Infectious and parasitic agents have been frequently associated with debilitating and wasting conditions in sheep. The prevalence of these agents has probably undermined the role of toxic causes as contributors to such disorders. In addition, many of these intoxications frequently produce acute clinical disease with specific and characteristic lesions, thus a causal relationship with the toxic substance may be relatively easy to establish. However, persistent exposure to some of these organic or inorganic toxic substances may lead to emaciation, ill-thrift, and poor external aspect. The anti-nutritional factors and alkaloids of several plants, including pyrrolizidine alkaloids, among others, have also been associated with emaciation and/or poor general performance in sheep flocks.
- sheep
- intoxications
- poisonous plants
- wasting
1. Introduction
There are several diseases of sheep that may cause wasting, leading to decreased productivity and economic losses in sheep flocks as a result. Johne’s disease, maedi-visna, caseous lymphadenitis, and several parasitic diseases, amongst others, have been traditionally included under the umbrella of the so-called “thin ewe syndrome”, a concept that encompasses several diseases with wasting as the main clinical sign [1][2][3][4]. Hence, this concept has included mostly infectious and parasitic disorders, probably underestimating the role of other disease causes.
There are several diseases of sheep that may cause wasting, leading to decreased productivity and economic losses in sheep flocks as a result. Johne’s disease, maedi-visna, caseous lymphadenitis, and several parasitic diseases, amongst others, have been traditionally included under the umbrella of the so-called “thin ewe syndrome”, a concept that encompasses several diseases with wasting as the main clinical sign [1,2,3,4]. Hence, this concept has included mostly infectious and parasitic disorders, probably underestimating the role of other disease causes.
Commercial diets must be correctly formulated to prevent either toxicity or deficiencies due to excess or lack of certain mineral components, respectively [5]. There are also pernicious chemical and biological products that cause acute toxicity in the short-term, but exposure to some of them over longer periods of time may also contribute to wasting and poor general performance [6]. Certain plants, including grasses, legumes, and shrubs, contain anti-nutritional and toxic compounds that, depending on the situation, may cause deleterious effects on animals that consume them [7][8]. Some of these substances are non-lethal, but their consumption during long periods of time, especially with concomitant confinement and/or food scarcity, may lead to emaciation as well.
Commercial diets must be correctly formulated to prevent either toxicity or deficiencies due to excess or lack of certain mineral components, respectively [5]. There are also pernicious chemical and biological products that cause acute toxicity in the short-term, but exposure to some of them over longer periods of time may also contribute to wasting and poor general performance [6]. Certain plants, including grasses, legumes, and shrubs, contain anti-nutritional and toxic compounds that, depending on the situation, may cause deleterious effects on animals that consume them [7,8]. Some of these substances are non-lethal, but their consumption during long periods of time, especially with concomitant confinement and/or food scarcity, may lead to emaciation as well.
2. Inorganic and Organic Toxic Compounds
2.1. Copper
Sheep are very limited in their ability to excrete copper (Cu). Indeed, sheep tend to accumulate Cu in the liver along time, thus being extraordinarily susceptible to chronic Cu intoxication [9][10]. This can occur mainly in three forms: (i) Excessive consumption due to contamination of drinking water and/or food with Cu-containing compounds (e.g., Cu sulfate); (ii) Low levels of dietary molybdenum (Mb), which increase the rate of Cu absorption in the gut, since Mb tends to combine with sulfate and Cu, making the latter insoluble; (iii) Concomitant exposure to hepatic toxins, such as plants containing pyrrolizidine alkaloid (PA) [9]. Interestingly, chronic Cu poisoning in sheep is a long-term intoxication with an acute clinical presentation [10].
Sheep are very limited in their ability to excrete copper (Cu). Indeed, sheep tend to accumulate Cu in the liver along time, thus being extraordinarily susceptible to chronic Cu intoxication [9,10]. This can occur mainly in three forms: (i) Excessive consumption due to contamination of drinking water and/or food with Cu-containing compounds (e.g., Cu sulfate); (ii) Low levels of dietary molybdenum (Mb), which increase the rate of Cu absorption in the gut, since Mb tends to combine with sulfate and Cu, making the latter insoluble; (iii) Concomitant exposure to hepatic toxins, such as plants containing pyrrolizidine alkaloid (PA) [9]. Interestingly, chronic Cu poisoning in sheep is a long-term intoxication with an acute clinical presentation [10].
Cu accumulates in hepatocyte lysosomes and is actively incorporated and stored in new hepatocytes when others die and release it. However, there is a threshold level at which the organ cannot cope with this turnover rate and significant hepatocellular necrosis ensues. Subsequently, plasma Cu levels increase, causing a sudden crisis of intravascular hemolysis, anemia, and death. Sheep may show no clinical abnormalities before the hemolytic crisis occurs, although in certain cases there could be an elevation of hepatic enzymes such as plasma aspartate transaminase (AST) and gamma-glutamyl transferase (GGT), with anorexia and ill-thrift prior to this crisis [6][10]. Weight loss and hypoxaemia were detected in all sheep involved in an experimental Cu intoxication two weeks before the hemolytic crisis started [11].
Cu accumulates in hepatocyte lysosomes and is actively incorporated and stored in new hepatocytes when others die and release it. However, there is a threshold level at which the organ cannot cope with this turnover rate and significant hepatocellular necrosis ensues. Subsequently, plasma Cu levels increase, causing a sudden crisis of intravascular hemolysis, anemia, and death. Sheep may show no clinical abnormalities before the hemolytic crisis occurs, although in certain cases there could be an elevation of hepatic enzymes such as plasma aspartate transaminase (AST) and gamma-glutamyl transferase (GGT), with anorexia and ill-thrift prior to this crisis [6,10]. Weight loss and hypoxaemia were detected in all sheep involved in an experimental Cu intoxication two weeks before the hemolytic crisis started [11].
Lesions are very characteristic once the hemolytic crisis occurs, and include widespread icterus, yellow to orange and friable liver, dark, “gunmetal-colored” kidneys, and dark red urine. Histologically, there is hepatic centrilobular necrosis and renal tubular necrosis with hemoglobin casts in the tubular lumina [9]. It is important to identify animals in which the hemolytic crisis has not started, since once this occurs the treatment has very few chances of being successful [6]. Sheep with high blood Cu may be treated with intravenous ammonium tetramolybdate to decrease the Cu:Mb ratio. Furthermore, including Mb salts to the concentrate ration may help to prevent new cases and decrease mortality in the flock [6][10][12]. Recently, it has been proposed that dietary zinc (Zn) supplementation at 300 mg/kg dry matter may prevent accumulation of Cu in the liver of sheep exposed to high concentrations of this element [11].
Lesions are very characteristic once the hemolytic crisis occurs, and include widespread icterus, yellow to orange and friable liver, dark, “gunmetal-colored” kidneys, and dark red urine. Histologically, there is hepatic centrilobular necrosis and renal tubular necrosis with hemoglobin casts in the tubular lumina [9]. It is important to identify animals in which the hemolytic crisis has not started, since once this occurs the treatment has very few chances of being successful [6]. Sheep with high blood Cu may be treated with intravenous ammonium tetramolybdate to decrease the Cu:Mb ratio. Furthermore, including Mb salts to the concentrate ration may help to prevent new cases and decrease mortality in the flock [6,10,12]. Recently, it has been proposed that dietary zinc (Zn) supplementation at 300 mg/kg dry matter may prevent accumulation of Cu in the liver of sheep exposed to high concentrations of this element [11].
2.2. Nitrogen-Containing Compounds
2.2.1. Nonprotein Nitrogen Sources
These substances encompass all non-protein sources of nitrogen (N), such as urea, from which sheep are able to synthetize proteins. Therefore, ovine rations are occasionally supplemented with urea and other similar substances to take advantage of this process [13]. Normally, the ruminal microbiota converts these N sources into ammonia, which is then used to produce amino acids and proteins. However, if the ingested amount of non-protein N exceeds the metabolic capacity of the rumen to produce proteins, these substances persist in the ruminal fluid and are turned into excessive ammonium, which increases the ruminal pH above 8. Free ammonia is also absorbed, causing hyperammonemia and toxicity. In most cases, this is an acute process related to the sudden incorporation of the N source into the diet. Excessive salivation, tremors, ataxia, and bloating are described. Subacute or chronic clinical signs are rare, and may range from mild nervous signs to lethargy, anorexia, and poor external aspect. Advanced emaciation is uncommonly observed with urea poisoning, but periods of fasting and malnutrition associated with other concomitant processes may predispose to urea intoxication [14][15].
These substances encompass all non-protein sources of nitrogen (N), such as urea, from which sheep are able to synthetize proteins. Therefore, ovine rations are occasionally supplemented with urea and other similar substances to take advantage of this process [13]. Normally, the ruminal microbiota converts these N sources into ammonia, which is then used to produce amino acids and proteins. However, if the ingested amount of non-protein N exceeds the metabolic capacity of the rumen to produce proteins, these substances persist in the ruminal fluid and are turned into excessive ammonium, which increases the ruminal pH above 8. Free ammonia is also absorbed, causing hyperammonemia and toxicity. In most cases, this is an acute process related to the sudden incorporation of the N source into the diet. Excessive salivation, tremors, ataxia, and bloating are described. Subacute or chronic clinical signs are rare, and may range from mild nervous signs to lethargy, anorexia, and poor external aspect. Advanced emaciation is uncommonly observed with urea poisoning, but periods of fasting and malnutrition associated with other concomitant processes may predispose to urea intoxication [14,15].
There are no diagnostically relevant gross or histologic findings, but the demonstration of elevated ruminal pH (8 or above) and high ammonia concentration in serum and/or aqueous humor supports a diagnosis [13]. Treatment strategies are focused on reducing the ruminal pH. Vinegar, given orally or instilled directly into the rumen by gastric tubing, has been able to successfully control some clinical cases [6].
2.2.2. Nitrates and Nitrites
Sheep may be exposed to nitrates by consuming nitrate accumulating plants, decaying organic matter, and/or certain fertilizers [6]. Recently, nitrate supplementation has been proposed as an effective method to reduce methane emissions by ruminants [16]. Some examples of nitrate accumulating plants and their effects are described later in this paper (see
Section 3.4 Nitrate-Accumulating Plants). Nitrate-containing fertilizers may contaminate drinking water, which becomes one of the main sources of exposure for animals. Ruminal microbiota transforms nitrates into the more toxic nitrites, which are absorbed, inducing the formation of methemoglobin in blood and a subsequent decrease of tissue oxygenation. The clinical course is most commonly acute, with rapid pulse, cyanosis, and weakness. Exposure to sublethal doses of nitrates over time may lead to weight loss and reproductive problems, thus contributing to general ill-thrift and poor performance [17].
Nitrate-Accumulating Plants). Nitrate-containing fertilizers may contaminate drinking water, which becomes one of the main sources of exposure for animals. Ruminal microbiota transforms nitrates into the more toxic nitrites, which are absorbed, inducing the formation of methemoglobin in blood and a subsequent decrease of tissue oxygenation. The clinical course is most commonly acute, with rapid pulse, cyanosis, and weakness. Exposure to sublethal doses of nitrates over time may lead to weight loss and reproductive problems, thus contributing to general ill-thrift and poor performance [17].
Necropsy findings are suggestive of nitrate intoxication if the carcass is fresh enough. A brownish discoloration in muscles, lungs, and/or brain, with brown, dense blood (i.e., “chocolate-like”) may be found. These changes are due to methemoglobinemia, and since methemoglobin rapidly turns into hemoglobin, they tend to disappear shortly after death [18]. Elevated serum, plasma, and/or ocular fluid nitrate concentrations are often diagnostic. Ocular fluid may be analyzed by qualitative nitrate strips as a screening procedure, followed by a quantitate confirmatory test in positive cases [18]. Treatment is focused on reducing methemoglobin to hemoglobin. Intravenous injection of an aqueous solution of methylene blue has proven to be effective for this purpose [6].
2.3. Fluoride/Fluorine
Fluoride (F) is the monovalent anion of fluorine and the form that commonly occurs in nature as part of different minerals [19]. Livestock may be chronically exposed by grazing on pastures close to industrial sites with F emissions, such as aluminum factories, or in active volcanic areas [20]. The condition is better described in cattle, but sheep might develop a similar syndrome [21]. F tends to accumulate in bones and teeth of livestock, and thus exostosis and malformations may appear in long bones and mandible, whereas teeth develop wearing and discoloration. These lesions may lead to loss of condition due to stiffness, lameness, and apprehension and chewing problems due to poor dentition. Botha et al. [22] described an outbreak of fluorosis that affected sheep and cattle. Sheep developed pitting of the enamel and abnormally elevated plasma levels of urea and alkaline phosphatase. Icelandic sheep experimentally poisoned with F for 20 weeks did not develop gross or microscopic lesions in bones or teeth, although loss of appetite and recumbency were seen [23]. Poor skin and hair coat have also been associated with chronic F exposure [19]. Elevated urine and/or plasma F concentrations together with compatible lesions support a diagnosis of fluorosis. The cornerstone of the therapeutic approach is removing the animals from the contaminated pastures. In addition, calcium or aluminum salts may be added to the concentrate to reduce F solubility and prevent its accumulation [6].
3. Toxic Plants
3.1. Pyrrolizidine Alkaloid-Containing Plants
PA-containing plants are among the most common causes of plant poisoning in livestock. More than 350 PA have been identified in more than 6000 forage species with predominance in three families: Compositae (e.g.,
Senecio
spp.), Leguminosae (e.g.,
Crotalaria
spp.), and Boraginaceae (e.g.,
Heliotropium
spp.,
Cynoglossum
spp.,
Amsinckia
spp.,
Echium
spp.,
Trichodesma
spp., and
The plants most often involved in PA toxicosis in sheep are
Heliotropium europaeum
and
Echium plantagineum [9]. In some regions of Australia, these two plants are considered the main cause of death in sheep due to plant poisoning [24][25][26], while in Brazil the most important PA-containing plants are
[9]. In some regions of Australia, these two plants are considered the main cause of death in sheep due to plant poisoning [50,51,52], while in Brazil the most important PA-containing plants are
Crotalaria retusa
(
A) and
Senecio brasiliensis [27][28][29]. Other PA-containing plants associated with loss of condition in sheep in a variety of geographical locations include
[53,54,55]. Other PA-containing plants associated with loss of condition in sheep in a variety of geographical locations include
Amsinckia intermedia [30],
[56],
Crotalaria mucronata [31],
[57],
Senecio cineraria [32],
[58],
Senecio sanguisorbae [33],
[59],
Senecio madagascariensis [25],
[51],
Senecio erraticus [36],
[62],
Heliotropium ovalifolium [37],
[63],
Heliotropium amplexicaule [38],
[64],
Heliotropium dasycarpum [39], and
[65], and
Echium plantagineum [24][40][41].
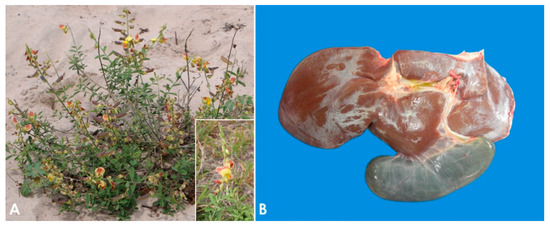
Figure 1.
A
Crotalaria retusa
B
PA-containing plants are non-palatable, and they only become a problem for livestock if good forage sources are unavailable and/or if they contaminate the harvested hay or other foodstuffs [8]. Furthermore, sheep are considered markedly resistant to PA poisoning because their ruminal flora is able to detoxify these compounds, and glutathione conjugation in the liver is very efficient [9][42]. For this reason, sheep grazing has been traditionally proposed as a method of biological control for several invasive species of PA-containing plants [43][44][45][46]. However, if the use of sheep with this purpose is indiscriminate, poisoning may ensue [40][41].
PA-containing plants are non-palatable, and they only become a problem for livestock if good forage sources are unavailable and/or if they contaminate the harvested hay or other foodstuffs [8]. Furthermore, sheep are considered markedly resistant to PA poisoning because their ruminal flora is able to detoxify these compounds, and glutathione conjugation in the liver is very efficient [9,68]. For this reason, sheep grazing has been traditionally proposed as a method of biological control for several invasive species of PA-containing plants [69,70,71,72]. However, if the use of sheep with this purpose is indiscriminate, poisoning may ensue [66,67].
The clinical manifestations of sheep grazing PA-containing plants are variable. While some animals may present loss of appetite, pale mucous membranes, gradual loss of condition, apathy, emaciation, and death; others can live without obvious clinical signs and die suddenly [8][39]. Jaundice and varying degrees of photodermatitis characterized by crusty lesions in the ears and nose can also be observed [29][39], and stressful events such as lambing or shearing could precipitate sudden death. Clinico-pathologic changes include anemia, transient elevations in the activity of serum AST, sorbitol dehydrogenase, alkaline phosphatase, and GGT, as well as increased concentrations of serum bilirubin, bile acids, and copper [9][29][39][47].
The clinical manifestations of sheep grazing PA-containing plants are variable. While some animals may present loss of appetite, pale mucous membranes, gradual loss of condition, apathy, emaciation, and death; others can live without obvious clinical signs and die suddenly [8,65]. Jaundice and varying degrees of photodermatitis characterized by crusty lesions in the ears and nose can also be observed [55,65], and stressful events such as lambing or shearing could precipitate sudden death. Clinico-pathologic changes include anemia, transient elevations in the activity of serum AST, sorbitol dehydrogenase, alkaline phosphatase, and GGT, as well as increased concentrations of serum bilirubin, bile acids, and copper [9,55,65,73].
The pathogenesis of poisoning by PA correlates with hepatic dehydration of highly reactive dehydropyrrolizidine alkaloids, which are powerful alkylating agents that react with cellular proteins and cross-link DNA, resulting in cellular dysfunction, abnormal mitosis, and tissue necrosis [9][39][48]. Ingestion of high doses of PA during a short period of time leads to acute poisoning [49]. Emaciation is generally associated with prolonged exposure, which induces two morphologically different liver patterns: 1. Phasic pattern (usually seasonal and the most common in field cases) associated with repetitive exposure to PA, which leads to hepatic atrophy with formation of regenerative nodules; multifocal, tan, 2–5 mm in diameter nodules and a distended gallbladder can be observed in necropsy. 2. Chronic pattern, which occurs with prolonged exposure to low doses of PA, and it is characterized by a firm, fibrotic and atrophic liver without nodular regeneration; the liver could look grossly normal or slightly smaller than usual, with a mild gray-yellowish discoloration due to thickening of Glisson’s capsule, and increased firmness due to parenchymal fibrosis (
The pathogenesis of poisoning by PA correlates with hepatic dehydration of highly reactive dehydropyrrolizidine alkaloids, which are powerful alkylating agents that react with cellular proteins and cross-link DNA, resulting in cellular dysfunction, abnormal mitosis, and tissue necrosis [9,65,74]. Ingestion of high doses of PA during a short period of time leads to acute poisoning [75]. Emaciation is generally associated with prolonged exposure, which induces two morphologically different liver patterns: 1. Phasic pattern (usually seasonal and the most common in field cases) associated with repetitive exposure to PA, which leads to hepatic atrophy with formation of regenerative nodules; multifocal, tan, 2–5 mm in diameter nodules and a distended gallbladder can be observed in necropsy. 2. Chronic pattern, which occurs with prolonged exposure to low doses of PA, and it is characterized by a firm, fibrotic and atrophic liver without nodular regeneration; the liver could look grossly normal or slightly smaller than usual, with a mild gray-yellowish discoloration due to thickening of Glisson’s capsule, and increased firmness due to parenchymal fibrosis (
Figure 1B). Chronic exposure may also induce lipidosis, ketosis, or promote secondary hepatogenic Cu poisoning [8][9][39][50]. Other necropsy findings include hydropericardium, ascites, icterus, hydrothorax, and mesenteric edema.
B). Chronic exposure may also induce lipidosis, ketosis, or promote secondary hepatogenic Cu poisoning [8,9,65,76]. Other necropsy findings include hydropericardium, ascites, icterus, hydrothorax, and mesenteric edema.
Microscopic changes associated with PA poisoning include centrilobular hepatocyte necrosis if the intoxication is acute. In the phasic form, regenerative nodules, minimal to marked periportal and peribiliary fibrosis, bile duct hyperplasia, megalocytosis, and nuclear invaginations can be seen. In the chronic or prolonged exposure-related form, variable hepatocyte atrophy, slight bile duct hyperplasia, and minimal periportal fibrosis are observed [8][9][29][39][48][51].
Microscopic changes associated with PA poisoning include centrilobular hepatocyte necrosis if the intoxication is acute. In the phasic form, regenerative nodules, minimal to marked periportal and peribiliary fibrosis, bile duct hyperplasia, megalocytosis, and nuclear invaginations can be seen. In the chronic or prolonged exposure-related form, variable hepatocyte atrophy, slight bile duct hyperplasia, and minimal periportal fibrosis are observed [8,9,55,65,74,77].
3.2. Indolizidine Alkaloids (Swainsonine-Containing Plants)
Indolizidine alkaloids are found in several species of three genera in the Fabaceae (Fabales) family, including
Astragalus
spp.,
Oxytropis
spp., and
Swainsona spp. Swainsonine, is the main bioactive principle found in these genera of plants. In addition, swainsonine has been documented in two other plant families, the Convolvulaceae (Solanales) and the Malvaceae (Malvales) [8]. Toxic potential is attributed to several species, including poison pea (
spp. Swainsonine, is the main bioactive principle found in these genera of plants. In addition, swainsonine has been documented in two other plant families, the Convolvulaceae (Solanales) and the Malvaceae (Malvales) [8]. Toxic potential is attributed to several species, including poison pea (
Swainsona
spp.), locoweeds (
Astragalus
spp. and
Oxytropis
spp.), broomweed (
Sida carpinifolia
) and several species belonging to the morning glory family (
Ipomoea
spp.;
Figure 2A) [52][53][54][55]. The alkaloid is found in every part of the plant, with highest concentrations in the leaves, flowers, and seeds [53]. However, many of the species within these families are nontoxic and considered important forage resources.
A) [78,79,80,81]. The alkaloid is found in every part of the plant, with highest concentrations in the leaves, flowers, and seeds [79]. However, many of the species within these families are nontoxic and considered important forage resources.
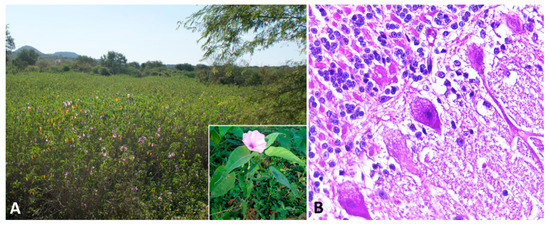
Figure 2.
A
Ipomoea carnea
fistulosa
B
Swainsonine is a potent inhibitor of two lysosomal enzymes, alpha-D-mannosidase and Golgi mannosidase II, which are important in the metabolism of saccharides and the formation of glycoproteins. The inhibition of alpha-mannosidase causes cells to accumulate oligosaccharides, whereas the inhibition of Golgi mannosidase II affects the normal structure of glycoproteins [56]. As a result, oligosaccharide glycosylated proteins accumulate in the cells of the brain and many other organs, interfering with normal cellular function [57][58]. Other similar polyhydroxylated alkaloids with different stereochemical configurations, such as castanospermine and calystegines, have been identified in several plants and are specific inhibitors of other enzymes [8][59][32].
Swainsonine is a potent inhibitor of two lysosomal enzymes, alpha-D-mannosidase and Golgi mannosidase II, which are important in the metabolism of saccharides and the formation of glycoproteins. The inhibition of alpha-mannosidase causes cells to accumulate oligosaccharides, whereas the inhibition of Golgi mannosidase II affects the normal structure of glycoproteins [82]. As a result, oligosaccharide glycosylated proteins accumulate in the cells of the brain and many other organs, interfering with normal cellular function [83,84]. Other similar polyhydroxylated alkaloids with different stereochemical configurations, such as castanospermine and calystegines, have been identified in several plants and are specific inhibitors of other enzymes [8,49,85].
Sheep intoxicated with indolizidine alkaloids-containing plants are lethargic, anorexic, reluctant to move, and may show neurologic signs that range from subtle anxiety to seizures [54][60][61]. Loss of condition and emaciation are typical and may also occur in situations of plentiful feed [60][62][63]. In an experimental study, sheep that received
Sheep intoxicated with indolizidine alkaloids-containing plants are lethargic, anorexic, reluctant to move, and may show neurologic signs that range from subtle anxiety to seizures [80,86,87]. Loss of condition and emaciation are typical and may also occur in situations of plentiful feed [86,88,89]. In an experimental study, sheep that received
Oxytropis sericea refused up to 50% of their ration after 3 weeks, which resulted in significantly reduced weight gains [64].
refused up to 50% of their ration after 3 weeks, which resulted in significantly reduced weight gains [90].
Neurogenic anorexia, inability to eat properly, and impairment of glycosylation and secretion of intestinal and pancreatic exocrine enzymes, with subsequent poor digestion and absorption, are the main mechanisms related to weight loss or reduced weight gains in poisoned sheep [65][61][64]. Sheep become cachectic and they develop more severe neurologic signs after continuous exposure to indolizidine alkaloids for weeks or months [8]. Other clinical signs such as infertility, reproductive failure, and abortions are also reported. Ewes poisoned later in gestation often give birth to small and weak lambs [53].
Neurogenic anorexia, inability to eat properly, and impairment of glycosylation and secretion of intestinal and pancreatic exocrine enzymes, with subsequent poor digestion and absorption, are the main mechanisms related to weight loss or reduced weight gains in poisoned sheep [85,87,90]. Sheep become cachectic and they develop more severe neurologic signs after continuous exposure to indolizidine alkaloids for weeks or months [8]. Other clinical signs such as infertility, reproductive failure, and abortions are also reported. Ewes poisoned later in gestation often give birth to small and weak lambs [79].
Affected sheep normally have no prominent gross lesions other than emaciation with marked loss of adipose tissue. Histologically, widespread cytoplasmic vacuolation in different locations, especially epithelia and nervous tissues, may be detected. Neuronal vacuolation is most severe in cerebellar Purkinje cells (
Figure 2B) and large neurons of the basal ganglia [60][61][62][64][66][67]. Purkinje cells also present axonal dystrophy and abnormal neuritic processes, which may include meganeurite formation at the axonal hillock, aberrant synapses, and dendritic outgrowths. Some cerebellar Purkinje cells are lost (empty axonal baskets) and undergo subsequent axonal degeneration and spheroid formation [8][68].
B) and large neurons of the basal ganglia [86,87,88,90,91,92]. Purkinje cells also present axonal dystrophy and abnormal neuritic processes, which may include meganeurite formation at the axonal hillock, aberrant synapses, and dendritic outgrowths. Some cerebellar Purkinje cells are lost (empty axonal baskets) and undergo subsequent axonal degeneration and spheroid formation [8,93].
Swainsonine-containing plant poisoning may be tentatively diagnosed by demonstrating the presence of swainsonine in the serum, coupled with decreased serum alpha-mannosidase activity [69]. Lectin histochemistry in liver biopsies could be an effective diagnostic method, even in animals without neurological signs [70]. An accurate postmortem diagnosis may be done by demonstrating the presence of characteristic cytoplasmic vacuoles in the cerebellar Purkinje cells, lymphocytes, liver, thyroid gland, and several other tissues.
Swainsonine-containing plant poisoning may be tentatively diagnosed by demonstrating the presence of swainsonine in the serum, coupled with decreased serum alpha-mannosidase activity [94]. Lectin histochemistry in liver biopsies could be an effective diagnostic method, even in animals without neurological signs [95]. An accurate postmortem diagnosis may be done by demonstrating the presence of characteristic cytoplasmic vacuoles in the cerebellar Purkinje cells, lymphocytes, liver, thyroid gland, and several other tissues.
3.3. Calcinogenic Glycoside-Containing Plants
Sheep grazing calcinogenic plants develop a progressive debilitating disease with widespread mineralization of soft tissues called enzootic calcinosis. The main species of calcinogenic plants that affect sheep are
Cestrum diurnum in southern United States [7],
in southern United States [7],
Trisetum flavescens in Bavarian and Austrian Alps [71][72],
in Bavarian and Austrian Alps [96,97],
Solanum malacoxylon
(
Figure 3A) in Argentina and Uruguay [73],
A) in Argentina and Uruguay [98],
Solanum escuriali in Australia [74],
in Australia [99],
Solanum verbascifolium in South Africa [75],
in South Africa [100],
Nierembergia veitchii in Brazil [76][77], and
Nierembergia repens
and
Nierembergia rivularis in Uruguay [78]. In some countries such as Israel, India and Central Western Brazil, outbreaks caused by undetermined calcinogenic plants occurred in sheep [73][79].
in Uruguay [103]. In some countries such as Israel, India and Central Western Brazil, outbreaks caused by undetermined calcinogenic plants occurred in sheep [98,104].
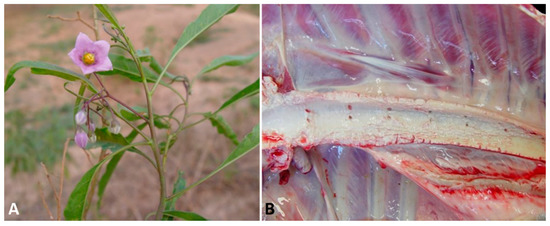
Figure 3.
A
Solanum malacoxylon
B
Solanum malacoxylon
The leaves of these calcinogenic plants contain a glycoside related to 1,25-dihydroxycholecalciferol (calcitriol) or a calcitriol-like compound with noxious biologic activity. Calcitriol is the active form of vitamin D (cholecalciferol) and acts by increasing calcium absorption from the gastrointestinal tract and bone resorption, and by decreasing calcium excretion in the kidneys [53]. Intoxication by these plants produces rapid wasting and marked elevations of the calcium and phosphate levels in the blood [53], causing metastatic calcification in the heart, aorta, lungs, kidneys, and joints. As the renal metastatic calcification progresses, there is an increase in blood urea nitrogen and creatinine on serum chemistry analyses. Radiology may aid in revealing calcification in several organs, including the vascular walls of the limbs [53][71].
The leaves of these calcinogenic plants contain a glycoside related to 1,25-dihydroxycholecalciferol (calcitriol) or a calcitriol-like compound with noxious biologic activity. Calcitriol is the active form of vitamin D (cholecalciferol) and acts by increasing calcium absorption from the gastrointestinal tract and bone resorption, and by decreasing calcium excretion in the kidneys [79]. Intoxication by these plants produces rapid wasting and marked elevations of the calcium and phosphate levels in the blood [79], causing metastatic calcification in the heart, aorta, lungs, kidneys, and joints. As the renal metastatic calcification progresses, there is an increase in blood urea nitrogen and creatinine on serum chemistry analyses. Radiology may aid in revealing calcification in several organs, including the vascular walls of the limbs [79,96].
Poisoning is progressive, and the first clinical signs observed are anorexia, depression, weakness, weight loss, infertility, cardiac arrhythmias, cachexia, stiffness, impaired stilted gait, kyphosis, and recumbence. Musculoskeletal abnormalities, including slight flexion of the forelimbs while walking, abnormally straight hind limbs, and/or knee walking, may be severe in some sheep. Increased respiratory rate, abdominal distension due to ascites, and reduced rumen motility are also described. Death generally occurs after a protracted clinical course [71][77][78][79]. The majority of these signs are related to hypercalcemia and subsequent soft tissue calcification. Death may occur as a result of emaciation and weakness, as well as secondary to cardiac and/or pulmonary insufficiency [53][80].
Poisoning is progressive, and the first clinical signs observed are anorexia, depression, weakness, weight loss, infertility, cardiac arrhythmias, cachexia, stiffness, impaired stilted gait, kyphosis, and recumbence. Musculoskeletal abnormalities, including slight flexion of the forelimbs while walking, abnormally straight hind limbs, and/or knee walking, may be severe in some sheep. Increased respiratory rate, abdominal distension due to ascites, and reduced rumen motility are also described. Death generally occurs after a protracted clinical course [96,102,103,104]. The majority of these signs are related to hypercalcemia and subsequent soft tissue calcification. Death may occur as a result of emaciation and weakness, as well as secondary to cardiac and/or pulmonary insufficiency [79,105].
At necropsy, depletion of fat deposits is noted, and mineralization can be visualized as aggregates of a gritty, white to tan material on the surfaces of several organs and tissues, particularly in the aorta (
Figure 3B), valvular and mural endocardium, lungs, and kidneys [72]. Mineralization may be also observed in the uterine, omasal, ruminal, and reticular serosae [77]. Histologically, systemic tissue mineralization is readily detected as fine, granular, basophilic deposits, and the extent of tissue damage is related to the severity and duration of the hypercalcemia. In the arteries, mineralization is frequently observed in the subintimal media, in which chondroid and osseous metaplasia occasionally occur. Mineral may be found in the renal parenchyma, pulmonary alveolar septa, bronchiolar epithelium, endocardium, gastric and intestinal musculature, and other tissues. In response to chronic hypercalcemia, the thyroid C cells often become hyperplastic and the parathyroid gland may undergo atrophy [8][53][71][72][77][78].
B), valvular and mural endocardium, lungs, and kidneys [97]. Mineralization may be also observed in the uterine, omasal, ruminal, and reticular serosae [102]. Histologically, systemic tissue mineralization is readily detected as fine, granular, basophilic deposits, and the extent of tissue damage is related to the severity and duration of the hypercalcemia. In the arteries, mineralization is frequently observed in the subintimal media, in which chondroid and osseous metaplasia occasionally occur. Mineral may be found in the renal parenchyma, pulmonary alveolar septa, bronchiolar epithelium, endocardium, gastric and intestinal musculature, and other tissues. In response to chronic hypercalcemia, the thyroid C cells often become hyperplastic and the parathyroid gland may undergo atrophy [8,79,96,97,102,103].
A tentative diagnosis of enzootic calcinosis can be made by measuring serum levels of 1,25-dihydroxycholecalciferol, which may be detectable in recently exposed animals [53]. Demonstration of mineral deposits in tissues by ultrasonography is also a valid in vivo approach [71]. Postmortem visualization of mineral deposits in the heart, aorta, lung, or kidney, either at necropsy or microscopically, coupled with a history of calcinogenic plant exposure, is probably the most reliable diagnostic method. The prognosis is unfavorable once extensive calcification has occurred, since there is no treatment. Prevention and control may be attempted by removing these weeds from the grazing areas.
A tentative diagnosis of enzootic calcinosis can be made by measuring serum levels of 1,25-dihydroxycholecalciferol, which may be detectable in recently exposed animals [79]. Demonstration of mineral deposits in tissues by ultrasonography is also a valid in vivo approach [96]. Postmortem visualization of mineral deposits in the heart, aorta, lung, or kidney, either at necropsy or microscopically, coupled with a history of calcinogenic plant exposure, is probably the most reliable diagnostic method. The prognosis is unfavorable once extensive calcification has occurred, since there is no treatment. Prevention and control may be attempted by removing these weeds from the grazing areas.
