Mangiferin is a naturally occurring C-glucosylxantone that has substantial potential for the treatment of various diseases. It possesses antioxidant, anti-infection, anti-cancer, anti-diabetic, cardiovascular, neuroprotective properties and it also increases immunity.
- cancer
- drug delivery systems
- mangiferin
- molecular mechanisms
- polymer systems
1. Introduction
-
Introduction
Mangiferin, also known as alpizarin or quinomine, belongs to the group of organic class compounds of xanthones. Its chemical formula is C19H18O11 and its average molecular weight is 422.34. Until the 1960s, its structure remained unknown [1][2][3][1,2,3]. Only recently, X-ray diffraction analysis of mangiferin has been conducted [4][5][4,5]. It is a polyphenol linked to glucose residues via an abnormal C-C bond (Figure 1).
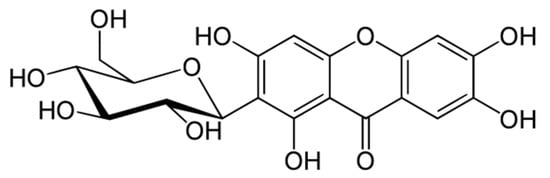
Figure 1.
Molecular structure of mangiferin.
According to the IUPAC nomenclature, mangiferin is identified as: 1,3,6,7-tetrahydroxy-2-[3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl]-9H-xanten-9-one.
In 1908, a pigment—mangiferin—was first isolated from mangoes (Mangifera indica L., Anacardisaceae) [3][6][3,6]. Mangiferin was also found in many other plants, in particular, in the families Anacardiaceae and Gentianaceae [7]. In mangoes, mangiferin is viable as a crystal in the leaves, cores and rind of the trunk [2][8] [2,8] and in the pods and seeds of the fruit [8].
Mangiferin is slightly soluble in ethanol, water and insoluble in some non-polar solvents (for example, n-hexane or diethyl ether) [9]. In water, mangiferin solubility is only at 0.111 mg/mL [10].
In the traditional medicine of several countries such as China, India and Cuba, mangiferin-rich plants have been grown and actively used to treat many diseases such as cardiovascular diseases, diabetes [11], many types of infection and cancer [7][12][13][14][15][7,12,13,14,15].
2. Anti-Cancer Effects of Mangiferin and Mechanisms of Anti-Cancer Action
-
Anti-Cancer Effects of Mangiferin and Mechanisms of Anti-Cancer Action
There is a great deal of evidence of mangiferin’s anti-cancer activity against many malignant tumors in in vitro and in vivo models. This action is carried out by mangiferin through a variety of mechanisms. Depending on the type of cancer cell and its growth pathways, mangiferin has different modes of action. Sometimes mangiferin simply disrupts the signaling of transcription factors, sometimes it blocks, stabilizes or activates certain enzymes or specific proteins, or it can also protect DNA from injury. The simultaneous implementation of these activities helps mangiferin easily arrest the cell cycle, promoting apoptosis of many types of cancer. Not only that, but it can also work synergistically with other cancer drugs to increase their activity on chemotherapy-resistant cancers. In the following, the specific mechanisms and pathways of mangiferin’s anti-cancer activities are covered in more detail.
2.1. Antioxidant and Anti-Inflammatory Effects of Mangiferin
Mangiferin reduces the inflammatory response mainly by intervention in the NF-κB pathway [16]. Mangiferin interferes with different steps in the NF-κB activation pathways, which are classical or alternative. The classical pathway is controlled by the IκB kinase complex and p50, while the alternative pathway is controlled by inhibitors of kappa B kinase (IKKa) and p52. Mangiferin also inhibits other factors including TNFR1—Tumor Necrosis Factor Receptor type-1-Associated Death Domain protein (TRADD), TNFR-Associated Factor 2 (TRAF2), factors of NCK Interacting Kinase (NIK) and IKK that induce Secreted Embryonic Alkaline Phosphatase (SEAP) expression, however, there is no significant effect on p65 which is responsible for the expression of SEAP. In addition, mangiferin also inhibits MAPKs p38, the kinase is modulated with an extracellular signal (ERK) and kinase phosphorylation at the Jun N c terminal [17], thereby reducing the MAPK (Mitogen Activated Protein Kinase) signal [16]. There is already evidence of the anti-tumor effects of mangiferin via multiple signaling pathways, which include nuclear NF-κB signaling and cyclooxygenase-2 (COX-2) protein expression [18]. In anticancer activity, mangiferin induces an apoptosis effect, possibly by activation of caspases. Disorders and imbalances between cell proliferation and apoptosis have been identified as the causes of tumor initiation.
Mangiferin enhances mRNA expression of the Peroxisome Proliferator Activated Receptor Gamma (PPARgamma) gene [19] [19] and minifies the transcriptional activation of COX-2. It has been demonstrated by in vitro studies of the MDA-MB-231 cells that mangiferin may play a beneficial role in modulating the regulation of PPARgamma as well as COX-2 [18].
Mangiferin, in a dose-dependent manner, prevents the depletion of total nucleotides, ATP damage, as well as restored energy charge potential in H2O2-treated erythrocytes. Mangiferin also protects hepatocytes from free radical-mediated hypoxia/reoxygenation damage by forming a complex of mangiferin: Fe3+ complexes and neutralizing free radicals [20][21][20,21].
Mangiferin reduces lipid peroxidase and increases levels of antioxidant enzymes so it is also effective in vitro against glycated protein/chelate iron-induced toxicity in human umbilical vein endothelial cells [22].
Numerous data about activity mangiferin in in vitro and in in vivo models have been published.
Mangiferin inhibits the initiation, promotion and metastasis of cancer, by targeting pro-inflammatory transcription factors, growth factors, cell-cycle proteins, cytokines, kinases, adhesion molecules, chemokines and inflammatory enzymes [23], at the same time it also activates the estrogen receptor alpha (ERα) [24].
Its anti-cancer effect is confirmed in mouse models in doses of 5–10 mg/kg (i.p. or i.m.) against ascitic fibrosarcoma in Swiss mice [25], 50 and 100 mg/kg (oral) against Benzo(a)pyrene induced lung carcinogenesis [26][27] [26,27] and 100 mg/kg (oral) against ER-negative breast cancer [28].
2.2. Mouse Melanoma
Mangiferin inhibits spontaneous metastasis and tumor growth in the highly metastatic malignant cancer B16BL6 model (mouse melanoma) in doses 50, 100 and 200 mg/kg (mice, orally, 21 days) [29]. Mangiferin also suppresses the nuclear translocation of NF-κB and minimizes the expression of phosphorylated NF-κB-inducing kinase (NIK), inhibitors of kappa B kinase (IKK) and kappa B (IκB) and conversely increases the expression of IκB protein in vivo. In vivo experiments, also confirmed the inhibitory effect of mangiferin on matrix metalloproteinases (MMP) MMP-1, MMP-2, MMP-9 and MMP-14 and very late antigens (VLAs) VLA-4, VLA-5 and VLA-6, which are highly overexpressed in metastatic malignancies. Treatment with mangiferin enhances the expression of cleaved caspase-3, cleaved Poly ADP ribose polymerase-1 (PARP-1), p53 proteins such as p53 upregulated modulator of apoptosis (PUMA—is a key regulator of apoptosis) and phosphorylated p53 proteins, and at the same time, it also reduces the expression of Survivin and Bcl-associated X (Bcl-xL) proteins in vivo. The above results confirm that mangiferin is highly selective in blocking the NF-κB pathway through inhibition of NIK activation, thus inhibiting tumor metastasis and growth. Oral mangiferin administration did not show toxicity since there were no differences in body weight between sham control, tumor control and mangiferin-treated groups, at the same time, it also did not exhibit any side effects at all.
2.3. Acute Myeloid Leukemia
Chemotherapy-induced oxidative damage correlates with the development of secondary malignancies such as acute myeloid leukemia (AML).
Mangiferin possesses antileukemic and preventive effects in HL-60 leukemia cells. Mangiferin activates the G2/M phase cell cycle arrest by modulation of the CDK1 (Cyclin-Dependent Kinase 1)-cyclin B1 signaling pathway in a dose-dependent manner. At higher concentrations, it induces Wee1 mRNA expression, significantly suppressing mRNA expression of Chk1 (Checkpoint kinase 1) and cdc25C, and remarkably inhibits the phosphorylation of Ataxia Telangiectasia and Rad3-related protein (ATR), Chk1, Wee1, Akt and Erk1/2. In addition, mangiferin decreases the activation of cyclin B1 and cdc25C, and protein expression levels of Akt and Wee1 via ATRChk1 [30].
Mangiferin is a dose-dependent and time-dependent agent that increases Nrf2 (Nuclear factor erythroid 2-Related Factor 2) expression and protein stabilization in human HL-60 myeloid leukemia cells in vitro. Furthermore, it also inhibits the proliferation and degradation of blood cells through the increasing of the stability of the Nrf2 protein [31].
In HL-60 cells, mangiferin (50 μM) increases Nrf2 protein accumulation, enhances Nrf2 binding of antioxidant response elements (AREs), modulates NQO1 (NAD(P)H: quinine reductases) expression and restricts intracellular ROS levels. It also lowers oxidative stress and relieves etoposide-induced cytotoxicity in mononuclear human umbilical cord blood cells [32].
2.4. Glioma Cells
Mangiferin promotes miR-15b and inhibits MMP-7, MMP-9 and EMT (epithelial-to-mesenchymal transition), which significantly limits proliferation and increases apoptosis in U87, U373MG and CRT-MG glioma cells. Mangiferin can also influence VEGF-A (Vascular Endothelial Growth Factor) transcription to modulate angiogenesis via NF-κB [33][34][33,34].
2.5. Prostate Cancer
In the LNCaP prostate carcinoma cells, in androgen-sensitive humans, mangiferin significantly reduces TNFα-induced MMP-9 activity, relieves NF-κB activity and inhibits nuclear translocation of the NF-κB subunits, p65 and p50 [35]. It is known that MMP-7 and MMP-9 are also strong promoters of cancer progression and metastasis of malignant tumor cells [36].
Whereas in PC3 prostate cancer cells, mangiferin promotes apoptosis and induces the caspase-3 activity, significantly reduces Bcl-2 expression levels and enhances miR-182 expression [37].
2.6. Colon Carcinoma
Mangiferin causes a reduction of NF-κB activation in HT29 cells rendered resistant to oxaliplatin [38].
In cells of colorectal cancer HT29 and cervical cancer HeLa, mangiferin treatment induces a delay in the S phase, which is an important phase of the cell cycle responsible for DNA synthesis [38].
Mangiferin (0.1% in diet) in rat colon carcinogenesis induced by the carcinogen azoxymethane, significantly inhibits the aberrant crypt foci development in rats, significantly lowers the incidence and multiplicity of intestinal neoplasms and reduces cell proliferation in colonic mucosa [39].
2.7. Hepatocellular Carcinoma
Mangiferin has potent cytoprotective and antigenotoxic effects against CdCl2 induced toxicity in the HepG2 cell line and may decrease in CdCl2 induced reactive oxygen species levels and resultant oxidative stress [40].
Mangiferin inhibits human hepatocellular carcinoma cells BEL-7404 also through G2/M phase cell cycle arrest [41][42][41,42].
Mangiferin lowers the levels of total bilirubin, AST, SGPT, SGOT and alkaline phosphatase (ALP) in hepatic damage. DEN (diethylnitrosamine)-treated rats showed decreased levels of total protein, serum albumin and globulin. Mangiferin also lowers the levels of tumor markers carcinoembryonic antigen and alpha-fetoprotein [42].
The antioxidant efficiency of mangiferin in DEN-induced rat liver carcinogenesis was evaluated [43].
Oral administration of mangiferin suppresses orthotopic hepatic tumor growth in vivo. The inhibitory effect of mangiferin was mediated through the transcriptional repression of LEF1 (Lymphoid Enhancer Binding Factor 1) via the β-catenin-independent Wnt pathway, with downregulation of MYC (MYC Proto-Oncogene, BHLH Transcription Factor), axin2, MMP2 and CCND1 [44].
2.8. Breast Carcinoma
Mangiferin inhibits the growth of MCF-7 breast cancer cells, in a time-dependent manner by downregulating the CDK1-cyclin Bl signaling pathway and inducing G2/M phase cell-cycle arrest [45]. It induces apoptotic cell death by inhibition of the protein kinase C (PKC)-NF-κB pathway. In vivo experiments performed on the MCF-7 xenograft rat model confirmed these results. The mitochondrial cytochrome C level was reduced under mangiferin treatment, which means that apoptosis can be reduced through the mitochondrial pathway. This pathway also supports the increase of caspase-3, -8, -9 and the decreased expression of procaspase-3, -8, -9 activity.
In C57BL/6J mice, mangiferin reduces tumor volume by 89.4% with a dose of 100 mg/kg, which is close to the effect of the chemotherapeutic drug cisplatin (91.5%). At this dose, mangiferin extended the lifespan of the treated animals [45].
Mangiferin reduces cell viability, restricts metastatic potential, minifies MMP-7 and -9 expression, reverses EMT and interdicts the β-catenin pathway in breast cancer cell lines. It significantly reduces proliferation, weight and volume of tumors and enhances apoptosis, as well as also reducing the expression levels of MMP-7, MMP-9, β-catenin activity, vimentin and increasing E-cadherin expression in MDA-MB-231 xenograft mice to modulate angiogenesis [28].
In the cell line MDA-MB-231 of triple negative breast cancer, mangiferin suppresses the activation of classical NF-κB by IκB kinases (IKK) α/β via impairing IκB degradation, NF-κB translocation and NF-κB/DNA binding. In addition, mangiferin inhibits additional NF-κB pathways involved in cancer cell survival and resistance to therapy such as c-Jun N-terminal kinases (JNK) 1/2, MEK1, p90 ribosomal s6 kinase and mitogen- and stress-activated protein kinase 1[46] [46]. Vimang@ and mangiferin, when stimulated by TNF, both have the ability to reduce the production of IL-6 (Interleukin-6) and IL-8 (Interleukin-8), thereby reducing the inflammatory response.
2.9. Lung Carcinoma
Mangiferin possesses growth-inhibitory and apoptosis-inducing effects against both A549 cells (25 µg/mL) and in A549 xenograft mice in vivo (100 mg/kg, i.p., two weeks). Mangiferin exhibits anti-cancer properties by inducing G2/M phase cell cycle arrest through the cyclin-dependent kinase 1-cyclin B1 signaling pathway downregulation and apoptotic cell death by inhibiting the PKC-NF-κB pathway [47].
An 18-week diet containing mangiferin (oral, 100 mg/kg) significantly improved the high levels of glycoprotein components, membrane lipid peroxidation and ATPases in animals (male Swiss albino mice) with lung carcinoma. It increased the levels of glutathione (GSH), glutathione transferase (GST), quinone reductase (QR), uridine 5′-diphosphate-glucuronosyl transferase (UDP-GT), catalase (CAT), superoxide dismutase, GSH reductase, GSH peroxidase, vitamin E and vitamin C [26][27][26,27].
A study on lymphocytes, polymorphonuclear cells (PMN) and macrophages from B(a)P-treated mice by oral mangiferin twice a week (50 mg/kg and 100 mg/kg) for four weeks confirmed the effects of enhanced lipid peroxidation and decreased activity of catalase and superoxide dismutase. Mangiferin also played an immunoprotective role determined by the reduction of oxidative stress, inducing an intermediate response in lymphocytes, neutrophils and macrophages. The IgG and IgM levels were significantly increased and the IgA level was decreased [48].
The levels of glycoproteins, membrane ATPases and membrane lipid peroxidation were significantly decreased under the action of mangiferin (100 mg/kg) in the benzo(a)pyrene-induced lung carcinogenesis (Male Swiss albino mice) [49].
Adjustment of electron transport chain complexes and of key enzymes in the tricarboxylic acid cycle [50] [50] significantly decreases the levels of polyamines, protein carbonyl, nucleic acid content and lipid peroxidation was found after mangiferin treatment in animals (male Swiss albino mice, 100 mg/kg) [51].
In albino mice with lung carcinoma (caused by benzo(a)pyrene (BaP), 50 mg/kg), the use of mangiferin (100 mg/kg) was shown to decrease the activity of lysosomal enzymes such as β-glucuronidase, acid phosphatase, β-galactosidase and N-acetyl glucosaminidase [52].
2.10. Other Types of Cancer Diseases
In the human cell line nasopharyngeal carcinoma (CNE2 cells), different doses of mangiferin (from 12.5 to 200 μM) inhibited their proliferation through G2/M phase cell cycle arrest, induced early apoptosis, modulated the mRNA, Bcl-2 protein levels and Bax [53].
In human cervical cancer HeLa cells, mangiferin downregulated protein expression of BH3 (interacting domain death agonist), Bcl-2 and pro-caspase-3 and pro-caspase-8, thereby activating caspase-3, -7, -8 and -9, eventually leading to apoptosis [54].
In human neuroblastoma caused by the methylmercury(MeHg)-induced IMR-32 cell line, mangiferin significantly suppressed DNA damage, reduced oxidative stress and inhibited depolarization of the mitochondrial membrane, increased levels of GSH and glutathione S-transferase (GST), resulting in a significant decrease in malondialdehyde formation [55].
2.11. Synergic Action
The synergistic effects of the major chemotherapeutic drugs enhance drug use potential and may allow lower dosages of drugs, thereby reducing toxicity and providing higher selective toxicity to malignant cells, as well as decreasing the extent of side effects [56].
Mangiferin enhances the activity of pro-apoptotic agents such as cisplatin, vincristine, doxorubicin, etoposide, Adriamycin and AraC in lymphoma U-937 cells [57].
It was demonstrated that mangiferin enhances the activity of hesperidin, even in low concentration in HeLa cells via increased regulation of the TRADD and TNFR superfamily member 25, which are related to the external apoptotic pathway [58].
Mangiferin increases the apoptotic effect of oxaliplatin via NF-kB inhibition in HeLa and HT29 cells [38]. The addition of mangiferin in a 10 µg/mL dose has been investigated in HeLa, HT29 and MCF7 cancer cell lines. This addition of mangiferin reduces oxaliplatin IC50 values in HT29 (3.4-fold) and HeLa (1.7-fold) cells. Mangiferin causes a reduction of NF-κB activation in HT29 cells rendered resistant to oxaliplatin.
Mangiferin (25 µg/mL) enhances the antiproliferative effects of cisplatin (12.5 µg/mL) on lung cancer A549 cells. Notably, mangiferin exerts anti-cancer effects in vivo, where it was able to markedly decrease the volume and weight of subcutaneous tumor mass and expand the lifespan (100 mg/kg) of xenograft mice [47].
The higher mangiferin concentrations (10, 25 or 50 μM) together with doxorubicin were able to modulate MCF-7 breast cancer cells via the reduction of their viability and the inhibition of the activity of P-glycoprotein (P-gp) for a period of 96 h [59].
Adverse effects such as the myelosuppression of etoposide were reduced under the action of mangiferin in HL-60 cells, as well as a lack of wild type p53 [28].
2.12. Summary of Anti-Cancer Activities of Mangiferin
Thus, there is much evidence of the anti-cancer activity of mangiferin against many malignancies in in vitro and in vivo models. The dose usually used in animals is 100 mg/kg. The main molecular pathway responsible for the anti-cancer activity is its interaction with NF-kB on various steps. Mangiferin induces G2/M phase cell cycle arrest through the cyclin-dependent kinase 1-cyclin B1 signaling pathway. Another pathway is the downregulation of MMP-7 and MMP-9, which are responsible for cancer progression and metastasis of malignant tumor cells. Activation of caspases-3, -8, -9 is probably the main pathway leading to apoptosis of cancer cells.
Several studies revealed that the use of mangiferin in combination with other anti-cancer drugs leads to a synergic mode of action and fewer side-effects. These important studies may indicate the way to overcome the resistance to some chemotherapeutic drugs.
