumor microenvironment (TME).
2. D Scaffolds and Hydrogels
Cells in 3D scaffolds exhibit similar responses to chemotherapy and radiotherapy as cells in vivo and may be used as an important tool in cancer biology. Three-dimensional scaffolds provide signaling and physical support to the attached cells. Various types of synthetic and natural polymers have been used for hydrogel preparation depending on their biocompatibility, water absorbing ability and gel strength [89]. Several biopolymers were used to generate porous scaffolds, which include collagen, gelatin, silk, chitosan, Matrigel and alginate [103]. Additionally, the synthetic polymers, such as PAG, PLGA and PLA, were used for 3D structures generation. However, culturing cells in scaffolds may not capture the cell-to-cell interactions present in aggregated tumors such as in spheroids.
Cells in 3D scaffolds exhibit similar responses to chemotherapy and radiotherapy as cells in vivo and may be used as an important tool in cancer biology. Three-dimensional scaffolds provide signaling and physical support to the attached cells. Various types of synthetic and natural polymers have been used for hydrogel preparation depending on their biocompatibility, water absorbing ability and gel strength [7]. Several biopolymers were used to generate porous scaffolds, which include collagen, gelatin, silk, chitosan, Matrigel and alginate [8]. Additionally, the synthetic polymers, such as PAG, PLGA and PLA, were used for 3D structures generation. However, culturing cells in scaffolds may not capture the cell-to-cell interactions present in aggregated tumors such as in spheroids.
In the study of A. Stratmann et al. [84], a combined in vitro and in silico lung tumor model based on a biological tissue scaffold was generated. Two cell lines with (HCC827) or without (A549) an activating mutation of the
In the study of A. Stratmann et al. [9], a combined in vitro and in silico lung tumor model based on a biological tissue scaffold was generated. Two cell lines with (HCC827) or without (A549) an activating mutation of the
EGFR
, exhibiting different sensitivities to the EGFR inhibitor gefitinib were cultured on a small intestinal submucosa. The in silico models of the two different tumor subgroups, with activating
EGFR
mutation or with
Kras mutation, resulted in two different gefitinib responses, reflected in proliferation and apoptosis status. Furthermore, the application of TGFβ1 induced tumor cell invasion and EMT in 3D model, which was visible in mesenchymal cell morphology and modified expression of fibronectin, E-cadherin, β-catenin and mucin-1. The authors demonstrated that the combined in vitro and in silico model represents a powerful tool for analysis of signaling networks, especially involved in proliferation, apoptosis, invasion and EMT [84].
mutation, resulted in two different gefitinib responses, reflected in proliferation and apoptosis status. Furthermore, the application of TGFβ1 induced tumor cell invasion and EMT in 3D model, which was visible in mesenchymal cell morphology and modified expression of fibronectin, E-cadherin, β-catenin and mucin-1. The authors demonstrated that the combined in vitro and in silico model represents a powerful tool for analysis of signaling networks, especially involved in proliferation, apoptosis, invasion and EMT [9].
A microphysiologic 3D lung model was established by E. Wallstabe et al. [85] and resembled architectural and phenotypical features of primary tumor. Three-dimensional tumors were generated from A549 cell line on a biological scaffold with intact basement membrane (BM) on SISmuc platform. The antitumor function of receptor tyrosine kinase-like orphan receptor 1-specific (ROR1-specific) CAR T cells was evaluated. Authors found that ROR1–CAR T cells are able to penetrate and migrate through a tumor mass and confer a potent antitumor effect over a several-day period. The detected antitumor activity of ROR1–CAR T was specific and very potent against A549 lung cancer. This microphysiologic 3D lung model may be involved in preclinical CAR T cell research [85].
A microphysiologic 3D lung model was established by E. Wallstabe et al. [10] and resembled architectural and phenotypical features of primary tumor. Three-dimensional tumors were generated from A549 cell line on a biological scaffold with intact basement membrane (BM) on SISmuc platform. The antitumor function of receptor tyrosine kinase-like orphan receptor 1-specific (ROR1-specific) CAR T cells was evaluated. Authors found that ROR1–CAR T cells are able to penetrate and migrate through a tumor mass and confer a potent antitumor effect over a several-day period. The detected antitumor activity of ROR1–CAR T was specific and very potent against A549 lung cancer. This microphysiologic 3D lung model may be involved in preclinical CAR T cell research [10].
In another study, cells from tumor resections of six patients’ NSCLC were cultured on human embryonic stem cell-qualified Matrigel-coated plates [86]. In this in vitro culturing system, two important elements of the tumor microenvironment were mimicked: lung fibroblast-derived extracellular matrix and physiological hypoxia (5% O
In another study, cells from tumor resections of six patients’ NSCLC were cultured on human embryonic stem cell-qualified Matrigel-coated plates [11]. In this in vitro culturing system, two important elements of the tumor microenvironment were mimicked: lung fibroblast-derived extracellular matrix and physiological hypoxia (5% O
2). The stabilization of HIF1-α was assessed during the experiment. Although Western blot analysis revealed stabilization of HIF1-α in the hypoxia environments (2D and 3D) after 72 hours, there was a considerable decrease in HIF1-α stabilization in the 3D environment compared to the 2D environment. This 3D system allowed isolation and rapid expansion of stromal progenitors from patient lung tumor resections. These progenitor cell populations in the TME-like environment may be anticancer drug targets, which limits their effects on promoting cancer metastasis [86].
). The stabilization of HIF1-α was assessed during the experiment. Although Western blot analysis revealed stabilization of HIF1-α in the hypoxia environments (2D and 3D) after 72 hours, there was a considerable decrease in HIF1-α stabilization in the 3D environment compared to the 2D environment. This 3D system allowed isolation and rapid expansion of stromal progenitors from patient lung tumor resections. These progenitor cell populations in the TME-like environment may be anticancer drug targets, which limits their effects on promoting cancer metastasis [11].
Synthetic scaffolds constructed from porous poly(lactic-co-glycolic acid) (PLGA) microparticles were also successfully used as substrates for A549 lung cancer cell culture. Additionally, these tumor models were screened in vitro for their therapeutic efficacies [87]. However, hypoxia status was not verified in the abovementioned study.
Synthetic scaffolds constructed from porous poly(lactic-co-glycolic acid) (PLGA) microparticles were also successfully used as substrates for A549 lung cancer cell culture. Additionally, these tumor models were screened in vitro for their therapeutic efficacies [12]. However, hypoxia status was not verified in the abovementioned study.
Another study highlighted the use of synthetic scaffolds for assessing lung cancer cell adhesion, polarity and morphology. NSCLC cells derived from metastatic pleural fluid (NCI-H460) were cultured on polyester-based composite, the Variotis tissue scaffold. In this 3D model, cells showed enhanced expression of a membrane protein related to hypoxia CAIX, which reflects functional changes and demonstrates exchanges between cancer cells and their environment [88].
Another study highlighted the use of synthetic scaffolds for assessing lung cancer cell adhesion, polarity and morphology. NSCLC cells derived from metastatic pleural fluid (NCI-H460) were cultured on polyester-based composite, the Variotis tissue scaffold. In this 3D model, cells showed enhanced expression of a membrane protein related to hypoxia CAIX, which reflects functional changes and demonstrates exchanges between cancer cells and their environment [13].
In another study sodium alginate–gelatin (SA-GL) hydrogel was used to print NSCLC patient derived xenograft cells and lung CAFs co-cultures. Both cells were mixed with the hydrogel, printed and showed high printability and cell viability [89]. Spheroid size distribution after 15 days was in the diameter range of 50–1100 µm, which may allow generation of a hypoxic core. The cellular crosstalk was confirmed in this model by overexpression of vimentin, α-SMA and loss of E-cadherin, which promotes EMT. Tumor stroma interactions to mimic in vivo tumor microenvironments were provided in this study.
In another study sodium alginate–gelatin (SA-GL) hydrogel was used to print NSCLC patient derived xenograft cells and lung CAFs co-cultures. Both cells were mixed with the hydrogel, printed and showed high printability and cell viability [7]. Spheroid size distribution after 15 days was in the diameter range of 50–1100 µm, which may allow generation of a hypoxic core. The cellular crosstalk was confirmed in this model by overexpression of vimentin, α-SMA and loss of E-cadherin, which promotes EMT. Tumor stroma interactions to mimic in vivo tumor microenvironments were provided in this study.
In a subsequent study, the 3D tissue-like construct was also used to evaluate the metabolic response of lung cancer cells to ionizing radiation [90]. Cells-in-Gels-in-Paper (CiGiP) were prepared by stacking multiple sheets of paper containing cell-embedded hydrogels and efficiently generated a gradient of oxygen and nutrients that decreased monotonically in the stack. A549 cells used in the CiGiP model showed increased levels of HIF1-α, decreased proliferation and reduced sensitivity to ionizing radiation. Authors identified three isogenic variants of A549 cells based on their metabolic radiosensitivity, which are known to differ in migration and proliferation in vivo [90].
In a subsequent study, the 3D tissue-like construct was also used to evaluate the metabolic response of lung cancer cells to ionizing radiation [14]. Cells-in-Gels-in-Paper (CiGiP) were prepared by stacking multiple sheets of paper containing cell-embedded hydrogels and efficiently generated a gradient of oxygen and nutrients that decreased monotonically in the stack. A549 cells used in the CiGiP model showed increased levels of HIF1-α, decreased proliferation and reduced sensitivity to ionizing radiation. Authors identified three isogenic variants of A549 cells based on their metabolic radiosensitivity, which are known to differ in migration and proliferation in vivo [14].
Taken together, abovementioned studies have confirmed that 3D scaffold models can accommodate the TME oxygen content in a gradient-dependent manner for better therapeutic approaches.
3. Microfluidic Devices
Microfluidic technology provides exquisite control of any physical and chemical parameter of the cell culture in the device at the micrometer scale. This technology combined with 3D cell cultures has the potential to better replicate in vivo responses, as it effectively reproduces cell–cell and cell–matrix interactions, diffusion gradients of drugs, nutrients, oxygen, pH, and dynamic changes in microenvironmental parameters such as stiffness [104]. Different platforms can control oxygen concentration in three ways: introducing oxygen scavenging chemicals, altering the diffusional distance of oxygen or incorporating relatively oxygen impermeable materials.
Microfluidic technology provides exquisite control of any physical and chemical parameter of the cell culture in the device at the micrometer scale. This technology combined with 3D cell cultures has the potential to better replicate in vivo responses, as it effectively reproduces cell–cell and cell–matrix interactions, diffusion gradients of drugs, nutrients, oxygen, pH, and dynamic changes in microenvironmental parameters such as stiffness [15]. Different platforms can control oxygen concentration in three ways: introducing oxygen scavenging chemicals, altering the diffusional distance of oxygen or incorporating relatively oxygen impermeable materials.
Lung cancer metastasis was studied in a multiorgan microfluidic device [105]. A549 cells cultured inside the microfluidic device formed cancer mass and showed the EMT features. These cells were further used to evaluate the potential to invade the distant organs (brain, bone, liver). The microfluidic system was also used to monitor tumor models during anticancer treatment under varying oxygen conditions. Higher uptake of doxorubicin under a cycling hypoxia profile than under either chronic hypoxia was observed in breast cancer cells.
Lung cancer metastasis was studied in a multiorgan microfluidic device [16]. A549 cells cultured inside the microfluidic device formed cancer mass and showed the EMT features. These cells were further used to evaluate the potential to invade the distant organs (brain, bone, liver). The microfluidic system was also used to monitor tumor models during anticancer treatment under varying oxygen conditions. Higher uptake of doxorubicin under a cycling hypoxia profile than under either chronic hypoxia was observed in breast cancer cells.
The study of Ch. Chang et al. [91] highlights the use of a polydimethylsiloxane–polycarbonate (PDMS–PC) hybrid microfluidic device for A549 cell culture under combinations of chemical and oxygen gradients. The drug testing results showed an increase in A549 cell apoptosis due to the hypoxia-activated cytotoxicity of tirapazamine. In addition, it was confirmed that the oxygen gradient plays an essential role during cell moving because the A549 cell migration assay demonstrated an aerotactic behavior. The authors claimed the device is promising to advance the control of in vitro microenvironments and allows to study of cellular responses under various physiological conditions simultaneously.
The study of Ch. Chang et al. [17] highlights the use of a polydimethylsiloxane–polycarbonate (PDMS–PC) hybrid microfluidic device for A549 cell culture under combinations of chemical and oxygen gradients. The drug testing results showed an increase in A549 cell apoptosis due to the hypoxia-activated cytotoxicity of tirapazamine. In addition, it was confirmed that the oxygen gradient plays an essential role during cell moving because the A549 cell migration assay demonstrated an aerotactic behavior. The authors claimed the device is promising to advance the control of in vitro microenvironments and allows to study of cellular responses under various physiological conditions simultaneously.
Another study harnessed the power of a 3D microfluidic chip to observe real-time changes in lung cancer cells after exposition to cobalt chloride (CoCl
2), which simulates a hypoxic microenvironment [92]. It was demonstrated that Netrin-1 mediated EMT of A549 and PC9 cells in vitro, may be related to the phosphoinositide 3 kinase/AKT pathway, but only in a hypoxic microenvironment. The higher concentration of Netrin-1 was also found in NSCLC patients’ sera. Taken together, this finding obtained with the use of 3D microfluidic chip provided evidence that Netrin-1 promotes hypoxia-induced EMT in lung cancer cells and may be a potential therapeutic target.
), which simulates a hypoxic microenvironment [18]. It was demonstrated that Netrin-1 mediated EMT of A549 and PC9 cells in vitro, may be related to the phosphoinositide 3 kinase/AKT pathway, but only in a hypoxic microenvironment. The higher concentration of Netrin-1 was also found in NSCLC patients’ sera. Taken together, this finding obtained with the use of 3D microfluidic chip provided evidence that Netrin-1 promotes hypoxia-induced EMT in lung cancer cells and may be a potential therapeutic target.
An effective drug sensitivity test platform was designed by Z. Xu et al. [93] on microfluidic chip-based, 3D co-culture. A mixture of lung cancer and stromal cell lines, and cells from fresh lung cancer tissues were cultured in 3D under conditions mimicking the TME in vivo. The cells were treated with a panel of anticancer drugs according to a gradient concentration generator inside the chips to screen the appropriate chemotherapy schemes. The authors assayed the sensitivity to different anti-cancer drugs in parallel and accurately determined the appropriate dose of single and combined-drug chemotherapy schemes for eight patients. The presented microfluidic chip-based 3D co-culture may be useful to screen the appropriate chemotherapy schemes to guide individualized treatment in lung cancer.
An effective drug sensitivity test platform was designed by Z. Xu et al. [19] on microfluidic chip-based, 3D co-culture. A mixture of lung cancer and stromal cell lines, and cells from fresh lung cancer tissues were cultured in 3D under conditions mimicking the TME in vivo. The cells were treated with a panel of anticancer drugs according to a gradient concentration generator inside the chips to screen the appropriate chemotherapy schemes. The authors assayed the sensitivity to different anti-cancer drugs in parallel and accurately determined the appropriate dose of single and combined-drug chemotherapy schemes for eight patients. The presented microfluidic chip-based 3D co-culture may be useful to screen the appropriate chemotherapy schemes to guide individualized treatment in lung cancer.
A novel multi-flow microfluidic (MFM) system for the separation of circulating tumor cells (CTCs) from six out of eight NSCLC patients with high purity was discovered by J. Zhou et al. [94]. This device was constructed and configured based on the phenomenal effect of size-dependent inertial migration and allowed separation of CTCs from patients’ blood.
A novel multi-flow microfluidic (MFM) system for the separation of circulating tumor cells (CTCs) from six out of eight NSCLC patients with high purity was discovered by J. Zhou et al. [20]. This device was constructed and configured based on the phenomenal effect of size-dependent inertial migration and allowed separation of CTCs from patients’ blood.
All abovementioned studies significantly confirmed that microfluidic systems are a promising platform for the hypoxia TME investigation due to the precise oxygen control facilitated by their small size scales.
4. 3D Bioprinting
3D bioprinting is an advanced fabrication technology that is used for creating tissues of one or more cell types that can mimic the 3D geometry and structure of native tissues. Three-dimensional bioprinting has emerged as a promising method to create reproducible but complex biological constructs by printing cell-laden hydrogel matrix precursors or bio-inks.
In the previously described study of A. Mondal et al. [89], SA-GL hydrogel was used to print NSCLC patient-derived xenograft cells and lung CAFs co-cultures. Rheological optimization of SA-GL hydrogel enhanced printability and viability of NSCLC xenograft cells and CAF co-culture, which allowed the 3D co-culture spheroid formation within the printed scaffold. Therefore, this model can be used for conducting high-throughput drug screening and other pre-clinical applications [78].
In the previously described study of A. Mondal et al. [7], SA-GL hydrogel was used to print NSCLC patient-derived xenograft cells and lung CAFs co-cultures. Rheological optimization of SA-GL hydrogel enhanced printability and viability of NSCLC xenograft cells and CAF co-culture, which allowed the 3D co-culture spheroid formation within the printed scaffold. Therefore, this model can be used for conducting high-throughput drug screening and other pre-clinical applications [21].
Another tumor-like lung cancer model was successfully created by X. Wang et al. [95] to evaluate the feasibility of utilizing it in biomedical applications. Three-dimensional bioprinting was used to fabricate a cell-laden hydrogel grid scaffold structure, using gelatin–sodium alginate lung cancer cell A549/95-D suspension as the bio-ink. Cell viability after the printing process remained over 90%, showing that the temperature and pressure changes the cells encountered during the 3D printing process, caused no serious damage. Biological properties of the printed cells, cell invasion and migration capabilities were checked by scratch test. Additionally,
Another tumor-like lung cancer model was successfully created by X. Wang et al. [22] to evaluate the feasibility of utilizing it in biomedical applications. Three-dimensional bioprinting was used to fabricate a cell-laden hydrogel grid scaffold structure, using gelatin–sodium alginate lung cancer cell A549/95-D suspension as the bio-ink. Cell viability after the printing process remained over 90%, showing that the temperature and pressure changes the cells encountered during the 3D printing process, caused no serious damage. Biological properties of the printed cells, cell invasion and migration capabilities were checked by scratch test. Additionally,
MMP2
and
MMP9
genes expression was assessed. Results showed that both properties were improved in 3D printed cells compared to 2D cultured cells. Although the gelatin–sodium alginate system could simulate the extracellular structure and environment to a certain extent, this model can be only cultured for up to 28 days, as later, the structure would become disintegrated.
In a very interesting study of R. Utama et al., the formation of matrix-embedded multicellular spheroids prepared in high-throughput (HTP) was described [96]. Authors developed an enabling technology consisting of a bespoke drop-on-demand 3D bioprinter capable of HTP printing of 96-well plates of spheroids. This bioprint gave a high cell number and high cell viability. The HTP bioprinting of embedded spheroids was derived from various cell types, also human NSCLC H460 cells. Three-dimensional printed matrix-embedded spheroids features were compared to manually prepared spheroids by different approaches. H&E staining was performed on spheroid cross sections and showed that the cell arrangements and populations were very similar in both 3D bioprinted and manual spheroids at both days 3 and 6. Spheroids stained with phalloidin for F-actin organization and SYTOX green for nuclei were used to explore the cell arrangement, with no significant difference found between 3D bioprinted and manual spheroids. To visualize the organization of the spheroids, immunostaining for the cell proliferation protein Ki67 and the nucleus staining was prepared. Proliferating cells were consistently found on the periphery of the spheroid during the entire 6 days of investigation in both types of spheroids. Additionally, the percentage of the apoptotic marker cleaved caspase-3-positive cells was 0.4 and 1.7% for the 3D bioprinted and manual spheroid, respectively. Further, the authors checked the presence of hypoxic cells in the 3D bioprinted spheroids by HIF1-α immunolabeling. The analysis via fluorescence-activated cell sorting (FACS) showed equivalent positive cell populations of 5% in both the 3D bioprinted and manual spheroids. These data confirmed that generated the 3D bioprinted spheroids carry the important hypoxia characteristic of a 3D spheroid model. Additionally, the opportunity of HTP drug screening was investigated on neuroblastoma spheroids, exposed to doxorubicin. It was shown that sensitivity to spheroid size, embedding and how spheroids conform to the embedding affect the response toward doxorubicin [96].
In a very interesting study of R. Utama et al., the formation of matrix-embedded multicellular spheroids prepared in high-throughput (HTP) was described [23]. Authors developed an enabling technology consisting of a bespoke drop-on-demand 3D bioprinter capable of HTP printing of 96-well plates of spheroids. This bioprint gave a high cell number and high cell viability. The HTP bioprinting of embedded spheroids was derived from various cell types, also human NSCLC H460 cells. Three-dimensional printed matrix-embedded spheroids features were compared to manually prepared spheroids by different approaches. H&E staining was performed on spheroid cross sections and showed that the cell arrangements and populations were very similar in both 3D bioprinted and manual spheroids at both days 3 and 6. Spheroids stained with phalloidin for F-actin organization and SYTOX green for nuclei were used to explore the cell arrangement, with no significant difference found between 3D bioprinted and manual spheroids. To visualize the organization of the spheroids, immunostaining for the cell proliferation protein Ki67 and the nucleus staining was prepared. Proliferating cells were consistently found on the periphery of the spheroid during the entire 6 days of investigation in both types of spheroids. Additionally, the percentage of the apoptotic marker cleaved caspase-3-positive cells was 0.4 and 1.7% for the 3D bioprinted and manual spheroid, respectively. Further, the authors checked the presence of hypoxic cells in the 3D bioprinted spheroids by HIF1-α immunolabeling. The analysis via fluorescence-activated cell sorting (FACS) showed equivalent positive cell populations of 5% in both the 3D bioprinted and manual spheroids. These data confirmed that generated the 3D bioprinted spheroids carry the important hypoxia characteristic of a 3D spheroid model. Additionally, the opportunity of HTP drug screening was investigated on neuroblastoma spheroids, exposed to doxorubicin. It was shown that sensitivity to spheroid size, embedding and how spheroids conform to the embedding affect the response toward doxorubicin [23].
In the presented study, the authors demonstrated 3D bioprinted spheroids that possessed important in vivo tumor-like characteristics found in manually prepared spheroids. Moreover, it was confirmed that the 3D bioprinting may be a robust HTP platform to screen biological and therapeutic parameters in the future.
5. Fluorescence Imaging of 3D Lung Cancer Models
Standard O
2
imaging techniques described previously in
Section 4b, have been applied clinically for tissue voxels assessment, but they are not useful for individual cell monitoring. Fluorescence imaging is a noninvasive method, which is highly advantageous for the study of 3D dimensional systems. During the 3D model generation, there has constantly been a need to control the tumor parameters. Phenotypic characterization of tumor spheroids, ECM accumulation or hypoxia occurrence can be visualized by light-sheet fluorescence microscopy. Recently, the 3D-3 model, based on the alginate microencapsulation strategy, composed from NSCLC cells, CAF and monocytes, were monitored over time by fluorescence imaging [106]. It was demonstrated that the 3D-3-culture recreates an invasive and the immunosuppressive TME, with accumulation of cytokines/chemokines, ECM elements and matrix metalloproteinases, supporting cell migration and promoting cell–cell interactions within the alginate microcapsules. The effectiveness of chemotherapeutic treatment of 3D-3-culture were also visualized by immunofluorescence.
b, have been applied clinically for tissue voxels assessment, but they are not useful for individual cell monitoring. Fluorescence imaging is a noninvasive method, which is highly advantageous for the study of 3D dimensional systems. During the 3D model generation, there has constantly been a need to control the tumor parameters. Phenotypic characterization of tumor spheroids, ECM accumulation or hypoxia occurrence can be visualized by light-sheet fluorescence microscopy. Recently, the 3D-3 model, based on the alginate microencapsulation strategy, composed from NSCLC cells, CAF and monocytes, were monitored over time by fluorescence imaging [24]. It was demonstrated that the 3D-3-culture recreates an invasive and the immunosuppressive TME, with accumulation of cytokines/chemokines, ECM elements and matrix metalloproteinases, supporting cell migration and promoting cell–cell interactions within the alginate microcapsules. The effectiveness of chemotherapeutic treatment of 3D-3-culture were also visualized by immunofluorescence.
In another study, confocal fluorescence microscopy was used to monitoring lung cancer cells with CD44 expression and showed varying invasiveness into the 3D hydrogel [107]. The tested biomimetic 3D hydrogel platform enabled to quantitative analysis of cell invasion and viability at the individual cell level and was developed using automated data acquisition methods. Within this system more detailed analyses of cellular responses to drug treatments are possible, allowing for more effective drug screens.
In another study, confocal fluorescence microscopy was used to monitoring lung cancer cells with CD44 expression and showed varying invasiveness into the 3D hydrogel [25]. The tested biomimetic 3D hydrogel platform enabled to quantitative analysis of cell invasion and viability at the individual cell level and was developed using automated data acquisition methods. Within this system more detailed analyses of cellular responses to drug treatments are possible, allowing for more effective drug screens.
The fluorescence imaging for detection of hypoxic cell was commonly applied. The HIF-binding sequences were used to transcriptionally control the expression of fluorescent proteins under hypoxia in order to detect hypoxic cells in human prostate [108] and breast cancer [109] models. The unique system was generated by I. Godet et al. to track the fate of hypoxic cells that undergo reoxygenation in the bloodstream and lung [109]. This system allowed to permanently mark cells when they become hypoxic by triggering a fluorescent switch from red (DsRed, red fluorescent protein) to green (GFP, green fluorescence protein). Additionally, the fluorescence imaging indicated that cells exposed to hypoxia migrated away from the core of the spheroid, where they become hypoxic, to the more oxygenated periphery of the spheroid. The ability of the system to fate-map hypoxic cells was tested in 2D and in 3D spheroids, as well as in orthotopic and mouse models of breast cancer. The developed fate-mapping hypoxia system can be applied to another types of cancer that are critically affected by hypoxia.
The fluorescence imaging for detection of hypoxic cell was commonly applied. The HIF-binding sequences were used to transcriptionally control the expression of fluorescent proteins under hypoxia in order to detect hypoxic cells in human prostate [26] and breast cancer [27] models. The unique system was generated by I. Godet et al. to track the fate of hypoxic cells that undergo reoxygenation in the bloodstream and lung [27]. This system allowed to permanently mark cells when they become hypoxic by triggering a fluorescent switch from red (DsRed, red fluorescent protein) to green (GFP, green fluorescence protein). Additionally, the fluorescence imaging indicated that cells exposed to hypoxia migrated away from the core of the spheroid, where they become hypoxic, to the more oxygenated periphery of the spheroid. The ability of the system to fate-map hypoxic cells was tested in 2D and in 3D spheroids, as well as in orthotopic and mouse models of breast cancer. The developed fate-mapping hypoxia system can be applied to another types of cancer that are critically affected by hypoxia.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Cheng, T.-Y.D.; Cramb, S.M.; Baade, P.D.; Youlden, D.R.; Nwogu, C.; Reid, M.E. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J. Thorac. Oncol. 2016, 11, 1653–1671.
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594.
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.; Yatabe, Y.; Powell, C.A.; Beer, D.; Riely, G.; Garg, K.; et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: International Multidisciplinary Classification of Lung Adenocarcinoma: Executive Summary. Proc. Am. Thorac. Soc. 2011, 8, 381–385.
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157.
- Marhuenda, E.; Campillo, N.; Gabasa, M.; Martínez-García, M.A.; Campos-Rodríguez, F.; Gozal, D.; Navajas, D.; Al-caraz, J.; Farré, R.; Almendros, I. Effects of Sustained and Intermittent Hypoxia on Human Lung Cancer Cells. Am. J. Respir. Cell Mol. Biol. 2019, 61, 540–544.
- Mondal, A.; Gebeyehu, A.; Miranda, M.; Bahadur, D.; Patel, N.; Ramakrishnan, S.; Rishi, A.K.; Singh, M. Characteriza-tion and printability of Sodium alginate -Gelatin hydrogel for bioprinting NSCLC co-culture. Sci. Rep. 2019, 9, 1–12.
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083.
- Stratmann, A.T.; Fecher, D.; Wangorsch, G.; Göttlich, C.; Walles, T.; Walles, H.; Dandekar, T.; Dandekar, G.; Nietzer, S.L. Establishment of a human 3D lung cancer model based on a biological tissue matrix combined with a Boolean in silico model. Mol. Oncol. 2013, 8, 351–365.
- Wallstabe, L.; Göttlich, C.; Nelke, L.C.; Kühnemundt, J.; Schwarz, T.; Nerreter, T.; Einsele, H.; Walles, H.; Dandekar, G.; Nietzer, S.L.; et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tu-mor models. JCI Insight 2019, 4.
- Saforo, D.; Omer, L.; Smolenkov, A.; Barve, A.; Casson, L.; Boyd, N.L.; Clark, G.; Siskind, L.; Beverly, L.J. Primary lung cancer samples cultured under microenvironment-mimetic conditions enrich for mesenchymal stem-like cells that pro-mote metastasis. Sci. Rep. 2019, 9, 4177.
- Kuriakose, A.E.; Hu, W.; Nguyen, K.T.; Menon, J.U. Scaffold-based lung tumor culture on porous PLGA microparticle substrates. PLoS ONE 2019, 14, e0217640.
- Zhang, M.; Boughton, P.; Rose, B.; Lee, C.-S.; Hong, A.M. The Use of Porous Scaffold as a Tumor Model. Int. J. Biomater. 2013, 2013, 396056.
- Simon, K.A.; Mosadegh, B.; Minn, K.T.; Lockett, M.R.; Lockett, M.R.; Boucher, D.M.; Hall, A.B.; Hillier, S.M.; Udagawa, T.; Eustace, B.K.; et al. Metabolic response of lung cancer cells to radiation in a paper-based 3D cell culture system. Biomaterials 2016, 95, 47–59.
- Grist, S.M.; Nasseri, S.S.; Laplatine, L.; Schmok, J.C.; Yao, D.; Hua, J.; Chrostowski, L.; Cheung, K.C. Long-term monitor-ing in a microfluidic system to study tumour spheroid response to chronic and cycling hypoxia. Sci. Rep. 2019, 9, 1–13.
- Xu, Z.; Li, E.; Guo, Z.; Yu, R.; Hao, H.; Xu, Y.; Sun, Z.; Li, X.; Lyu, J.; Wang, Q. Design and Construction of a Multi-Organ Microfluidic Chip Mimicking the in vivo Microenvironment of Lung Cancer Metastasis. ACS Appl. Mater. Interfaces 2016, 8, 25840–25847.
- Chang, C.-W.; Cheng, Y.-J.; Tu, M.; Chen, Y.-H.; Peng, C.-C.; Liao, W.-H.; Tung, Y.-C. A polydimethylsiloxane-polycarbonate hybrid microfluidic device capable of generating perpendicular chemical and oxygen gradients for cell culture studies. Lab. Chip 2014, 14, 3762–3772.
- Jin, X.; Luan, H.; Chai, H.; Yan, L.; Zhang, J.; Wang, Q.; Cao, L. Netrin 1 interference potentiates epithelial-to-mesenchymal transition through the PI3K/AKT pathway under the hypoxic microenvironment conditions of non-small cell lung cancer. Int. J. Oncol. 2019, 54, 1457–1465.
- Xu, Z.; Gao, Y.; Hao, Y.; Li, E.; Wang, Y.; Zhang, J.; Wang, W.; Gao, Z.; Wang, Q. Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials 2013, 34, 4109–4117.
- Zhou, J.; Kulasinghe, A.; Bogseth, A.; O’Byrne, K.; Punyadeera, C.; Papautsky, I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst. Nanoeng. 2019, 5, 1–12.
- Arai, K.; Eguchi, T.; Rahman, M.M.; Sakamoto, R.; Masuda, N.; Nakatsura, T.; Calderwood, S.K.; Kozaki, K.-I.; Itoh, M. A Novel High-Throughput 3D Screening System for EMT Inhibitors: A Pilot Screening Discovered the EMT Inhibitory Activity of CDK2 Inhibitor SU9516. PLoS ONE 2016, 11, e0162394.
- Wang, X.; Zhang, X.; Dai, X.; Wang, X.; Li, X.; Diao, J.; Xu, T. Tumor-like lung cancer model based on 3D bioprinting. 3 Biotech 2018, 8, 501.
- Utama, R.H.; Atapattu, L.; O’Mahony, A.P.; Fife, C.M.; Baek, J.; Allard, T.; O’Mahony, K.J.; Ribeiro, J.C.; Gaus, K.; Kavallaris, M. A 3D Bioprinter Specifically Designed for the High-Throughput Production of Matrix-Embedded Multicellar Spheroids. Iscience 2020, 23, 101621.
- Rebelo, S.P.; Pinto, C.; Martins, T.R.; Harrer, N.; Estrada, M.F.; Loza-Alvarez, P.; Cabeçadas, J.; Alves, P.C.; Gualda, E.J.; Sommergruber, W.; et al. 3D-3-culture: A tool to unveil macrophage plasticity in the tumour microenvironment. Biomaterials 2018, 163, 185–197.
- Tam, R.Y.; Yockell-Lelièvre, J.; Smith, L.J.; Julian, L.M.; Baker, A.E.G.; Choey, C.; Hasim, M.S.; Dimitroulakos, J.; Stanford, W.L.; Shoichet, M.S. Rationally Designed 3D Hydrogels Model Invasive Lung Diseases Enabling High-Content Drug Screening. Adv. Mater. 2019, 31, e1806214.
- Glunde, K.; Shah, T.; Winnard, P.T.; Raman, V.; Takagi, T.; Vesuna, F.; Artemov, D.; Bhujwalla, Z.M. Hypoxia Regulates Choline Kinase Expression through Hypoxia-Inducible Factor-1α Signaling in a Human Prostate Cancer Model. Cancer Res. 2008, 68, 172–180.
- Godet, I.; Shin, Y.J.; Ju, J.A.; Ye, I.C.; Wang, G.; Gilkes, D.M. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat. Commun. 2019, 10, 1–18.
