The MXenes are a kind of 2D metal carbide/nitride with an alternative atomic arrangement of Mn+1Xn (M = transition metals; X = C, N; n = 1, 2, 3, 4).
- MXene
- 2D materials
- organic hybrids
- energy storage
1. Structure and Surface Characters of MXenes
Take the Ti3C2Tx MXene as an example. The Ti3C2 skeleton can be regarded as an extracted layer from fcc metal carbides lattice along (111) plane (Figure 1). Since the first discovery of Ti3C2Tx MXene in 2011, more than 70 MXenes have been theoretically predicted or synthesized. As mentioned above, the synthesis of MXene follows a “top-down” protocol: selective etching of the A atoms from MAX phase ceramics. However, as the Mn+1Xn layers and A atoms are alternatively arranged in MAX phase ceramics, the removal of A atoms will break M-A bonds, thus results in high-active M atoms at the surface. As first presented by Gogotsi et al., the surface terminal groups (T groups, e.g., –F, –O, or –OH) are necessary for stabilizing MXenes due to the high activity of these coordination unsaturated surface metal atoms [1][22]. Furthermore, owning to these surface terminal groups, the MXenes with typical inorganic carbide skeleton usually presents similar surface physicochemical properties (e.g., hydrophilicity and oxidizability) [2][23].
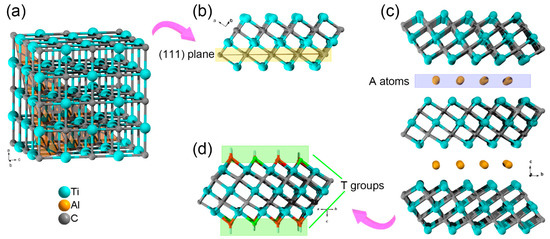
Figure 1. (a) A typical fcc lattice of TiC. (b) The extracted layer from fcc TiC lattice along (111) plane. (c) The side view of Ti3AlC2 lattice and (d) a monolayer of Ti3C2Tx MXene.
Attributing to the surface T groups, the fresh MXene flakes are negatively charged and highly hydrophilic, which will form the colloidal dispersion by surface electrostatic repulsion. Based on the double electrical layer theory, it is easy to speculate that the dispersity of MXene flakes will change along with different solution conditions. Also depends on the average oxidation states of the M and X elements, the Zeta potential of MXenes is varied. In the past several years, the oxidation of MXenes in water also arouses wide concerns, which is found to be isogenesis with their hydrophilic. Still exampled by Ti3C2Tx MXene, although TiO2 species are detected when annealing the MXene under either vacuum, inert atmosphere (Ar, N2), or oxidizing atmosphere (CO2, air), the oxidation can be dramatically slowed down by decrease the environment temperature. However, in an aqueous suspension, nearly half of the flakes degrade in five days, even at room temperature [3][4][24,25]. The degradation behavior of MXenes in the aqueous suspension then can be ascribed to the water or the synergy of water and dissolved oxygen molecules. Interestingly, Kathleen et al. found that with oxygen solubility orders of magnitude higher than water, the organic solvent (N,N-dimethylformamide (DMF), 1-methyl-2-pyrrolidinone (NMP), propylene carbonate (PC), and ethanol) did not cause observable oxidation of MXenes [5][26]. Therefore, a deeper insight into the relationship between the surface T groups on Ti3C2Tx MXene and their dispersion properties was presented, namely, the surface tension, polarity index, dielectric constant (ε), and cohesive energy density (δ). However, although some of the organic solvents were screened out to be a good medium for Ti3C2Tx MXene dispersion, the dispersibility of Ti3C2Tx MXene in nonpolar organic solvents is still an unsolved issue. What is more, the T groups usually have a random constituent and distribution, which makes the determination of surface properties more difficult.
Enlightened by traditional strategies towards surface compatibility regulation, the surface physicochemical properties of MXenes also can be dramatically altered by replacing the inorganic surface terminal groups with organic species. For example, di(hydrogenated tallow)benzyl methyl ammonium chloride (DHT)-treated Ti3C2Tx MXene has shown a good dispersibility in nonpolar organic solvents [6][27]. More importantly, the study opens up a new way for MXene-polymer hybrids, which have good interfacial compatibility. In the following section, based on the interaction between organic species and MXenes, the synthesis and corresponding surface physicochemical properties of MXene-organic hybrids will be briefly discussed.
2. MXene-Organic Hybrids through Covalent Interaction
Benefiting from the surface atomic layers of early transition metals with empty d orbital, the MXenes skeleton usually presents a strong affinity to electron donors. When the organic molecules with electron-donating groups are introduced, the original T groups can be replaced. To date, numerous small organic molecules are found to be able to covalently connect on MXenes.
Silane (RSiXn), one of the most commonly used organic coupling agents, has been widely applied for surface modification of inorganic materials. For silane, the R represents the functional group, such as an amino, alkane, aromatic group, epoxy group, and so on; X stands for hydrolysis group, containing Cl, OMe, OEt, etc. [7][28]. During the so-called “hydrolytic condensation” process (Figure 2a), X groups will first be hydrolyzed to form a siloxane (–SiOH) structure. Later, the siloxane goes through a dehydration condensation reaction to form oligomers. The –OH groups in oligomers will react with –OH groups on MXenes, forming an intermediate state. Finally, the Ti–O–Si covalent bonds are formed by dehydration condensation [7][8][28,29]. Meanwhile, different properties inherited from R groups can be introduced into the inorganic phase. In 2018, Zhao et al. reported a trimethoxy (1H,1H,2H,2H-perfluorodecyl) silane (PFDTMS) modified Ti3C2Tx MXene membrane prepared via a simple wet route [8][29], in which the water contact angle increased from 38.8° to 102.0° after PFDTMS grafting. Later, Sehyeong et al. have systematically studied the surface polarity regulation of Ti3C2Tx MXene by silane with different R groups. After replacing the surface T groups of Ti3C2Tx MXene with lipophilic octyltriethoxysilanes (OTS) [9][30], the OTS@MXene showed a water contact angle of 102.6° and exhibited excellent dispersion stability in non-polar hexane for more than four weeks. When methyltriethoxysilane (MTS), propyltriethoxyislane (PTS), and hexyltriethoxysilane (HTS) was selected, the surface hydrophobicity of these silane-MXene hybrids would decrease with shortening the alkyl chain. Beyond the randomly surface grafting, Zhang et al. designed a well-ordered vertically aligned Janus MXene-based aerogel, which has different wettability belong to two sides named VA-MXA [10][31]. After modifying the Ti3C2Tx MXene by fluorinated alkyl silane under vacuum conditions, the water contact angle of the upper layer increased from 44.6° to 133.2°. Also, the excessive concentration of silane clogged the available surface and decreased the hydroxyls on the surface of Ti3C2Tx, which can bring novel surface physicochemical properties [7][28]. In Tran and his co-workers’ work [11][32], the N,N-(diethylamino)dithiocarbamoyl-benzyl(trimethoxy) silane (SBDC) was used to functional Ti2CTx. Interestingly, the SBDC-MXene hybrid was applied as both substrate and iniferter, which allowed further polymerization of thermoresponsive polymer (poly(2-(dimethylamino)ethyl methacrylate (PDMAEMA)) on the surface of Ti2CTx. On the other hand, the silane molecules can not only change the surface hydrophilcity, but also alter the surface charged state (zeta potential) of MXenes. For instance, the aminosilane with positively charged amino group was demonstrated to have a critical role on the surface charge of aminosilane-Ti3C2Tx hybrids [12][13][33,34]. As reported by Hossein et al. the [3-(2-aminoethylamino)propyl]-trimethoxy silane (AEAPTMS)-Ti3C2Tx hybrids had a positive zeta potential of +62 mV at pH = 2.58, which is the highest zeta potential reported for MXenes to date. Similar to silane, the alkylphosphoric acid can also form covalent bonds with MXene through dehydration condensation reaction. Kim et al. demonstrated a simultaneous interfacial chemical grafting and phase transfer method for alkylphosphoric acid (CnPA, n = 3, 6, 8, 10, 12) grafting on the Ti3C2Tx flakes [14][35]. During the reaction, Ti–O–P bonds formed by interfacial nucleophilic addition and sequential condensation reaction between hydroxyl groups from Ti3C2Tx and phosphate group from CnPA. The pH and concentration of CnPA are found to influence the interfacial chemical reaction and phase transfer. Whereas the dispersion stability of the Ti3C2Tx-CnPA hybrids in nonpolar organic medium can be attributed to the steric stabilization and strong nonpolar interaction of long alkyl chains. Very recently, Sun et al. proposed new evidence on the existence of hydrogen bonds between Ti3C2Tx nanosheets and C8PA [15][36]. Therefore, it seems that the surface interaction between MXenes and coupling agents may be more complicated than we think.
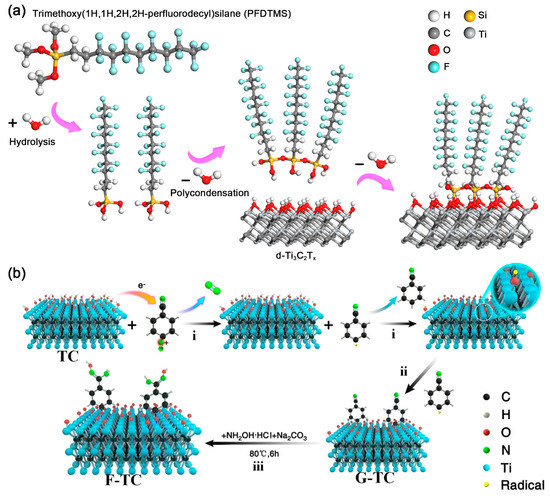
Figure 2. (a) Surface modification process of d-Ti3C2Tx nanosheets by PFDTMS; Reproduced with permission [8][29]. Copyright 2018, Royal Society of Chemistry. (b) Proposed mechanism of amidoxime functionalization for MXene Ti3C2Tx; Reproduced with permission [16][38]. Copyright 2020, American Chemical Society.
Diazonium salt is another commonly used reagent to covalently connect organic functional groups on MXenes. In 2020, Zhang et al. first proposed a detailed reaction mechanism between aryl diazonium salts and Ti3C2Tx MXene, in which the aromatic primary amine can undergo a diazotization reaction and the produced diazonium salts will covalently bond with MXenes. As shown in Figure 2b, the detailed reaction mechanism can be divided into three steps [17][16][37,38]: (1) an electron was transferred from Ti3C2Tx MXene to aryl diazonium salt, which resulted in the cleavage of diazonium to nitrogen and aromatic free radical; (2) the aromatic free radical received an additional H atom from Ti3C2Tx MXene, generating a Ti–O radical on the surface of MXene; (3) strong Ti–O–C covalent bonds formed between Ti–O radical and aromatic free radical. In fact, sulfanilic acid diazonium salts were introduced to get large-scale delaminated Ti3C2Tx multilayers as early as 2016 [18][19][20][39,40,41]. Compared with the pristine Ti3C2Tx, the phenylsulfonic acid grafted Ti3C2Tx MXene becomes amphiphilic material, which shows an increased solubility and excellent stability in water for more than one month. However, in consideration of the potential aromatic π interaction and the oxygen from the sulfonic group, the works did not give a detailed mechanism and solid evidence on the formation of MXene-aryl (Ti–O–C) linkages. Recently, Muhammad et al. compared the noncovalent and covalent interaction mechanism of 1-aminoanthraquinone (AQ) on Ti3C2Tx MXene (denoted as AQ@Ti3C2Tx and AQ-Ti3C2Tx, respectively) [21][42]. It is found that after the diazonium reaction, the amino group disappeared, and the covalent bond formed between AQ and Ti3C2Tx. As observed, the covalently attached AQ on Ti3C2Tx MXene offered better charge transportation, thus electrochemical performance. According to the abovementioned works, the diazonium reaction can be a useful way for the covalent connection of organic molecules onto the MXene nanosheets and further expands the applications of MXene-organic hybrids.
Taking advantage of the electrical conductivity and layered structure of MXenes, the MXene-polymer hybrids usually show improved properties when compared with the pure polymer, such as mechanical properties [22][23][24][25][43,44,45,46], flame retardancy [26][27][47,48], and responsiveness [11][28][32,49]. In early 2015, an MXene-organic hybrid with CO2 and temperature dual-responsive property was reported by Chen et al. [28][49]. The hybrid consisted of V2CTx MXene and self-initiated photo grafting and photopolymerized (SIPGP) poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) brushes. The hydroxyl groups on V2CTx MXene were acting as the photoactive sites, which allowed further growth of polymer brushes via SIPGP. When pre-modified MXene by SBDC [11][32], PDMAEMA brushes can also be connected onto the MXene surface. Zhang et al. [29] [50] utilized in situ free radical polymerization to prepare Ti3C2Tx/polyacrylamide (PAA) hybrid with acrylamide monomer and potassium persulfate as redox initiator, in which the Ti3C2Tx MXene was introduced as the crosslinker.
3. MXene-Organic Hybrids through Electrostatic Interaction
In addition to utilizing T groups of MXenes to covalently graft organic molecules by chemical reactions, the electrostatic interactions on negatively charged MXenes surface can also be utilized. Cui et al. synthesized the (3-aminopropyl) triethoxylsilane (APTES) grafted Si nanoparticles first [30][51], in which the covalently X groups on silane were consumed. However, by directly using the positively charged Y group (here, amino), the grafted Si nanoparticles were still self-assembled with Ti3C2Tx through electrostatic interaction. Xu et al. reported microcontact printing to get ultrathin MXene micropatterns [31][52]. The MXene ink was applied to a polydimethylsiloxane (PDMS) film with patterns of alternating grooves. Subsequently, transfer printing it onto APTES-modified coverslips. In contrast, Ti3C2Tx adsorbed onto coverslips by electrostatic interaction with amino groups.
As another kind of common-used organic molecules, alkylammonium salts including dodecyl trimethyl ammonium bromide (DTAB), tetradecyltrimethylammonium bromide (TTAB), cetyltrimethylammonium bromide (CTAB), stearyl trimethyl ammonium bromide (STAB), octadecyl trimethyl ammonium bromide (OTAB), dioctadecyl dimethyl ammonium chloride (DDAC), dihexadecyl dimethyl ammonium bromide (DDAB), and DHT are usually used to insert in MXene layers through electrostatic adsorption. After pillared by alkylammonium, the Ti3C2Tx MXene presented a dramatic increase in interlayer spacing [32][33][53,54]. Obviously, the interlayer spacing increased along with the increasing length of alkyl chains [34][55]. Bian et al. proposed that the positively charged ammonium group might mainly be anchored on the surface –O groups from Ti3C2Tx MXene [35][56], and the intercalation of alkylammonium can also affect the wettability of MXene (Figure 3a). Maybe because of the competitive adsorption of cations, the CTA cation pillared Ti3C2Tx MXene can only form a stable oil-in-water emulsion in neutral or basic condition. While in acidic conditions, the MXene will aggregate at the oil/water interface. In a current study reported by Michael et al., the DHT not only improved the organophilic nature of Ti3C2Tx, produced stable colloidal suspensions of Ti3C2Tx in nonpolar solvents but also shielded the MXene flakes from oxidation in water [6][27].
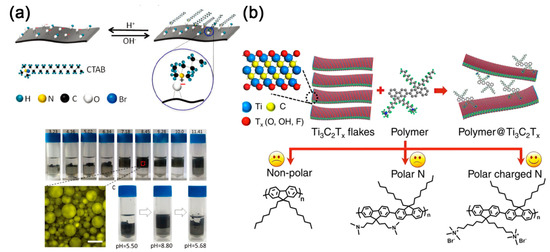
Figure 3. (a) Top: a possible scheme describing the interaction between cetyltrimethylammonium bromide (CTAB) and Ti3C2Tx MXene. Bottom: the emulsion stability as a function of the pH of the aqueous phase at a fixed weight ratio of CTAB to MXene; reproduced with permission [35][56]. Copyright 2018, Royal Society of Chemistry. (b) Schematic illustration of the interaction of the polymer with Ti3C2Tx layers (top) and the synthesized polymers with nonpolar, polar, and polar charged nitrogen lateral chain ends (bottom); Reproduced with permission [36][57]. Copyright 2017, American Chemical Society.
Amino acids are also studied as the organic part towards MXene-organic hybrids. Elumalai et al. proposed that the electrostatic interaction between –OH and –F groups on Ti3C2Tx MXene and the zwitterion consisted of amino acid molecules might be the driving force for the spontaneous intercalation [37][58]. The MXene hybrids contained negatively charged glycine (Gly), phenylalanine (Phe), tryptophan (Trp), and histidine (His) can form stable suspensions in water due to the interparticle electrostatic repulsion. Whereas aromatic (Phe, Trp, His) intercalation may also produce rutile TiO2@Ti3C2Tx by sonication. On the contrary, combined with first principle calculations (DFT), recently, Chen et al. studied the interaction between Gly and double-layer structure Ti3C2O2 [38][59]. From their analysis, the Ti and N atoms from Ti3C2O2 and Gly, respectively, were likely to share electrons and lead to the formation of Ti–N bonding. Obviously, the ionizable organic molecules are another good choice for preparing MXene-organic hybrids with controllable properties. However, the unclear interaction mechanism makes it an urgent task to be solved.
Carey and his coworkers prepared the 12-aminolauric acid (ALA), or DHT-modified Ti3C2Tx first (denoted as ALA-Ti3C2Tx and DHT-Ti3C2Tx) [39][40][60,61]. After the modification, MXene changed from hydrophilic to organophilic, so the intercalation of the ε-caprolactam monomer and 6-aminocaproic acid catalyst was easier [39][60]. Additionally, uniform Ti3C2Tx MXene/epoxy hybrids can be synthesized by ALA-Ti3C2Tx and DHT-Ti3C2Tx. The authors proposed that ALA and DHT can speed up the curing reaction of epoxy in the interlayer space, which further enlarged the interlayer space of the MXene layers [61].
Similarly, the aniline monomers are believed to electrostatically adsorb on and between the multilayer MXene nanosheets [41][62]. Fu et al. reported a graphene-encapsulated Ti2CTx@polyaniline (PANI) hybrid named GMP for supercapacitors [42][63]. The adsorbed aniline monomers can be chemical oxidative polymerized with ammonium persulfate as the oxidant to form Ti2CTx@PANI hybrid (Figure 4). For graphene encapsulation, CTAB was utilized to lower the surface energy and change the zeta potential of Ti2CTx@PANI. Recently, there is also evidence that the surface functional groups on MXenes will facilitate nucleation during the PANI polymerization [43][64].
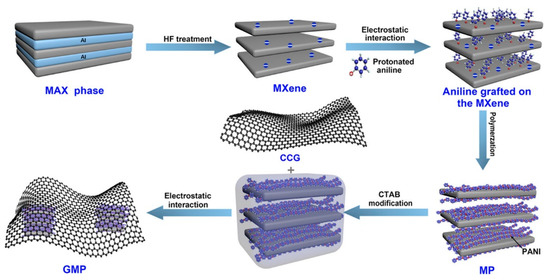
Figure 4. Scheme of the synthesis of graphene-encapsulated Ti2CTx@PANI hybrid (GMP); Reproduced with permission [42][63]. Copyright 2018, American Chemical Society.
When turning to the other common-used conducting polymer, poly(3,4-ethylene dioxythiophene) (PEDOT), Gogotsi and his co-workers revealed the mechanism of charge-transfer-induced polymerization of EDOT on the surface of Ti3C2Tx MXene [44][65]. Verified by the theoretical calculations, the parallel orientation of EDOT monomer on Ti3C2Tx surface presented the most stable configuration with the lowest binding energy of −1.02 eV. After adsorption, 0.34 electrons were transferred from EDOT to Ti3C2O2, which initiated the in situ polymerization of EDOT on Ti3C2O2. With the energy supplement via electrochemical reaction, Qin et al. demonstrated a one-step in situ electrochemical polymerization for Mo1.33C/PEDOT and Ti3C2Tx/PEDOT films with controlled thickness and micropattern [45][66]. However, due to the insoluble nature of PEDOT, the commercial product usually contains a mixture of poly(styrene sulfonate) (PSS). In consideration of the bicomponent structure and the ionic chain nature, the ex-suit blending method was more popular than in-suit polymerization towards MXene-PEDOT:PSS hybrids and the electrostatic interaction between PSS and MXene promoted the formation of 3D interconnected and reticulated structure. The hydrogen from –SO3H on PEDOT:PSS with –OH,–F on Ti3C2Tx and the electrostatic interaction between PSS and MXene were also found to jointly shield the coulombic attraction between PEDOT and PSS, which enhanced the inter-chain charge transport between PEDOT chains [46][67]. The Ti3C2Tx/(PEDOT:PSS) film can be fabricated by drop-casting [47][68], vacuum-assisted filtration [48][69], and simply filtration [49][50][70,71]. Recently, MXene/(PEDOT:PSS) hybrid was also fabricated into a fiber-shaped supercapacitor by a one-step wet-spinning approach [51][72].
By the ex-situ blending method, plenty of MXene/polymers hydrides can be prepared. Firstly reported by Gogotsi et al., charged poly-diallyl dimethyl ammonium chloride (PDDA) and electrically neutral polyvinyl alcohol (PVA) were blended with Ti3C2Tx by vacuum-assisted filtration [52][73]. Subsequently, the dispersibility of MXene sheets in the PVA matrix and the dielectric performance of MXene/PVA hybrids in X-band frequency was studied [53][74]. Boota et al. synthesized polyfluorene (PFO) derivatives containing nonpolar, polar nitrogen-containing, and charged nitrogen-containing lateral chains by Suzuki polycondensation reaction (Figure 3b) [36][57]. They have pointed out that both the electrostatic interactions and hydrogen bonds may be involved between MXene and PFO derivatives. However, the bulky methyl groups from polar polymers with charged nitrogen-containing ends prohibited the formation of the hydrogen bond. A physical vapor deposition technique, resonant infrared matrix-assisted pulsed laser evaporation (RIR-MAPLE), was performed by Ajnsztajn et al. to produce Ti3C2Tx/PFO transparent hybrid electrode [54][75]. Via RIR-MAPLE, the film exhibited the minimal phase segregation.
4. MXene-Organic Hybrids through Hydrogen Bonds and Other Supermolecular Interactions
Except for the strong inorganic-organic correlation provided by covalent and electrostatic interaction, the supermolecular interactions are also critical to the formation of MXene-organic hybrids. In 2016, a Ti3C2Tx/polypyrrole (PPy) hybrid with highly aligned PPy chains in between the Ti3C2Tx layers was prepared by unconventional oxidant-free polymerization (Figure 5a) [55][76]. Specifically, the key factor in the alignment process was the hydrogen bond, which might originate from the N–H group of the pyrrole ring and terminating oxygen or fluorine on the Ti3C2Tx surface. What is more, the fluorine on Ti3C2Tx will be doped in PPy chains, which can further enhance their electrochemical activity. Different from the oxidant-free chemical polymerization, Zhu et al. [56] [77] fabricated Ti3C2Tx/PPy hybrid film via electrophoretic deposition and electrochemical polymerization. The pronounced acidic character of the Ti3C2Tx surface generated hydrogen bonding between Ti3C2Tx and PPy during electrochemical polymerization. However, for the most traditional pyrrole polymerization [57] [78], FeCl3·6H2O was applied as an oxidant, and the strong oxidizing Fe3+ ions will result in the oxidation of Ti3C2Tx. Therefore, Zhang et al. only found the 3D TiO2@NC/Fe7S8 hybrids by in situ polymerization of pyrrole monomer with alkalized Ti3C2Tx.
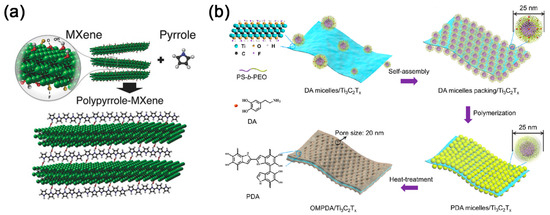
Figure 5. (a) Schematic illustration of pyrrole polymerization using MXene; reproduced with permission [55][76]. Copyright 2016, WILEY-VCH. (b) Schematic drawing depicting the preparation steps of OMPDA/Ti3C2Tx hybrid: (i) mixing the DAmi with Ti3C2Tx nanosheets, (ii) packing of DAmi and subsequent, (iii) direct polymerization of polydopamine (PDA) micelles on the surface of Ti3C2Tx nanosheets, and (iv) obtaining the OMPDA/Ti3C2Tx after heat treatment; Reproduced with permission [58][81]. Copyright 2020, American Chemical Society.
As confirmed by previous studies, quinones are important redox-active centers for the chemical reaction [59][79]. However, there are only a limited amount of quinones reserved in polydopamine (PDA), which is positively correlated to the oxidation state of PDA [60][80]. Interestingly, Ti3C2Tx/PDA hybrids were found to be an excellent precursor for o-benzoquinone after low-temperature heat treatment at 300 °C [59] [79]. After that, Li et al. further prepared vertically oriented ordered mesopores (OM) PDA/Ti3C2Tx hybrid (OMPDA/Ti3C2Tx) by using the PS-b-PEO block polymer as a soft-template (Figure 5b) [58][81]. In their report, the hydrogen bond also played a crucial role in the assembly of dopamine/PS-b-PEO micelles and Ti3C2Tx. Recently, Du and coworkers utilized Ti3C2Tx@PDA prepared by in situ polymerization as starting materials to hybrid with poly(ethylene glycol) (PEG)-based polyurethane (PU) [61][82]. The PDA is found to be a medium molecule that facilitated the interfacial compatibility of Ti3C2Tx flakes in PEG-based PU, where the Ti3C2Tx@PDA covalently bonded with PU during the process of copolymerization.
Beyond anchoring the organic species on the MXene surface, the hydrogen bond also functions as a flexible junction between the inorganic MXene and organic part. Hybrid consisting of Ti3C2Tx and polyacrylamide (PAM) was fabricated by Niu et al. [29][50]. In 2020, the exfoliated Ti3C2Tx nanosheets were reported for the first time that acted as a crosslinker instead of traditional organic molecules. In the hybrid, hydrogen bonding between the –CONH2 groups from PAM chains and the hydrophilic groups (–OH and –F) from the Ti3C2Tx nanosheets were observed. Zhang et al. simply prepared MXene hydrogel hybrids by mixing Ti3C2Tx MXene and commercial polyvinyl alcohol (PVA) hydrogel [62][83]. They indicated the secondary crosslinking between MXene and PVA and a dense network yielded by polymer chain entanglements. Xuan et al. improved this method by added MXene flakes into a homogeneous PVA solution [63][84], with the borate solution acting as the crosslinking agent, MXene bonding with PVA chains, and tetra-functional borate ion covalently. A similar interaction was also found in hydrophobically associated dry polyacrylamide (HAPAM) hydrogel [64][85]. In HAPAM hydrogel, hydrogen bonds between MXene and amide group of HAPAM became additional crosslinking points, which contributed to the hybrid double-network with N,N’-methylene diacryl amide (MBAA) crosslinker and affected the self-healing ability and temperature sensibility. When there were two or more different kinds of monomers or polymers together with MXene nanosheets, MXene-copolymeric hydrogels can also be prepared [65][66][67][86,87,88].
On the aspect of ex situ blending, Le et al. intertwined Ti3C2Tx MXene particles in tangled PPy nanowires through hydrogen bond [68][89]. The intercalated PPy chains between the conductive Ti3C2Tx sheets prevented the dense stacking of Ti3C2Tx. Meanwhile, the intercalation further improved the structural stability of PPy backbones. Moreover, Ti3C2Tx MXene and PANI hybrid was also prepared by mechanical blending [69][90]. The lightweight Ti3C2Tx/PANI hybrids for EMI shielding was obtained by simply mixing PANI powder with MXene powder. The authors suggested that an equal concentration of both materials would be beneficial for enhanced interfacial interaction between them.
Except for hydrogen, the polymer chain entanglements, ionic interactions, covalent bonding were also reported, and the role of MXene sheets in hydrogel varies [65][86]. MXene polymer hydrogel was gradually developed with improved performance. The typical interactions between MXene and organic modified species are summarized in Table 1.
Table 1. Summary of the interactions between MXene and organic species.
| Interaction Type | Typical Modifier | Binding Forms | |||
|---|---|---|---|---|---|
| Covalent interaction | Silane | a | Si–O–M | ||
| Diazonium salts | C–O–M | ||||
| Alkyl phosphoric acid | P–O–M | ||||
| Electrostatic interaction | Alkyl ammonium salts | b | R | 4 | N⊕ ⊝T–M |
| Amino acids | –H | 3 | N⊕ ⊝T–M | ||
| PFO | R | 4 | N⊕ ⊝T–M | ||
| PANI | –H | 3 | N⊕ ⊝T–M | ||
| PEDOT:PSS | SO | 3 | H⊕ ⊝T–M | ||
| Hydrogen bonds | Pyrrole/PPy | NH···O–M | |||
| Dopamine/PDA | OH···O–M | ||||
| PAM | CONH | 2 | ···O–M | ||
| PVA | OH···O–M | ||||
| PSS | SO | 3 | H···O–M |
a M represents a metal atom from the surface layer of MXenes. b R represents any alkyl group.
