Glycerol (C3H8O3), also known as propane-1,2,3-triol, is a significant biomolecule [1]. It is chemically classified as a ‘polyol with a molar mass of 92.09382 g/mol, a density of 1.26 g/cm3, and a boiling point of 554 °F (290 °C). In this section, we shall highlight some key roles this molecule plays in the biochemistry of life.
- glycerol
- phosphorylation
- catalysis
- extremophiles
- halophiles
- origin of life
- meteorites
1. Introduction
Glycerol is produced by human physiology and is a product of other living organisms. It is the structural backbone of lipid molecules (triacylglycerols) and is considered to be an important metabolite in living organisms [1]. It is synthesized from sn-glycerol-3-phosphate in the presence of an enzyme called glycerol-3-phosphate phosphatase [2]. sn-glycerol-3-phosphate is formed from 1,3-dihydroxyacetone phosphate (DHAP, hereafter), a glycolytic product, catalyzed by the enzyme glycerol phosphate dehydrogenase or from dihydroxyacetone (DHA, hereafter), catalyzed by glycerol dehydrogenase [1][3][4]. Such biochemical reactions are in equilibrium and hence are reversible depending on the substrate and product levels in the reactions. The enzymes that catalyze the reactions of glycerol are tissue-specific: e.g., glycerol kinase and glycerol dehydrogenase are chiefly found in the liver and kidney [1][3][4]. Small amounts of enzymes such as glycerol kinase and glycerol dehydrogenase have been found in skeletal muscle [5] as well as intestinal mucosa [6][7] while glycerol phosphate dehydrogenase is mainly found in skeletal muscle, the liver, and adipose tissue [6][7].
Another biochemical process for the formation of glycerol is the de-esterification reaction (or also known as lipolysis) of triacylglycerols [8] that mainly takes places in adipose tissue, skeletal muscle, the liver, and in blood circulating through blood vessels that are lined with lipoprotein lipase [8]. The most significant form of glycerol is sn-glycerol-3-phosphate, which directly takes part in the biochemical synthesis of lipids [1].
The key metabolic pathway of lipogenesis (formation of triacylglycerols) occurs in adipose tissue, liver, muscle, heart, and the pancreas, and requires glycerol and its derivatives such as glycerol phosphates and fatty acid esters of glycerol (acylglycerols). Such metabolic pathways maintain and control energy homeostasis in the living organisms [9]. It has been found that white adipose tissues (WAT) secrete copious amounts of glycerol [10][11]. As mentioned earlier, glycerol is also produced as a by-product of lipolysis of triacylglycerols [8][10][12]. While WAT’s capacity to regenerate glycerol is extremely limited [10][13], glycerol is the key substrate for hepatic gluconeogenesis as well as for many other biochemical reactions taking place in living organisms [10][14]. In addition to it taking part in hepatic gluconeogenesis, glycerol is also catalyzed by the enzyme glycerol kinase to form sn-glycerol-3-phosphate that then takes part in the biosynthesis of acylglycerols [10].
Glycerol is also the main low molecular weight compound in birds’ eggs, completely replacing glucose during the first stages of embryonic development [10][15]. Furthermore, in many species of plants and yeasts, formation of glycerol from sn-glycerol-3-phosphate favors its accumulation in living cells as a response to metabolic stress caused by harsh environmental conditions [10][16]. In WAT, glycerol becomes the waste product of lipolysis and thus gets converted into glucose, thus providing a significant source of glucose for hibernating animals such as the black bear [17]. The North American fish species rainbow smelt (Osmerus mordax) is known to depress the freezing point of body fluids by using a mixture of antifreeze protein and glycerol [18][19][20].
In some fungi and algae, the intracellular glycerol concentration can reach as high as 7–8 M, which might act as a stress factor in certain yeasts (or fungi) [21][22]. The significance of glycerol, its metabolism, and its transportation in yeasts (e.g., Saccharomyces cerevisiae) has been extensively discussed [21][23]. In Saccharomyces cerevisiae and many other yeasts, glycerol is synthesized in the cytosol of the cell by the reduction of DHAP to sn-glycerol-3-phosphate which undergoes dephosphorylation to form glycerol [24][25][26]. Glycerol is also an important nutrient for the cell-wall-less bacteria of the genus Mycoplasma [27]. This particular genus belongs to pathogens that derive glycerol from the lipids of their hosts (humans or animals) [27].
As discussed above, glycerol serves as the backbone of lipid molecules. It plays a central role in the formation of acylglycerols (simple lipids) and phospholipids (complex lipids). Both simple lipids and complex lipids play a central role in the formation of cell membranes in the living organisms and phosphorylated derivatives of glycerol. For example, glycerol phosphates play a significant role in the formation of cell membranes in living beings [1]. Glycerol phosphates (e.g., sn-glycerol-3-phosphate or sn-glycerol-1-phosphate and glycerol-2-phosphate) together with proteins make up the membranes of cells. Glycerol phosphate (with phosphate at the terminal carbon of glycerol) constitutes an essential part of the phospholipids.
Glycerol also plays a crucial role in the various metabolic pathways such as cellular respiration and photosynthesis. In vivo, glycerol is converted into products such as DHA, glyceraldehyde, and glyceric acid. These oxidized products of glycerol sustain metabolic pathways. For example, in glycolysis, glycerol is oxidized to DHA, the simplest ketone sugar, and this molecule actively takes part in respiration in higher plants and animals [28]. Its phosphorylated derivative, DHAP, is also an important intermediate in metabolic pathways such as glycolysis and the Calvin cycle, which is part of photosynthesis.
Another oxidation product of glycerol is glyceraldehyde, which is an intermediate in the metabolism of fructose sugar. Its phosphorylated derivative glyceraldehyde-3-phosphate is considered to be a high-energy intermediate that facilitates the generation of ATP (adenosine triphosphate) [29]. Similarly, glyceric acid and its phosphorylated derivatives such as 2-phosphoglyceric acid, 3-phosphoglyceric acid, 2,3-diphosphoglyceric acid, and 1,3-diphosphoglyceric acid all play central roles in glycolysis [30]. The bacterial species Gluconobacter oxydans is capable of converting glycerol into glyceric acid, implying a facilitated conversion of glycerol into glyceric acid [31].
2. The Varieties of Glycerol Used by Modern Biochemistry
In order to understand the origin and evolution of early membranes, it is important to understand the role of glycerol phosphates in phospholipids. Phospholipids are amphiphilic macromolecules that contain fatty acids linked to glycerol phosphate. Glycerol phosphates constitute the polar part of the phospholipids and may also link through a phosphate diester to form a structure called a ‘head group’. Important examples of phospholipids include phosphatidic acids, phosphatidylethanolamines, phosphatidylcholines, and phospohatidylserine.
Glycerol phosphate is an achiral molecule, whereas phospholipids have a stereogenic center at the second carbon of glycerol and on the basis of this stereogenic center, different types of glycerol phosphates are used in archaea compared to eukaryotes and bacteria. The naturally occurring enantiomer of the diacylglycerol phospholipids found in eukaryotes and bacteria is always D (also known as sn-glycerol-3-phosphate) [32][33], whereas the naturally occurring enantiomer of the phospholipidic isoprenoid glycerol ethers in archaeal cell membranes is L (also referred as sn-glycerol-1-phosphate) [34]. Bacteria and eukaryotes share similar membrane biochemistry, with ester-linked fatty acid-based phospholipids that comprise of sn-glycerol-3-phosphate. These sn-glycerol-3-phosphate based phospholipids were considered to be universal, but studying the archaeal biochemistry revealed that archaeal phospholipids are formed of sn-glycerol-1-phosphate that is connected to isoprenoid chains via ether linkage [34]. It is perhaps due to this chemical difference that there are different phospholipid biosynthesis pathways.
The plausible studies of the origin of phospholipids and glycerol phosphates would help in understanding the evolution of membranes and their various structural aspects. Perhaps one of the intriguing questions is indeed the structural disparity in the cell membranes of eukaryotes and bacteria versus archaea. However, it is important to mention here that certain prebiotic synthesis reactions reported to have started from achiral glycerol and fatty acids or aldehydes and achiral condensing agents and yielded the racemic mixtures of the phospholipids [32][33][34], and have generally not focused on chirality.
Cell membranes in bacteria, archaea, and eukaryotes are composed of phospholipids as bilayers; however, in some thermophilic prokaryotes they are present as monolayers. Various proteins are embedded in these lipid layers [35]. Despite the similarity in the basic composition of phospholipids in archaea and eukaryotes as well as bacteria, there is remarkable difference with respect to chemical compositions and structures. As mentioned above, in eukaryotes and bacteria, sn-glycerol-3-phosphate is chemically linked via an ester linkage to fatty acids.
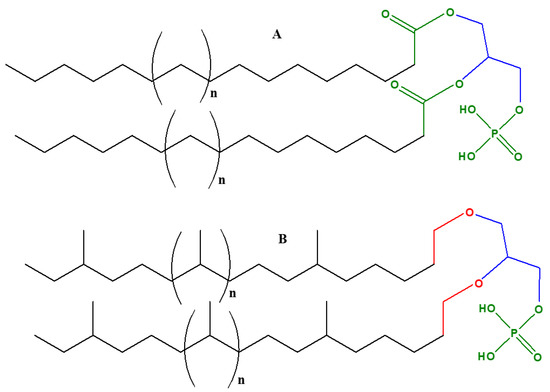
In archaea, there are three distinctive features of phospholipids that differ from other organisms [35][36][37]:
- Figure 2. The basic phospholipid structural differences of the archaea versus eukaryotes and bacteria: (A) generic structure of phospholipids in most eukaryotes and bacteria that contain unbranched fatty acid chains linked up with sn-glycerol-3-phosphate (D-glycerol). The bond between fatty acids and glycerol phosphate is an ester (shown as green color), (B) generic structure of phospholipids in archaea contains ‘branched isoprenoid’ units, linked up with sn-glycerol-1-phosphate (L- glycerol) via ether linkages, shown as red color. “n” represents generic number for the isoprenoid or fatty acid units.
What is a plausible explanation for the formation of these two forms of glycerol phosphates (both sn-glycerol-1-phosphate and sn-glycerol-3-phosphate) between the major domains of life? An answer may lie in their biochemical synthesis. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH, hereafter) is a dehydrogenase enzyme that directs/catalyzes the biosynthesis of sn-glycerol-3-phosphate in eukaryotes and bacteria, whereas glyceraldehyde-1-phosphate dehydrogenase (G1PDH) is responsible for the synthesis of sn-glycerol-1-phosphate in archaea. It is an open question as to which form of glycerol phosphate existed first in biology: sn-glycerol-1-phosphate may have preceded sn-glycerol-3-phosphate (or vice versa), or both may have co-existed [35]. This feeds into a larger question that relates to the issue of a ‘cenancestor’ (defined as the ancestor species from which all modern organisms plausibly evolved): was it archaeal or was it bacterial? Both the G3PDH and G1PDH dehydrogenases and their presence in the cenancestor could plausibly be a cause of the diversification of archaea and bacteria [35].
Furthermore, the stereospecificity of lipids found in archaea and their unique structure has been hypothesized to be more stable in extreme environments, providing the organism better chances of survival [37]. However, archaea are also present in mesophilic (growing in moderate temperatures) and neutrophilic environments (living in neutral environments e.g., pH = 6.5–7.3) where the significance of a chemical variation in cell membrane composition is lessened [38]. With these points in mind, there are three hypotheses that attempt to explain the plausible origin of sn-glycerol-1-phosphate and sn-glycerol-3-phosphate cell membranes [35].
The first hypothesis posits the prebiotic synthesis of glycerol phosphate in a non-chiral fashion on a surface that would have then become the “first acellular metabolists” [38][39][40]. Due to non-chiral synthesis both sn-glycerol-1-phosphate and sn-glycerol-3-phosphate would have been used in phospholipid syntheses at the initial stages of the formation of cell membranes. Later, the organisms could plausibly evolve their respective enzymes (G1PDH or G3PDH) to catalyze the reduction of DHAP for specific glycerol phosphate, either sn-glycerol-1-phosphate or sn-glycerol-3-phosphate. These primitive organisms would eventually evolve into eukaryotes via archaeal-bacterial symbiotic associations and the acquired membranes would have passed onto the eukaryotes.
The second hypothesis was presented by Martin and Russell [41] who favor a hydrothermal origin of life and, according to them, iron monosulphide bubbles around hydrothermal vents could be considered as the earliest cellular compartments. In this scenario, the origin of life and metabolic activities were more geochemically-driven and many chemical activities could be successfully carried out within the iron monosulphide bubbles. It has further been proposed that this cell-like entity could perform metabolic activities and, at a certain stage, even became capable of forming RNA and the associated necessary proteins, but still thrived on iron monosulphide bubbles to act as cellular compartment and support its developing membranes or simple compartments. In this scenario, the chirality of glycerol phosphate was selected much later than the origin of life and may have been done at random.
The third hypothesis suggests the formation of a lipid membrane during a pre-cell stage (Figure 3). This membrane was a racemic mixture of chiral ancient lipid molecules that over the course of time gave rise to enantiomeric lipids that would have selected for a specific handedness depending on the evolution of the organisms, and eventually would have replaced the racemic mixture of lipids [35][38][39][40][41][42].
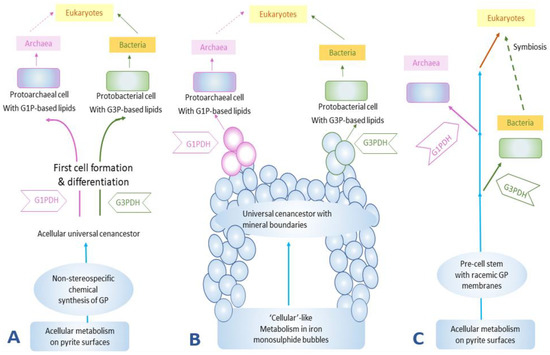
Figure 3. The formation of lipid membranes in primitive organisms: (A) the emergence of cellular life on pyrites, or more generally ‘minerals’ [40], (B) the emergence of life on monosulphide bubbles around hydrothermal vents [41], and (C) the non-stereospecific formation of the GP and the subsequent evolution of enzymes GIPDH and G3PDH to catalyze the formation of stereospecific GP [42]. GP stands for glycerol phosphates, G1P stands for sn-glycerol-1-phosphate, and G3P stands for sn-glycerol-3-phosphate. This figure was redrawn and reprinted with permission from [35]; Copyright © 2004 Elsevier.
The prebiotic synthesis of phospholipids has undergone further studies specifically concerning the issue of the symmetry breaking of phospholipids [43]. These experiments have discussed issues such as the generation of pure enantiomeric lipid compounds from chiral or achiral reactants or catalysts. According to this study, in the evolution and development of membrane systems, the enzymatic activity appears to be the main strategy to get enantiomeric pure lipid type molecules. The study further suggests that the occurrence of two genes encoding for G1PDH and G3PDH could be responsible for the buildup of an evolutionary tree. Evolution of these two specific genes would have been the potential source of evolution in the membranes. Gene encoding for G3PDH in eukaryotes may have originated from G3PDH gene that is found in archaea revealing that archaea might have originated earlier than eukaryotes [43]. Archaea and bacteria might have evolved separately, a suggestion based on their distinctively different genes coding for G1PDH and G3PDH. A consequence of this hypothesis is that the catalytic biosynthesis pathways of homochiral sn-glycerol-1-phosphate or sn-glycerol-3-phosphate are more efficient than those that form racemic glycerol phosphate mixtures. Additionally, it is quite possible that both G1PDH and G3PDH would have emerged separately during evolution [43].
3. Significance of Glycerol in the Biochemistry of Extremophiles
Extremophiles are organisms that can thrive in severe environmental conditions such as extreme temperatures (both exceptionally cold or hot), extreme pH (highly acidic or alkaline), or other harsh environments such as presence of salts/oxidants/radiation and many other harsh conditions [44]. Extremophiles thriving in various harsh conditions help to understand the various forms of life and their adaptations. Moreover, studying extremophiles also has implications for the origin and evolution of early life on the Earth [45][46] and thus these organisms are a subject of interest with relevance for this review article. This section will specifically highlight the significance of glycerol in the biochemistry of some extremophiles.
3.1. Role of Glycerol in the Biochemistry of Microalgae, Archaea and Other Organisms
Glycerol is a product of photosynthesis in the genus Dunaliella (red algae represented as D. hereafter). CO2 is fixed to form glycerol [47][48] in this process, as confirmed by the use of labeled 14CO2. The speciation of 14CO2-fixed products demonstrated most of the 14C was present in glycerol. It was also found that glycerol’s generation was directly proportional to an increase in salinity [47][48]. Studies with D. parva, D. tertiolecta [47][49][50], and D. viridis [51][52] revealed that the intracellular glycerol concentration is dependent on the salinity of the growth medium. D. parva cells were adapted to grow in concentration of 1.5–2 M NaCl and had about 2–2.2 M glycerol in the intracellular medium, whereas cells of D. salina grown in 4 M NaCl contained about 7.8 M glycerol (same as 72% glycerol solution in water by weight) [53]. Figure 4 shows the natural habitat of the aforementioned halophilic organisms, also known as red algae.

Figure 4. Images of Lake Chaxa: (A) a salt-flat lake in Chile wherein red algae impart a reddish hue to the lake, (B) higher salt contents and salt crusts that can easily be seen as large white deposits. This lake is the habitat of halophilic organisms including the red algae Dunaliella. These species thrive in high salt conditions, and the briny lake is an ideal habitat for these halophiles. Photo credit to Chris Hunkeler. Both images are licensed under the creative commons Attribution-Share Alike license.
A schematic diagram of the ‘glycerol cycle’ in the genus Dunaliella is represented as Figure 5A. The starting substrate is DHAP. Four specific enzymes catalyze the overall synthesis of glycerol, including (1) NAD+ dependent dehydrogenase or DHAP reductase, (2) glycerol-3-phosphate phosphatase, (3) DHA reductase, and 4) DHA kinase [47]. In the presence of light, glycerol is synthesized via photosynthesis, followed by the Calvin cycle (the light independent phase of photosynthesis) and, in the absence of light, the starch in the organism is converted into glycerol [47][54][55][56] (Figure 5).
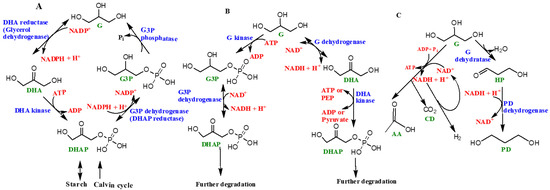
Figure 5. Schematic diagram showing the role of glycerol in various living organisms: (A) ‘glycerol cycle’in the genus Dunaliella, (B) glycerol’s metabolic pathways in aerobic heterotrophic prokaryotes, and (C) fermentation of glycerol in Halanaerobium saccharolyticum. The abbreviations used are as follows: G (glycerol), DHAP (1,3-dihydroxyacetone phosphate), G3P (sn-glycerol-3-phosphate), DHA (1,3-dihydroxyacetone), PEP (phosphoenolpyruvate), HP (3-hydroxypropionaldehyde), PD (1,3-propanediol), AA (acetic acid), CD (carbon dioxide), and H2 (hydrogen gas). Enzyme names are shown in blue while organics (products or reactants) are shown as green, and co-enzymatic or high energy molecules (such as PEP) based reactions are shown as red. For more information see [47]. Figure reproduced, redrawn (with minor modifications) with permission from [47]; Copyright © 2017 John Wiley and Sons.
Co-enzyme NAD+ (nicotinamide adenine dinucleotide) assists in various metabolic reactions and exists in another form, NADP+, which undergoes reduction by accepting electrons and hydrogen (e.g., H−) atoms to form NADPH. NADH and NADPH (nicotinamide adenine dinucleotide phosphate) are hence the biochemical reducing agents that drive this reaction. ATP (adenosine triphosphate), also known as the ‘energy currency’ of life, is needed in the process, which hydrolyzes ATP into ADP (adenosine diphosphate) to release energy.
Enzymes such as glycerol-3-phosphate dehydrogenase and glycerol-3-phosphate phosphatase help with the catalytic conversion of glycerol from DHAP, followed by the re-conversion of glycerol to DHAP, which is catalyzed by DHA reductase and DHA kinase [47][49][50]. These cyclic processes are highly energy dependent and need ATP to sustain and complete the cycle. Different steps of the glycerol cycle could happen in different locations in the cell, owing to the distribution of various enzymes, for example, enzymes like glycerol-3-phosphate dehydrogenase and quite possibly glycerol-3-phosphate phosphatase are located in the chloroplasts whereas DHA reductase is located in the cytoplasm. However, the locations and distributions of enzymes such as glycerol-3-phosphate phosphatase and DHA kinase are uncertain and still under further scrutiny. In the presence of light, glycerol can be synthesized by the products of photosynthesis, whereas in the dark starch hydrolyzes into glycerol [47][48][49][50][51][52][53][54][55][56] (Figure 5A).
Glycerol is also consumed by prokaryotes such as halophilic aerobic archaea that may catabolize glycerol by two pathways (Figure 5B). One path involves the synthesis of sn-glycerol-3-phosphate and subsequent formation of DHAP [47]. The major enzymes involved include glycerol kinase, a potential sn-glycerol-3-phosphate ABC transport system, and glycerol 3-phosphate dehydrogenase [47][57][58]. Alternatively, glycerol first converts into DHA with the help of glycerol dehydrogenase enzyme [59]. The synthesized DHA can potentially get phosphorylated and subsequently fed into certain Embden–Meyerhof pathways (a form of glycolytic pathway) or converted into metabolic intermediates including sn-glycerol-1-phosphate, which make up the backbone of archaeal phospholipids [47][58][59].
Certain halophilic archaea such as Halanaerobium saccharolyticum (and many others) have been demonstrated to generate energy by the fermentation of glycerol through its conversion into acetate, H2 and CO2 [47]. This is an anaerobic pathway. Figure 5C shows the fermentation of glycerol in Halanaerobium saccharolyticum. The aforementioned features of glycerol are attributed to its simplicity and excellent solubility in water, thus making it an excellent source of carbon for these organisms. A majority of halophilic bacterial species were found to be highly dependent on glycerol for nutrition [57]. Halophilic archaea consume glycerol by two different pathways: phosphorylation to sn-glycerol-3-phosphate and eventual conversion into DHAP and glycerol conversion into DHA. Both paths require their own sets of enzymes [58][59].
Another fascinating feature of glycerol is its help in maintaining the osmotic pressure in algae, where it acts as a solvent to regulate cellular metabolism and maintains enzymatic and other cellular activities under these saline concentrations [47][48][49]. This special feature of glycerol is seen in extremophiles living in highly saline environments.
There are so many other organisms that thrive on glycerol by consuming it as a nutrient. One such remarkable is Anaerobium acetethylicum, a strictly anaerobic, mesophilic bacterium which converts glycerol into useful compounds such as ethanol and hydrogen [60][61][62].
Certain fungal xerophiles such as Aspergillus penicillioides and Xeromyces bisporus are known to thrive in high solute environments and/or alternatively at very low water availability, by retaining high levels of glycerol for their osmotic adjustments [63][64]. Furthermore, in such species, glycerol is also known to facilitate the xerophile spore germination [64]. Similarly, halotolerant yeast Debaryomyces hansenii is also known to produce copious amounts of glycerol under increased saline concentrations [65]. This behavior is similar to that of genus Dunaliella of the red algae.
It is important to mention here that the utilization of glycerol as a substrate to derive energy and nutrients by various organisms has been documented previously in the scientific literature already available. Furthermore, the discussion of how non-extremophiles consume glycerol is beyond the scope of the current paper. The primary focus of this review article is extremophiles, owing to their relevance from an evolutionary viewpoint where they lie close to the root of the tree of life.
References
- Robergs, R.A.; Griffin, S.E. Glycerol Biochemistry, Pharmacokinetics and Clinical and Practical Applications. Sports Med. 1998, 26, 145–167.
- Mugabo, Y.; Zhao, S.; Seifried, A.; Gezzar, S.; Al-Mass, A.; Zhang, D.; Lamontagne, J.; Attane, C.; Poursharifi, P.; Iglesias, J.; et al. Identification of a mammalian glycerol-3-phosphate phosphatase: Role in metabolism and signaling in pancreatic β-cells and hepatocytes. Proc. Natl. Acad. Sci. USA 2016, 113, E430–E439.
- Elia, M.; Khan, K.; Calder, G.; Kurpad, A. Glycerol exchange across the human forearm assessed by a Combination of tracer and arteriovenous exchange techniques. Clin. Sci. 1993, 84, 99–104.
- Bortz, W.M.; Paul, P.; Haff, A.C.; Holmes, W.L. Glycerol turnover and oxidation in man. J. Clin. Investig. 1972, 51, 1537–1546.
- Newsholme, E.A.; Taylor, K. Glycerol kinase activities from vertebrates and invertebrates. Biochem. J. 1969, 112, 465–474.
- Frank, M.S.B.; Nahata, M.C.; Hilty, M.D. Glycerol: A review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy 1981, 1, 147–160.
- Lin, E.C. Glycerol utilization and its regulation in mammals. Annu. Rev. Biochem. 1977, 46, 765–795.
- Oscai, L.B.; Essig, D.A.; Palmer, W.K. Lipase regulation of muscle triglyceride hydrolysis. J. Appl. Physiol. 1990, 69, 1571–1577.
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474.
- Rotondo, F.; Ho-Palma, A.C.; Remesar, X.; Fernández-López, J.A.; Romero, M.D.M.; Alemany, M. Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover. Sci. Rep. 2017, 7, 8983.
- Jansson, P.A.; Larsson, A.; Smith, U.; Lönnroth, P. Glycerol production in subcutaneous adipose tissue of lean and obese hu-mans. J. Clin. Investig. 1992, 89, 1610–1617.
- Langin, D. Control of fatty acid and glycerol release in adipose tissue lipolysis. Comptes Rendus Biol. 2006, 329, 598–607.
- Chakrabarty, K.; Bombeck, C.T.; Sigel, B.; Tauber, J.W.; Jeffay, H. Glycerokinase activity in human adipose-tissue as related to obesity. Int. J. Obesity 1984, 8, 609–622.
- Ross, B.D.; Hems, R.; Krebs, H.A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem. J. 1967, 102, 942–951.
- Roca, P.; Sáinz, F.; González, M.; Alemany, M. Energetic components in the unincubated egg fractions of several avian spe-cies. Comp. Biochem. Physiol. B 1982, 72, 439–443.
- Saito, H.; Posas, F. Response to hyperosmotic stress. Genetics 2012, 192, 289–318.
- Engelking, L.R. Gluconeogenesis. In Textbook of Veterinary Physiological Chemistry, 3rd ed.; Academic Press: Cambridge, MA, USA, 2015; pp . 225–230.
- Driedzic, W.R.; Short, C.E. Relationship between food availability, glycerol and glycogen levels in low-temperature chal-lenged rainbow smelt Osmerus mordax. J. Exp. Biol. 2007, 210, 2866–2872.
- Ewart, K.V.; Fletcher, G.L. Isolation and characterization of antifreeze proteins from smelt (Osmerus mordax) and Atlantic herring (Clupea harengus harengus). Can. J. Zool. 1990, 68, 1652–1658.
- Raymond, J.A. Glycerol is a colligative antifreeze in some northern fishes. J. Exp. Zool. 1992, 262, 347–352.
- Klein, M.; Swinnen, S.; Thevelein, J.M.; Nevoigt, E. Glycerol metabolism and transport in yeast and fungi: Established knowledge and ambiguities. Environ. Microbiol. 2017, 19, 878–893.
- Cray, J.A.; Stevenson, A.; Ball, P.; Bankar, S.B.; Eleutherio, E.C.; Ezeji, T.C.; Singhal, R.S.; Thevelein, J.M.; Timson, D.J.; Hallsworth, J.E. Chaotropicity: A key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 2015, 33, 228–259.
- Xiberras, J.; Klein, M.; Nevoigt, E. Glycerol as a substrate for Saccharomyces cerevisiae based bioprocesses—Knowledge gaps regarding the central carbon catabolism of this ‘non-fermentable’ carbon source. Biotechnol. Adv. 2019, 37, 107378.
- Nevoigt, E.; Stahl, U. Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 1997, 21, 231–241.
- Blomberg, A. Metabolic surprises in Saccharomyces cerevisiae during adaptation to saline conditions: Questions, some answers and a model. FEMS Microbiol. Lett. 2000, 182, 1–8.
- Chen, X.; Fang, H.; Rao, Z.; Shen, W.; Zhuge, B.; Wang, Z.; Zhuge, J. Cloning and characterization of a NAD+-dependent glycerol-3-phosphate dehydrogenase gene from Candida glycerinogenes, an industrial glycerol producer. FEMS Yeast Res. 2008, 8, 725–734.
- Cedric Blötz, Jörg Stülke, Glycerol metabolism and its implication in virulence in Mycoplasma. FEMS Microbiol. Rev. 2017, 41, 640–652.
- Córdova, A.; Notz, W.; Barbas, C.F., 3rd. Direct organocatalytic aldol reactions in buffered aqueous media. Chem. Commun. 2002, 24, 3024–3025.
- Clough, S.R. Glyceraldehyde. In Encyclopedia of Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 448–449.
- Björkman, O. Comparative studies on photosynthesis in higher plants. In Photophysiology Current Topics in Photobiology and Photochemistry; Giese, A.C., Ed.; Academic Press: Cambridge, MA, USA, 1973; Volume 8, pp. 1–63.
- Chozhavendhan, S.; Devi, G.K.; Bharathiraja, B.; Kumar, R.P.; Elavazhagan, S. Assessment of crude glycerol utilization for sustainable development of biorefineries. In Refining Biomass Residues for Sustainable Energy and Bioproducts. Technology, Advances, Life Cycle Assessment, and Economics; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 195–212.
- Fayolle, D.; Altamura, E.; D’Onofrio, A.; Madanamothoo, W.; Fenet, B.; Mavelli, F.; Buchet, R.; Stano, P.; Fiore, M.; Strazewski, P. Crude phosphorylation mixtures containing racemic lipid amphiphiles self-assemble to give stable primitive compartments. Sci. Rep. 2017, 7, 18106.
- Weis, R.M.; McCollen, H.M. Two-dimensional chiral crystals of phospholipids. Nature 1984, 310, 47–49.
- Lombard, J.; López-García, P.; Moreira, D. The early evolution of lipid membranes and the three domains of life. Nat. Rev. 2012, 10, 507–515.
- Peretó, J.; López-García, P.; Moreira, D. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem. Sci. 2004, 29, 469–477.
- Kates, M. The phytanyl ether-linked polar lipids and isoprenoid neutral lipids of extremely halophilic bacteria. Prog. Chem. Fats Other Lipds 1977, 15, 301–342.
- Kate, M. Membrane lipids of archaea. In New Comprehensive Biochemistry; Kates, M., Kushner, D.J., Matheson, A.T., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; Volume 26, pp. 261–295.
- Koga, Y. Thermal Adaptation of the Archaeal and Bacterial Lipid Membranes. Archaea 2012, 2012, 789652.
- Jain, S.; Caforio, A.; Driessen, A.J. Biosynthesis of archaeal membrane ether lipids. Front. Microbiol. 2014, 5, 1–16.
- Koga, Y.; Kyuragi, T.; Nishihara, M. Did archaeal and bacterial cells arise independently from noncellular precursors? A hypothesis stating that the advent of membrane phospholipid with enantiomeric glycero-phosphate backbones caused the separation of the two lines of descent. J. Mol. Evol. 1998, 46, 54–63.
- Martin, W.; Russell, M.J. On the origins of cells: A hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. Lond. Ser. B 2003, 358, 59–83.
- Wächtershäuser, G. From pre-cells to eukarya—A tale of two lipids. Mol. Microbiol. 2003, 47, 13–22.
- Fiore, M.; Buchet, R. Symmetry Breaking of Phospholipids. Symmetry 2020, 12, 1–16.
- Rampelotto, P.H. Extremophiles and Extreme Environments. Life 2013, 3, 482–485.
- Merino, N.; Aronson, H.S.; Bojanova, D.P.; Feyhl-Buska, J.; Wong, M.L.; Zhang, S.; Giovannelli, D. Living at the Extremes: Extremophiles and the Limits of Life in a Planetary Context [published correction appears in Front. Microbiol. 2019, 10, 1785.
- Javaux, E.J. Extreme life on Earth—Past, present and possibly beyond. Res. Microbiol. 2006, 157, 37–48.
- Oren, A. Glycerol metabolism in hypersaline environments. Environ. Microbiol. 2017, 19, 851–863.
- Craigie, J.S.; McLachlan, J. Glycerol as a photosynthetic product in Dunaliella tertiolecta Butcher. Can. J. Bot. 1964, 42, 777–778.
- Ben-Amotz, A.; Avron, M. The Role of Glycerol in the Osmotic Regulation of the Halophilic Alga Dunaliella parva. Plant Physiol. 1973, 51, 875–878.
- Ben-Amotz, A. Adaptation of the unicellular alga Dunaliella parva to a saline environment. J. Phycol. 1975, 11, 50–54.
- Borowitzka, L.J.; Brown, A.D. The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. The role of glycerol as a compatible solute. Arch. Microbiol. 1974, 96, 37–52.
- Borowitzka, L.J.; Kessly, D.S.; Brown, A.D. The salt relations of Dunaliella. Further observations on glycerol production and its regulation. Arch Microbiol. 1977, 113, 131–138.
- Brown, A.D. Microbial water stress. Bacteriol. Rev. 1976, 40, 803–846.
- Ben-Amotz, A.; Avron, M. NADP specific dihydroxyacetone reductase from Dunaliella parva. FEBS Lett.1973, 29, 153–155.
- Ben-Amotz, A.; Sussman, I.; Avron, M. Glycerol production by Dunaliella. In New Trends in Research and Utilization of Solar Energy through Biological Systems; Mislin, H., Bachofen, R., Eds.; Springer: Basel, Switzerland, 1982; Volume 38, pp. 49–52.
- Goyal, A. Osmoregulation in Dunaliella, Part II: Photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta. Plant Physiol. Biochem. 2007, 45, 705–710.
- Da Costa, M.S.; Rainey, F.A.; Nobre, M.F. The Prokaryotes. In The Genus Thermus and Relatives; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 7, pp. 797–812.
- Falb, M.; Müller, K.; Königsmaier, L.; Oberwinkler, T.; Horn, P.; von Gronau, S.; Gonzalez, O.; Pfeiffer, F.; Bornberg-Bauer, E.; Oesterhelt, D. Metabolism of halophilic archaea. Extremophiles 2008, 12, 177–196.
- Rawal, N.; Kelkar, S.M.; Altekar, W. Alternative routes of carbohydrate metabolism in halophilic archaebacteria. Indian J. Biochem. Biophys. 1988, 25, 674–686.
- Patil, Y.; Junghare, M.; Muller, N. Fermentation of glycerol by Anaerobium acetethylicum and its potential use in biofuel production. Microb. Biotechnol. 2017, 10, 203–217.
- Patil, Y.; Junghare, M.; Pester, M.; Müller, N.; Schink, B. Characterization and phylogeny of Anaerobium acetethylicum gen. nov., sp. nov., a strictly anaerobic gluconate-fermenting bacterium isolated from a methanogenic bioreactor. Int. J. Syst. Evol. Microbiol. 2015, 65, 3289–3296.
- Hidalgo, M.; Puerta-Fernández, E. Fermentation of glycerol by a newly discovered anaerobic bacterium: Adding value to biodiesel production. Microb. Biotechnol. 2017, 10, 528–530.
- Stevenson, A.; Cray, J.A.; Williams, J.P.; Santos, R.; Sahay, R.; Neuenkirchen, N.; McClure, C.D.; Grant, I.R.; Houghton, J.D.; Quinn, J.P.; et al. Is there a common water-activity limit for the three domains of life? ISME J. 2015, 9, 1333–1351.
- Stevenson, A.; Hamill, P.G.; Medina, Á.; Kminek, G.; Rummel, J.D.; Dijksterhuis, J.; Timson, D.J.; Magan, N.; Leong, S.L.; Hallsworth, J.E. Glycerol enhances fungal germination at the water-activity limit for life. Environ. Microbiol. 2017, 19, 947–967.
- Norkrans, B.; Kylin, A. Regulation of the potassium to sodium ratio and of the osmotic potential in relation to salt tolerance in yeasts. J. Bacteriol. 1969, 100, 836–845, doi:10.1128/JB.100.2.836-845.1969.