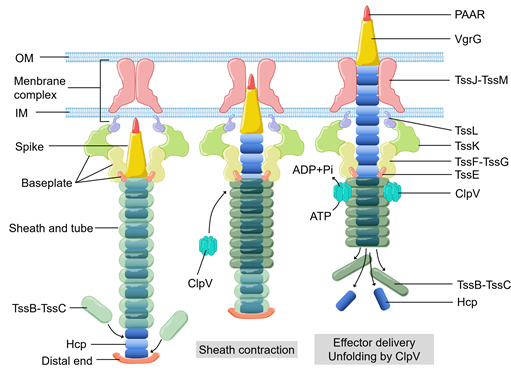
The bacterial type VI secretion system (T6SS) is a protein secretion apparatus widely distributed in Gram-negative bacterial species. Many bacterial pathogens employ T6SS to compete with the host and to coordinate the invasion process. The T6SS apparatus consists of a membrane complex and an inner tail tube-like structure that is surrounded by a contractile sheath and capped with a spike complex. A series of antibacterial or antieukaryotic effectors is delivered by the puncturing device consisting of a Hcp tube decorated by the VgrG/PAAR complex into the target following the con-traction of the TssB/C sheath, which often leads to damage and death of the competitor and/or host cells. As a tool for protein secretion and interspecies interactions, T6SS can be triggered by many different mechanisms to respond to various physiological conditions.
- bacteria
- stress response
- T6SS
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Bacteria can use secretion systems to transport individual proteins, as well as DNA–protein and protein–protein complexes into adjacent cells or the external medium. These secretion systems are critical for bacterial survival and adaptation to complex environmental conditions via their diversified secreted effectors, which are required for nutrition uptake, toxin delivery, cell-to-cell communication, and interspecies competition. Nine secretion systems (T1SS, T2SS, T3SS, T4SS, T5SS, T6SS, T7SS, T8SS (curli/Csg), and T9SS) have been identified in Gram-negative and Gram-positive bacteria [1–4][1][2][3][4]. Among them, the type VI secretion system (T6SS) (Figure 1), a complex contractile nano-machine similar to bacteriophage tail in structure, retains one of the most complicated secretion mechanisms [5]. T6SS is a needle-like multiprotein machine which is a member of contractile injection systems. Its structure consists of the membrane complex, the baseplate structure, and the tail tube sheath complex [6–8][6][7][8]. ClpV, a type of hexameric AAA+ ATPase, pulls the exposed N terminus of TssC and releases it from the contracted sheaths to replenish the pool of sheath subunits for starting a new cycle of assembly [9–11][9][10][11]. VgrG trimer recruits the hemolysin coregulated protein (Hcp) to become polymerized as the inner tube [5,12][12]. The type Ⅵ secretion system (T6SS) of bacteria was first detected in Rhizobium leguminosarum in 2003 [13,14][13][14]. In the next seventeen years, it was found that at least 1/4 Gram-negative bacteria species contain T6SS [15,16][15][16]. T6SS is often involved in multiple processes related to bacterial virulence [17–21][17][18][19][20][21]. To cope with external pressures, bacteria use T6SS to secret toxins into the external environment [3,22][22]. Moreover, T6SS has been shown to attack bacterial competitors [23–25][23][24][25], or to defeat the host defense mechanisms [26,27][26][27], in order to colonize a host niche and/or survive in competition.
2. Metal Ion Uptake for ROS Stress Response
Both the host immunity and the environmental factors such as heavy metals and antibiotics will lead to increased reactive oxygen species (ROS) levels in bacterial pathogens significantly [2]. The formation of ROS (e.g., hydrogen peroxide, hydroxyl radical, and superoxide) is inevitable in an oxygen-rich environment [28,29][28][29]. Other factors causing the generation of ROS include peptidoglycan recognition proteins [30], antimicrobial compounds with bactericidal activity [31], and attacks from competing bacteria and bacteriophages [32]. Elevated levels of ROS in cells will cause oxidative damage to DNA, proteins, lipids, and other macromolecules [33]. Bacteria are well known to respond to oxidative stress with the help of some regulatory proteins, like SoxR, SoxS, OxyR, or ZntR, which coordinate specific ROS stress tolerance mechanisms [34–36][34][35][36]. For example, the synthesis of antioxidant enzymes (e.g., peroxidase, glutaredoxin, uperoxide dismutase, thioredoxin) and small molecular weight antioxidants (e.g., β-carotene, tripeptide glutathione, vitamin C, and vitamin E) can be induced via these regulatory proteins to combat the adverse effects of ROS [33,37,38] [37][38].
Figure 1. The basic components, structural assembly, and effector delivery of the T6SS apparatus. OM, outer membrane. IM, inner membrane.
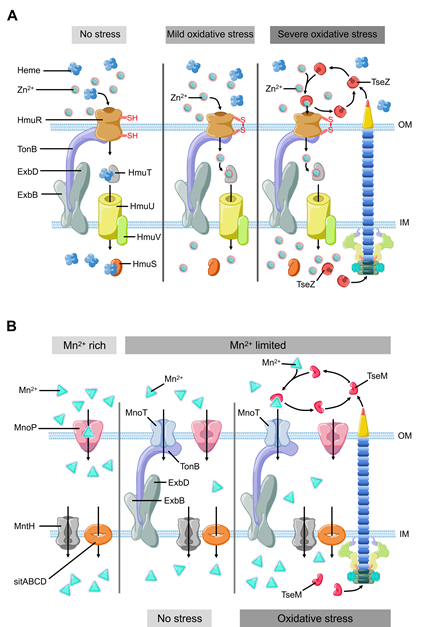
In recent years, it has been revealed that T6SS is involved in the process of oxidative stress response in bacterial pathogens, such as Vibrio anguillarum [39], Burkholderia thailandensis [40], Enterohemorrhagic E. coli [41], and Yersinia pseudotuberculosis [42]. Metal ions, such as zinc (Zn2+) and manganese (Mn2+), can be used as structural components or cofactors of antioxidant enzymes [35]. These metal ions also participate in the formation of antioxidant complexes [43–46][43][44][45][46]. Both metals help bacteria maintain redox balance and eliminate ROS [38]. Zinc (Zn2+) is an essential nutrient that participates in several mechanisms to maintain the redox homeostasis of bacteria, for instance, as a cofactor of superoxide dismutase [45,47][47]. Even though there are already well-studied systems involved in metal ion uptake and transportation, Si and colleges recently found that B. thailandensis employs T6SS to export TseZ as a Zn2+-binding effector under the condition of oxidative stress [48] (Figure 2A). The wild-type B. thalandensis could increase its survival rate and decrease the level of ROS by importing zinc ions when it was exposed to exogenous oxidative stress. The survival rate of B. thalandensis clpv4 mutant, which is unable to import zinc ions via HmuR, was lower than the wild-type strain and the mutant accumulated higher level of intracellular ROS than the wild-type strain [48]. This group demonstrated that T6SS effector TseZ interacts with HmuR (the outer membrane heme transporter) and further showed that the HmuRSTUV system is involved in the acquisition of Zn2+ under oxidative stress conditions [48]. HmuR is a type of dual-functional transporter which is regulated by redox [48]. HmuR transports heme iron under normal conditions and forms an intramolecular disulfide upon sensing oxidative stress [48]. The formation of the disulfide bond leads to a conformational change, which allows HmuR to bind TseZ, and more efficiently transfers the chelated Zn2+ into the cell [48,49][48][49]. This is a fine-tuned mechanism that allows B. thailandensis to response to different levels of oxidative stress [48]. In Y. pseudotuberculosis, the MarR family transcriptional regulator HpaR [50,51][50][51] directly binds the promoter of T6SS to upregulate its expression. T6SS can secrete YezP (a zinc-binding protein substrate) which binds extracellular Zn2+ and transports it into Y. pseudotuberculosis [42]. When wild-type Y. pseudotuberculosis was inoculated into C57BL/6 mice, the mortality rate of the mice exceeded 50% within two weeks. In contrast, the survival rate of mice infected by T6SS or yezP-deficient mutant strains increased significantly [42]. This means that the Y. pseudotuberculosis mutant lacking T6SS or yezP has defective virulence in the process of infection in mice. T6SS is involved in the transportation of zinc ions and is essential to increase the survival rate of bacteria during host infection [42]. Compared with the wild-type Y. pseudotuberculosis strains, the strains lacking hpaR showed lower level of virulence when it infects mice, which further confirmed that HpaR helps Y. pseudotuberculosis to coordinate stress response and adapt to the host environment [43]. Therefore, HpaR positively regulates T6SS to modulate the antioxidant activity of Y. pseudotuberculosis, and the uptake of divalent zinc ion via a yet unknown mechanism against oxidative stress [43].
Manganese (Mn2+) is another important micronutrient transition metal involved in many biochemical processes, especially in the process of resisting oxidative stress [44,46]. Mn2+ can be used as a cofactor or as a substitute of iron in certain iron-containing enzymes that reduce the ROS damage to bacteria [44,49,52,53] [52][53]. In mammalian hosts, Mn2+ are strictly restricted by the mechanism of nutritional immunity [54,55][54][55]. Bacteria also have several strategies to acquire manganese, including ATP-binding cassette family transporter [56], Mn2+-selective channel protein [57], and natural resistance-associated macrophage protein family transporter [58,59][58][59]. Recently, Si and colleagues reported that T6SS helps cells acquire manganese under oxidative stress in B. thailandensis [40]. Compared with the wild-type strain, the survival rate of B. thailandensis T6SS clpV4 mutant strains under oxidative stress was decreased significantly in manganese-rich environment [40]. This difference in survival rate is regulated by OxyR (a conservative regulator of oxidative stress) via an Mn2+- binding T6SS effector [40,42]. OxyR is known as an oxidative stress regulator that controls the expression of many important genes to resist oxidative stress, such as ahpCF, ccpA, dps, goRA, grxA, katG, and oxyS [60–64][60][61][62][63][64]. Under oxidative stress condition, B. thailandensis T6SS secretes TseM (Mn2+-binding effector) (Figure 2B), which binds Mn2+ to promote its uptake through MnoT (a TonB-dependent outer membrane transporter). Facing the oxidative stress from cumene hydroperoxide, compared with clpV4 mutant strains, wild-type strains exhibit higher survival rates, higher concentration of Mn2+ and lower intracellular ROS levels [40]. Thus, T6SS-mediated manganese uptake alleviates the attack from ROS, which significantly improves the survival rate of B. thailandensis under oxidative stress [40].
Figure 2. The model of T6SS-mediated stress response to environmental pressure. (A) B. thailandensis T6SS effector TseZ as a proteinaceous zincophore interacts with heme transporter HmuR to acquire zinc, which facilitates Zn2+ transportation to resist oxidative stress. HmuR forms intramolecular disulfide bond to transport zinc instead of heme under oxidative stress. (B) B. thailandensis T6SS effector TseM transports Mn2+ to resist oxidative stress with the help of outer membrane transporter MnoT. TseM is secreted into the extracellular milieu to scavenging Mn2+ and deliver it to MnoT when bacteria encounter oxidative stress.
3. Adaptation to Changes in Temperature and pH
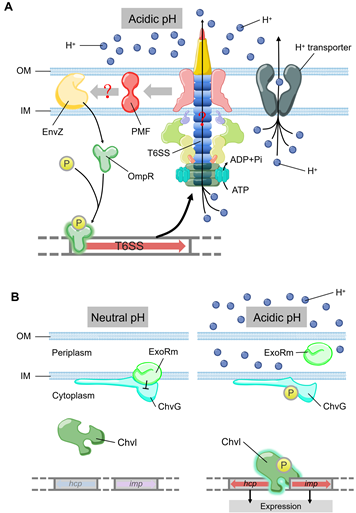
Low pH is a factor produced by the host’s defense mechanism when it is infected, which can limit the growth of pathogens. T6SS also plays a role in bacterial stress response towards low intracellular pH, an environmental stress encountered frequently by bacteria during phagocytosis. OmpR is a well characterized regulator from the EnvZ/OmpR two-component regulatory system, which regulates the expression of genes in response to changes in the osmolarity and pH [65]. A recent study showed that Y. pseudotuberculosis OmpR directly binds to the promoter of T6SS to regulate its expression [66,67][66][67] (Figure 3A). The survival rate of T6SS-deficient strains is significantly reduced under acidic conditions [67]. Additionally, OmpR-induced T6SS expression in a low pH environment is essential for the survival of Y. pseudotuberculosis [67]. Compared with the wild type, the survival rate of Y. pseudotuberculosis ompR mutant in acidic environment was significantly reduced, which was confirmed by measuring colony-forming unit (CFU) after growing for two hours in pH 4.0 buffer [67] [67]. In Y. pseudotuberculosis, ClpV4 may uses its ATPase activity to pump hydrogen ions to extracellular matrix to maintain intracellular pH homeostasis. This could be a general strategy for pathogens to resist acidic environments encountered in phagosomes [67]. In Agrobacterium tumefaciens, the ExoR-ChvG/ChvI cascade can activate T6SS in acidic conditions [68] (Figure 3B). ExoR associates with ChvG (transmembrane sensor kinase) in neutral pH environment, which inhibits the ChvG/ChvI two-component system signaling by physical interaction, resulting in decreased expression of T6SS and secretion activity [68]. At acidic pH, ExoR no longer inhibits the activity of ChvG, which phosphorylates the response regulator ChvI and thus positively regulates the expression of T6SS [68]. In α-Proteobacteria (including many symbionts and plant or animal pathogens), it may be a common phenomenon that ExoR-ChvG/ChvI cascade system regulates T6SS due to the extensive distribution and highly conservative nature of ExoR and ChvG/ChvI in these bacteria.
Figure 3. (A) The model of T6SS-mediated acid resistance in Y. pseudotuberculosis. EnvZ receives the acidic pH signal to promote the phosphorylation of OmpR, which activates the expression of T6SS. T6SS maintains the pH homeostasis by H+ extrusion via the ATPase activity of ClpV4. (B) Acidic pH can activate T6SS expression by ExoR-ChvG/ChvI. ExoR associates with ChvG and inhibites the activity of ChvG/Chvl two-component system in neutral pH, which reduces the expression and secretion of T6SS. In acidic pH, ExoR degrades from ChvG sensor kinase and then phosphorylates ChvI, which facilitates the expression of imp and hcp. The color of arrows represents the level of expression. Blue, no expression. Pink, low expression. Red, normal expression.
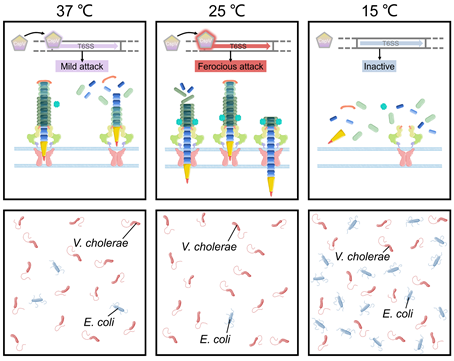
v. cholerae undergoes temperature fluctuations in its natural aquatic habitat and during the course of infection. Temperature is a key signal to control the outbreak of cholera. Recent studies showed that bacterial pathogens could employ T6SS to facilitate adaptation to temperature changes. For example, temperature is a vital environmental signal to regulate V. cholerae physiology and has a huge influence on its survival status and pathogenicity [69]. The V. cholerae cold shock protein CspV was shown to regulate T6SS and mediate interspecies killing when temperature is changed [69]. In the aquatic crustacean Daphnia magna infection model, CspV-deficient V. cholerae strains showed significantly reduced virulence that is mediated by T6SS [69]. The expression of V. cholerae T6SS-related genes such as hcp, which encodes a hemolysin-coregulated protein, was down-regulated at 15 °C and up-regulated at 25 °C [69,70][69][70]. Moreover, using E. coli as a prey strain in mixed culture with V. cholerae showed that the number of colony-forming unit of E. coli decreased by 5-fold at 37 °C, and by 16-fold at 25 °C, without significant change at 15 °C. During this competition process, expression of CspV was essential for V. cholerae to kill E. coli through T6SS [69] (Figure 4). Temperatures of 15°C and 25 °C simulated the living conditions of V. cholerae in the environment and 37 °C simulated the living environment in the human body [69]. Transcriptomic analysis showed that V. cholerae T6SS expression varied at different stages of the infection process when it invades the host and enters the environment from the host [69]. V. cholerae can respond to changes in temperature, thereby changing the ability to form biofilms and activate T6SS [69]. These processes in turn affect bacterial survival and pathogenicity. Temperature changes may be the key signal for V. cholerae to distinguish the host from the environment and to promote the expression of genes essential to survive in different environments. This is important to understanding the mechanism of pathogen survival in the host. In the continuation of the infection cycle, how to re-adapt to the environment after leaving the human body is also an important life process [69,71,72][71][72].
Figure 4. T6SS-mediated temperature adaptation and competition. The expression of T6SS-related genes in V. cholerae is regulated by temperature. V. cholerae showed significantly different virulence at different temperatures.
4. Interspecies and Intraspecies Competition
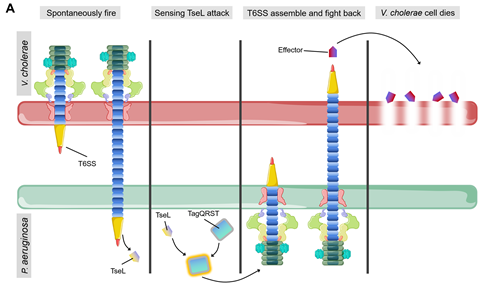
Bacteria can sense attack from T6SS of neighboring cells and activate their own stress response towards them [73] [73]. One of the most well characterized examples is that P. aeruginosa rapidly activates its own T6SS to fight against V. cholerae after being attacked by TseL (T6SS effector with phospholipase activity) from V. cholerae [26,74][74] (Figure 5A). Kamal and colleague suggested that TseL can activate the expression and assembly of P. aeruginosa T6SS to evoke a retaliatory response to V. cholerae [74]. Under T6SS attack from neighboring cells, P. aeruginosa activates its immunity-independent stress response pathways for self-protection [74]. On the other hand, the regulation cascade of TagQRST-PpkA-PppA-Fha1 can sense attack from V. cholerae T6SS [26,74–76][74][75][76]. TseL can be sensed by TagQRST which in turn induces P. aeruginosa retaliation against V. cholerae [26,74][74]. The stress response system of P. aeruginosa coordinates a strong defense against the effectors of V. cholerae and contributes to its survival during bacterial competition [74]. This novel type of immunity-independent stress response is similar to the innate immunity in bacteria [74].
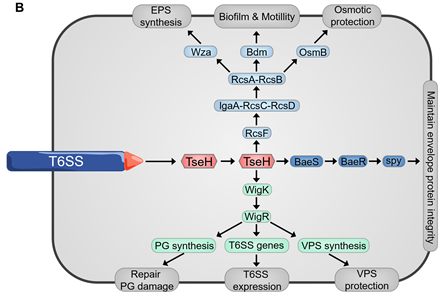
Since many T6SS systems attack bacterial cell wall, envelope stress response can often be evoked by bacteria to defend against these attacks [77–79][77][78][79] (Figure 5B). Envelope stress response maintains the membrane integrity to resist the damage induced by the effectors [74,79]. The V. cholerae TseH, a PAAR-dependent species-specific killing T6SS effector, was shown to damage the cell wall and activate two envelope stress response pathways, Rcs phosphorelay and BaeSR two-component system, of E. coli [77–79][77][78][79]. Rcs helps E. coli to defend against TseH-mediated attacks by activating the expression of osmotic-response genes and increasing the production of exopolysaccharides (EPS) [79]. Moreover, Rcs induces capsule synthesis and the formation of mucoid colonies, where the colanic acid capsule significantly increases E. coli resistance to the attack from T6SS [79,80][80]. V. cholerae employs a two-component system WigKR (VxrAB), to respond to cell wall damage and induces the expression of peptidoglycan-repair genes to protect itself from self-killing, as a type of immune-independent Gene defense [79,81,82] [81][82]. The wild-type V. cholerae strain can efficiently kill the wigKR mutant by delivering TseH [79]. WigKR positively regulates the formation of biofilms and the synthesis of Vibrio polysaccharide (VPS) [83,84][83][84]. Interestingly, the presence of vpsA has no impact on the sensitivity of wigR mutant to its own T6SS, which demonstrates that the protection from WigKR on bacteria is not due to the regulation of VPS but due to superseding VPS [79].
Figure 5. (A) Spontaneous firing from V. cholerae T6SS and retaliatory attack from P. aeruginosa. TagQRST cascade senses T6SS-delivered TseL and induces a counterstrike in P. aeruginosa. (B) After sensing the effector attack of T6SS, E. coli and V. cholerae induce envelope stress response through Rcs phosphorelay and BaeSR (blue) or WigKR (green). The induced genes produce protective effects in different ways. PG, peptidoglycan. EPS, exopolysaccharides. VPS, Vibrio polysaccharide.
Stress responses are also involved in intra-species competition mediated by T6SS. After two T6SS-expressing Salmonella Typhimurium stains, SL1344 (S1) and ATCC14028 (S2), were mixed and cultured, S1 could sense T6SS of S2 and activate RpoS and SoxRS coordinating stress response, including upregulation of expression of csgD, tolC, and hilA genes [73]. Interestingly, when S1 was co-cultured with S2 mutant lacking ClpV (an essential component of T6SS function), the expression of csgD, tolC, and hilA of S1 was no longer up-regulated [73]. CsgD, tolC, and hilA genes are involved in biofilm matrix production, chemical efflux, antibiotic tolerance, and epithelial invasion [73,85–89][85][86][87][88][89]. Similarly, V. cholerae stains with the same T6SS effector module sets can coexist and be compatible, while strains lacking cognate immunity genes are incompatible and outcompeted [90]. Toxigenic V. cholerae strains carry the AAA effector/immunity module [90], which provides the most effective killing of non-kin V. cholerae strains [91]. Interestingly, the predator V. cholerae strains incorporate the extracellular prey DNA released from the bacteria killed by T6SS. Therefore, Kostiuk and colleagues proposed a model: V. cholerae can exchange genetic information including T6SS effector modules during competition in the environment and generate a diverse genotypic pool with members of the same species, similar to genetic card reshuffling [91].
5. Involvement of T6SS in the Regulation of Host Immune Signaling Pathways
Inflammasome activation is a crucial defense mechanism of innate immune response, which is used by the host to fight against invading bacteria [92,93][92][93]. The host can trigger pyroptosiss by releasing pro-inflammatory cytokines during the activation of inflammasomes [94,95][94][95]. Inflammatory cytoplasmic content is released into extracellular environment after pyroptosis of myeloid cells, which further activates innate immune response and accelerates the clearance of bacteria in vivo [93,96][96]. Edwardsiella tarda can significantly inhibit the formation of NLRP3 inflammasome via T6SS [97]. EvpP (T6SS effector of Edwardsiella tarda) can inhibit Ca2+-dependent c-Jun N-terminal kinase (Jnk, a stress-responsive MAPK signaling pathway) activation, which results in the failure of ASC oligomerization and ultimately inhibits the activation of NLRP3 inflammasome [97]. The inhibition of EvpP-mediated NLRP3 inflammasome notably promoted bacterial colonization in vivo [97]. This is a mechanism adopted by pathogenic bacteria to block host signal transduction and evade from the innate immune response of the host [97].
The highly motile phagocytic cells, neutrophils and macrophages, can be recruited to the sites of tissue infection as the first-line of defence [98]. Morpholino can be used to further analyze the formation of inflammasome in neutrophils recruitment in zebrafish [99]. Neutrophils and macrophages are necessary for zebrafish to combat E. piscicida proliferation and infection. After using caspy- or IL-1β-morpholino knockdown larvae to suppress the development of neutrophils and macrophages in zebrafish larvae and comparing with that of control zebrafish, the survival rate of zebrafish larvae decreased significantly when it was infected by E. piscicida [99]. The T6SS effector EvpP inhibits the recruitment of neutrophils to promote E. piscicida proliferation and infection [99]. Furthermore, it is EvpP inhibiting the phosphorylation of Jnk-MAPK signal pathway that leads to the inhibition of neutrophils recruitment since the MAPK signal cascade can regulate the activation of gene transcription of a variety of proinflammatory and chemokines [99–101][99][100][101]. EvpP regulates the expression of ligand 8 (cxcl8a, also known as IL-8) and matrix metallopeptidase 13 (mmp13) by inhibiting the Jnk-MAPK signal cascade [99]. In zebrafish, cxcl8 is the most effective chemokine which guides neutrophils through the tissue matrix to reach the site of injury or infection [102]. Mmp13 also plays an important role in the recruitment of neutrophils [101]. The Jnk-MAPK signaling pathway plays an important role in E. piscicida clearance, neutrophil migration and downstream inflammasome activation in zebrafish [99]. EvpP inhibits the activation of the Jnk-caspy inflammasome pathway in zebrafish larvae, which in turn inhibits the recruitment of neutrophils and highlights the key role of myeloid cells in coping with E. piscicida infection in zebrafish [99].
Hemolysin-coregulated protein (Hcp) of Salmonella enteritidis is a structural component of the T6SS forming the inner tube which is critical to the secretion of effector proteins and the assembly of T6SS apparatus [103,104] [103][104]. Subcellular localization of Hcp in the cytoplasm was discovered by expressing plasmid carrying pEGFP-N1-hcp in BHK-21 cells, where Hcp regulates the gene expression along the TNF signaling pathway [105].
References
- Economou, A.; Christie, P.J.; Fernandez, R.C.; Palmer, T.; Plano, G.V.; Pugsley, A.P. Secretion by numbers: Protein traffic in prokaryotes. Mol. Microbiol. 2006, 62, 308–319.
- Green, E.R.; Mecsas, J. Bacterial Secretion Systems: An Overview. Microbiol. Spectr. 2016, 4, doi:10.1128/microbiolspec.vmbf-0012-2015.
- Lazzaro, M.; Feldman, M.F.; García Véscovi, E. A Transcriptional Regulatory Mechanism Finely Tunes the Firing of Type VI Secretion System in Response to Bacterial Enemies. mBio 2017, 8, e00559-17.
- Gorasia, D.G.; Hanssen, E.; Veith, P.D.; Reynolds, E.C. Structural Characterization of the Type IX Secretion System in Porphyromonas gingivalis. Methods Mol. Biol. 2021, 2210, 113–121.
- Leiman, P.G.; Basler, M.; Ramagopal, U.A.; Bonanno, J.B.; Sauder, J.M.; Pukatzki, S.; Burley, S.K.; Almo, S.C.; Mekalanos, J.J. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc. Natl. Acad. Sci. USA 2009, 106, 4154–4159.
- Bönemann, G.; Pietrosiuk, A.; Mogk, A. Tubules and donuts: A type VI secretion story. Mol. Microbiol. 2010, 76, 815–821.
- Cascales, E. Microbiology: And Amoebophilus Invented the Machine Gun. Curr. Biol. 2017, 27, R1170–R1173.
- Cherrak, Y.; Flaugnatti, N.; Durand, E.; Journet, L.; Cascales, E. Structure and Activity of the Type VI Secretion System. Microbiol. Spectr. 2019, 7, doi:10.1128/9781683670285.ch26
- Bönemann, G.; Pietrosiuk, A.; Diemand, A.; Zentgraf, H.; Mogk, A. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. Embo J. 2009, 28, 315–325.
- Pietrosiuk, A.; Lenherr, E.D.; Falk, S.; Bönemann, G.; Kopp, J.; Zentgraf, H.; Sinning, I.; Mogk, A. Molecular basis for the unique role of the AAA+ chaperone ClpV in type VI protein secretion. J. Biol. Chem. 2011, 286, 30010–30021.
- Basler, M.; Mekalanos, J.J. Type 6 secretion dynamics within and between bacterial cells. Science 2012, 337, 815.
- Renault, M.G.; Zamarreno Beas, J.; Douzi, B.; Chabalier, M.; Zoued, A.; Brunet, Y.R.; Cambillau, C.; Journet, L.; Cascales, E. The gp27-like Hub of VgrG Serves as Adaptor to Promote Hcp Tube Assembly. J. Mol. Biol. 2018, 430, 3143–3156.
- Bladergroen, M.R.; Badelt, K.; Spaink, H.P. Infection-blocking genes of a symbiotic Rhizobium leguminosarum strain that are involved in temperature-dependent protein secretion. Mol. Plant Microbe Interact. 2003, 16, 53–64.
- Filloux, A.; Hachani, A.; Bleves, S. The bacterial type VI secretion machine: Yet another player for protein transport across membranes. Microbiology (Reading) 2008, 154, 1570–1583.
- Boyer, F.; Fichant, G.; Berthod, J.; Vandenbrouck, Y.; Attree, I. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: What can be learned from available microbial genomic resources? BMC Genom. 2009, 10, 104.
- Journet, L.; Cascales, E. The type VI secretion system in Escherichia coli and related species. EcoSal Plus 2016, 7, 1–10.
- Schell, M.A.; Ulrich, R.L.; Ribot, W.J.; Brueggemann, E.E.; Hines, H.B.; Chen, D.; Lipscomb, L.; Kim, H.S.; Mrázek, J.; Nierman, W.C.; et al. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol. Microbiol. 2007, 64, 1466–1485.
- Nano, F.E.; Schmerk, C. The Francisella pathogenicity island. Ann. N. Y. Acad. Sci. 2007, 1105, 122–137.
- Fu, Y.; Waldor, M.K.; Mekalanos, J.J. Tn-Seq analysis of Vibrio cholerae intestinal colonization reveals a role for T6SS-mediated antibacterial activity in the host. Cell Host Microbe 2013, 14, 652–663.
- Li, J.; Yao, Y.; Xu, H.H.; Hao, L.; Deng, Z.; Rajakumar, K.; Ou, H.Y. SecReT6: A web-based resource for type VI secretion systems found in bacteria. Environ. Microbiol. 2015, 17, 2196–2202.
- Abby, S.S.; Cury, J.; Guglielmini, J.; Néron, B.; Touchon, M.; Rocha, E.P. Identification of protein secretion systems in bacterial genomes. Sci. Rep. 2016, 6, 23080.
- Pukatzki, S.; Ma, A.T.; Revel, A.T.; Sturtevant, D.; Mekalanos, J.J. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. USA 2007, 104, 15508–15513.
- English, G.; Trunk, K.; Rao, V.A.; Srikannathasan, V.; Hunter, W.N.; Coulthurst, S.J. New secreted toxins and immunity proteins encoded within the Type VI secretion system gene cluster of Serratia marcescens. Mol. Microbiol. 2012, 86, 921–936.
- Murdoch, S.L.; Trunk, K.; English, G.; Fritsch, M.J.; Pourkarimi, E.; Coulthurst, S.J. The opportunistic pathogen Serratia marcescens utilizes type VI secretion to target bacterial competitors. J. Bacteriol. 2011, 193, 6057–6069.
- Fu, Y.; Ho, B.T.; Mekalanos, J.J. Tracking Vibrio cholerae Cell-Cell Interactions during Infection Reveals Bacterial Population Dynamics within Intestinal Microenvironments. Cell Host Microbe 2018, 23, 274–281.e2.
- Basler, M.; Ho, B.T.; Mekalanos, J.J. Tit-for-tat: Type VI secretion system counterattack during bacterial cell-cell interactions. Cell 2013, 152, 884–894.
- Russell, A.B.; Hood, R.D.; Bui, N.K.; LeRoux, M.; Vollmer, W.; Mougous, J.D. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 2011, 475, 343–347.
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17.
- Imlay, J.A. Where in the world do bacteria experience oxidative stress? Environ. Microbiol. 2019, 21, 521–530.
- Kashyap, D.R.; Rompca, A.; Gaballa, A.; Helmann, J.D.; Chan, J.; Chang, C.J.; Hozo, I.; Gupta, D.; Dziarski, R. Peptidoglycan recognition proteins kill bacteria by inducing oxidative, thiol, and metal stress. PLoS Pathog. 2014, 10, e1004280.
- Dwyer, D.J.; Collins, J.J.; Walker, G.C. Unraveling the physiological complexities of antibiotic lethality. Annu. Rev. Pharmacol. toxicol. 2015, 55, 313–332.
- Dong, T.G.; Dong, S.; Catalano, C.; Moore, R.; Liang, X.; Mekalanos, J.J. Generation of reactive oxygen species by lethal attacks from competing microbes. Proc. Natl. Acad. Sci. USA 2015, 112, 2181–2186.
- D'Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell. Biol. 2007, 8, 813–824.
- Chiang, S.M.; Schellhorn, H.E. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch. Biochem. Biophys. 2012, 525, 161–169.
- Seo, S.W.; Kim, D.; Szubin, R.; Palsson, B.O. Genome-wide Reconstruction of OxyR and SoxRS Transcriptional Regulatory Networks under Oxidative Stress in Escherichia coli K-12 MG1655. Cell Rep. 2015, 12, 1289–1299.
- Wang, T.; Chen, K.; Gao, F.; Kang, Y.; Chaudhry, M.T.; Wang, Z.; Wang, Y.; Shen, X. ZntR positively regulates T6SS4 expression in Yersinia pseudotuberculosis. J. Microbiol. 2017, 55, 448–456.
- Si, M.; Zhang, L.; Chaudhry, M.T.; Ding, W.; Xu, Y.; Chen, C.; Akbar, A.; Shen, X.; Liu, S.J. Corynebacterium glutamicum methionine sulfoxide reductase A uses both mycoredoxin and thioredoxin for regeneration and oxidative stress resistance. Appl. Environ. Microbiol. 2015, 81, 2781–2796.
- Staerck, C.; Gastebois, A.; Vandeputte, P.; Calenda, A.; Larcher, G.; Gillmann, L.; Papon, N.; Bouchara, J.P.; Fleury, M. Microbial antioxidant defense enzymes. Microb. Pathog. 2017, 110, 56–65.
- Weber, B.; Hasic, M.; Chen, C.; Wai, S.N.; Milton, D.L. Type VI secretion modulates quorum sensing and stress response in Vibrio anguillarum. Environ. Microbiol. 2009, 11, 3018–3028.
- Si, M.; Zhao, C.; Burkinshaw, B.; Zhang, B.; Wei, D.; Wang, Y.; Dong, T.G.; Shen, X. Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc. Natl. Acad. Sci. USA 2017, 114, E2233–E2242.
- Wan, B.; Zhang, Q.; Ni, J.; Li, S.; Wen, D.; Li, J.; Xiao, H.; He, P.; Ou, H.Y.; Tao, J.; et al. Type VI secretion system contributes to Enterohemorrhagic Escherichia coli virulence by secreting catalase against host reactive oxygen species (ROS). PLoS Pathog. 2017, 13, e1006246.
- Wang, T.; Si, M.; Song, Y.; Zhu, W.; Gao, F.; Wang, Y.; Zhang, L.; Zhang, W.; Wei, G.; Luo, Z.Q.; et al. Type VI Secretion System Transports Zn2+ to Combat Multiple Stresses and Host Immunity. PLoS Pathog. 2015, 11, e1005020.
- Wang, Z.; Wang, T.; Cui, R.; Zhang, Z.; Chen, K.; Li, M.; Hua, Y.; Gu, H.; Xu, L.; Wang, Y.; et al. HpaR, the Repressor of Aromatic Compound Metabolism, Positively Regulates the Expression of T6SS4 to Resist Oxidative Stress in Yersinia pseudotuberculosis. Front. Microbiol. 2020, 11, 705.
- Aguirre, J.D.; Culotta, V.C. Battles with iron: Manganese in oxidative stress protection. J. Biol. Chem. 2012, 287, 13541–13548.
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759.
- Lisher, J.P.; Giedroc, D.P. Manganese acquisition and homeostasis at the host-pathogen interface. Front. Cell. Infect. Microbiol. 2013, 3, 91.
- Vanaporn, M.; Wand, M.; Michell, S.L.; Sarkar-Tyson, M.; Ireland, P.; Goldman, S.; Kewcharoenwong, C.; Rinchai, D.; Lertmemongkolchai, G.; Titball, R.W. Superoxide dismutase C is required for intracellular survival and virulence of Burkholderia pseudomallei. Microbiology 2011, 157, 2392–2400.
- Si, M.; Wang, Y.; Zhang, B.; Zhao, C.; Kang, Y.; Bai, H.; Wei, D.; Zhu, L.; Zhang, L.; Dong, T.G.; et al. The Type VI Secretion System Engages a Redox-Regulated Dual-Functional Heme Transporter for Zinc Acquisition. Cell Rep. 2017, 20, 949–959.
- DeShazer, D. A novel contact-independent T6SS that maintains redox homeostasis via Zn2+ and Mn2+ acquisition is conserved in the Burkholderia pseudomallei complex. Microbiol. Res. 2019, 226, 48–54.
- Alekshun, M.N.; Levy, S.B.; Mealy, T.R.; Seaton, B.A.; Head, J.F. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 A resolution. Nat. Struct. Biol. 2001, 8, 710–714.
- Grove, A. MarR family transcription factors. Curr. Biol. 2013, 23, R142–R143.
- Barnese, K.; Gralla, E.B.; Valentine, J.S.; Cabelli, D.E. Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds. Proc. Natl. Acad. Sci. USA 2012, 109, 6892–6897.
- Puri, S.; Hohle, T.H.; O'Brian, M.R. Control of bacterial iron homeostasis by manganese. Proc. Natl. Acad. Sci. USA 2010, 107, 10691–10695.
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537.
- Diaz-Ochoa, V.E.; Lam, D.; Lee, C.S.; Klaus, S.; Behnsen, J.; Liu, J.Z.; Chim, N.; Nuccio, S.P.; Rathi, S.G.; Mastroianni, J.R.; et al. Salmonella Mitigates Oxidative Stress and Thrives in the Inflamed Gut by Evading Calprotectin-Mediated Manganese Sequestration. Cell Host Microbe 2016, 19, 814–825.
- Sabri, M.; Caza, M.; Proulx, J.; Lymberopoulos, M.H.; Brée, A.; Moulin-Schouleur, M.; Curtiss, R, 3rd; Dozois, C.M. Contribution of the SitABCD, MntH, and FeoB metal transporters to the virulence of avian pathogenic Escherichia coli O78 strain chi7122. Infect. Immun. 2008, 76, 601–611.
- Hohle, T.H.; Franck, W.L.; Stacey, G.; O’Brian, M.R. Bacterial outer membrane channel for divalent metal ion acquisition. Proc. Natl. Acad. Sci. USA 2011, 108, 15390–15395.
- Champion, O.L.; Karlyshev, A.; Cooper, I.; Ford, D.C.; Wren, B.W.; Duffield, M.; Oyston, P.; Titball, R.W. Yersinia pseudotuberculosis mntH functions in intracellular manganese accumulation, which is essential for virulence and survival in cells expressing functional Nramp1. Microbiology (Reading) 2011, 157, 1115–1122.
- Kehres, D.G.; Zaharik, M.L.; Finlay, B.B.; Maguire, M.E. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 2000, 36, 1085–1100.
- Storz, G.; Altuvia, S. (1994). OxyR regulon. Methods Enzymol. 1994, 234, 217–223.
- Altuvia, S.; Weinstein-Fischer, D.; Zhang, A.; Postow, L.; Storz, G. A small, stable RNA induced by oxidative stress: Role as a pleiotropic regulator and antimutator. Cell 1997, 90, 43–53.
- Michán, C.; Manchado, M.; Dorado, G.; Pueyo, C. In vivo transcription of the Escherichia coli oxyR regulon as a function of growth phase and in response to oxidative stress. J. Bacteriol. 1999, 181, 2759–2764.
- Zheng, M.; Wang, X.; Doan, B.; Lewis, K.A.; Schneider, T.D.; Storz, G. Computation-directed identification of OxyR DNA binding sites in Escherichia coli. J. Bacteriol. 2001, 183, 4571–4579.
- Khademian, M.; Imlay, J.A. Escherichia coli cytochrome c peroxidase is a respiratory oxidase that enables the use of hydrogen peroxide as a terminal electron acceptor. Proc. Natl. Acad. Sci. USA 2017, 114, E6922–E6931.
- Gerken, H.; Charlson, E.S.; Cicirelli, E.M.; Kenney, L.J.; Misra, R. MzrA: A novel modulator of the EnvZ/OmpR two-component regulon. Mol. Microbiol. 2009, 72, 1408–1422.
- Flamez, C.; Ricard, I.; Arafah, S.; Simonet, M.; Marceau, M. Phenotypic analysis of Yersinia pseudotuberculosis 32777 response regulator mutants: New insights into two-component system regulon plasticity in bacteria. Int. J. Med. Microbiol. 2008, 298, 193–207.
- Zhang, W.; Wang, Y.; Song, Y.; Wang, T.; Xu, S.; Peng, Z.; Lin, X.; Zhang, L.; Shen, X. A type VI secretion system regulated by OmpR in Yersinia pseudotuberculosis functions to maintain intracellular pH homeostasis. Environ. Microbiol. 2013, 15, 557–569.
- Wu, C.F.; Lin, J.S.; Shaw, G.C.; Lai, E.M. Acid-induced type VI secretion system is regulated by ExoR-ChvG/ChvI signaling cascade in Agrobacterium tumefaciens. PLoS Pathog. 2012, 8, e1002938.
- Townsley, L.; Sison Mangus, M.P.; Mehic, S.; Yildiz, F.H. Response of Vibrio cholerae to Low-Temperature Shifts: CspV Regulation of Type VI Secretion, Biofilm Formation, and Association with Zooplankton. Appl. Environ. Microbiol. 2016, 82, 4441–4452.
- Basler, M.; Pilhofer, M.; Henderson, G.P.; Jensen, G.J.; Mekalanos, J.J. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 2012, 483, 182–186.
- Slamti, L.; Livny, J.; Waldor, M.K. Global gene expression and phenotypic analysis of a Vibrio cholerae rpoH deletion mutant. J. Bacteriol. 2007, 189, 351–362.
- Weber, G.G.; Kortmann, J.; Narberhaus, F.; Klose, K.E. RNA thermometer controls temperature-dependent virulence factor expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2014, 111, 14241–14246.
- Lories, B.; Roberfroid, S.; Dieltjens, L.; De Coster, D.; Foster, K.R.; Steenackers, H.P. Biofilm Bacteria Use Stress Responses to Detect and Respond to Competitors. Curr. Biol. 2020, 30, 1231–1244.e4.
- Kamal, F.; Liang, X.; Manera, K.; Pei, T.T.; Kim, H.; Lam, L.G.; Pun, A.; Hersch, S.J.; Dong, T.G. Differential Cellular Response to Translocated Toxic Effectors and Physical Penetration by the Type VI Secretion System. Cell Rep. 2020, 31, 107766.
- Casabona, M.G.; Silverman, J.M.; Sall, K.M.; Boyer, F.; Couté, Y.; Poirel, J.; Grunwald, D.; Mougous, J.D.; Elsen, S.; Attree, I. An ABC transporter and an outer membrane lipoprotein participate in posttranslational activation of type VI secretion in Pseudomonas aeruginosa. Environ. Microbiol. 2013, 15, 471–486.
- Hsu, F.; Schwarz, S.; Mougous, J.D. TagR promotes PpkA-catalysed type VI secretion activation in Pseudomonas aeruginosa. Mol. Microbiol. 2009, 72, 1111–1125.
- Laubacher, M.E.; Ades, S.E. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J. Bacteriol. 2008, 190, 2065–2074.
- Raffa, R.G.; Raivio, T.L. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 2002, 45, 1599–1611.
- Hersch, S.J.; Watanabe, N.; Stietz, M.S.; Manera, K.; Kamal, F.; Burkinshaw, B.; Lam, L.; Pun, A.; Li, M.; Savchenko, A.; et al. Envelope stress responses defend against type six secretion system attacks independently of immunity proteins. Nat. Microbiol. 2020, 5, 706–714.
- Stout, V.; Torres-Cabassa, A.; Maurizi, M.R.; Gutnick, D.; Gottesman, S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J. Bacteriol. 1991, 173, 1738–1747.
- Weaver, A.I.; Murphy, S.G.; Umans, B.D.; Tallavajhala, S.; Onyekwere, I.; Wittels, S.; Shin, J.H.; VanNieuwenhze, M.; Waldor, M.K.; Dörr, T.; et al. Genetic Determinants of Penicillin Tolerance in Vibrio cholerae. Antimicrob. Agents Chemother. 2018, 62, e01326-18.
- Dörr, T.; Alvarez, L.; Delgado, F.; Davis, B.M.; Cava, F.; Waldor, M.K. A cell wall damage response mediated by a sensor kinase/response regulator pair enables beta-lactam tolerance. Proc. Natl. Acad. Sci. USA 2016, 113, 404–409.
- Teschler, J.K.; Cheng, A.T.; Yildiz, F.H. The Two-Component Signal Transduction System VxrAB Positively Regulates Vibrio cholerae Biofilm Formation. J. Bacteriol. 2017, 199, e00139-17.
- Toska, J.; Ho, B.T.; Mekalanos, J.J. Exopolysaccharide protects Vibrio cholerae from exogenous attacks by the type 6 secretion system. Proc. Natl. Acad. Sci. USA 2018, 115, 7997–8002.
- Hermans, K.; Nguyen, T.L.; Roberfroid, S.; Schoofs, G.; Verhoeven, T.; De Coster, D.; Vanderleyden, J.; De Keersmaecker, S.C. Gene expression analysis of monospecies Salmonella typhimurium biofilms using differential fluorescence induction. J. Microbiol. Methods 2011, 84, 467–478.
- García, B.; Latasa, C.; Solano, C.; García-del Portillo, F.; Gamazo, C.; Lasa, I. Role of the GGDEF protein family in Salmonella cellulose biosynthesis and biofilm formation. Mol. Microbiol. 2004, 54, 264–277.
- Horiyama, T.; Yamaguchi, A.; Nishino, K. TolC dependency of multidrug efflux systems in Salmonella enterica serovar Typhimurium. J. Antimicrob. Chemother. 2010, 65, 1372–1376.
- Zhang, A.; Rosner, J.L.; Martin, R.G. Transcriptional activation by MarA, SoxS and Rob of two tolC promoters using one binding site: A complex promoter configuration for tolC in Escherichia coli. Mol. Microbiol. 2008, 69, 1450–1455.
- Thijs, I.M.; De Keersmaecker, S.C.; Fadda, A.; Engelen, K.; Zhao, H.; McClelland, M.; Marchal, K.; Vanderleyden, J. Delineation of the Salmonella enterica serovar Typhimurium HilA regulon through genome-wide location and transcript analysis. J. Bacteriol. 2007, 189, 4587–4596.
- Unterweger, D.; Miyata, S.T.; Bachmann, V.; Brooks, T.M.; Mullins, T.; Kostiuk, B.; Provenzano, D.; Pukatzki, S. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat. Commun. 2014, 5, 3549.
- Kostiuk, B.; Unterweger, D.; Provenzano, D.; Pukatzki, S. T6SS intraspecific competition orchestrates Vibrio cholerae genotypic diversity. Int. Microbiol. 2017, 20, 130–137.
- Chen, K.W.; Schroder, K. Antimicrobial functions of inflammasomes. Curr. Opin. Microbiol. 2013, 16, 311–318.
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142.
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426.
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832.
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142.
- Chen, H.; Yang, D.; Han, F.; Tan, J.; Zhang, L.; Xiao, J.; Zhang, Y.; Liu, Q. The Bacterial T6SS Effector EvpP Prevents NLRP3 Inflammasome Activation by Inhibiting the Ca2+-Dependent MAPK-Jnk Pathway. Cell Host Microbe 2017, 21, 47–58.
- Li, L.; Yan, B.; Shi, Y.Q.; Zhang, W.Q.; Wen, Z.L. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J. Biol. Chem. 2012, 287, 25353–25360.
- Tan, J.; Yang, D.; Wang, Z.; Zheng, X.; Zhang, Y.; Liu, Q. EvpP inhibits neutrophils recruitment via Jnk-caspy inflammasome signaling in vivo. Fish Shellfish Immunol. 2019, 92, 851–860.
- Walz, A.; Peveri, P.; Aschauer, H.; Baggiolini, M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem. Biophys. Res. Commun. 1987, 149, 755–761.
- Zhang, Y.; Bai, X.T.; Zhu, K.Y.; Jin, Y.; Deng, M.; Le, H.Y.; Fu, Y.F.; Chen, Y.; Zhu, J.; Look, A.T.; et al. In vivo interstitial migration of primitive macrophages mediated by JNK-matrix metalloproteinase 13 signaling in response to acute injury. J. Immunol. 2008, 181, 2155–2164.
- de Oliveira, S.; Boudinot, P.; Calado, Â.; Mulero, V. Duox1-derived H2O2 modulates Cxcl8 expression and neutrophil recruitment via JNK/c-JUN/AP-1 signaling and chromatin modifications. J. Immunol. 2015, 194, 1523–1533.
- Hood, R.D.; Singh, P.; Hsu, F.; Güvener, T.; Carl, M.A.; Trinidad, R.R.; Silverman, J.M.; Ohlson, B.B.; Hicks, K.G.; Plemel, R.L.; et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 2010, 7, 25–37.
- Mougous, J.D.; Cuff, M.E.; Raunser, S.; Shen, A.; Zhou, M.; Gifford, C.A.; Goodman, A.L.; Joachimiak, G.; Ordoñez, C.L.; Lory, S.; et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 2006, 312, 1526–1530.
- Zheng, L.; Wang, S.; Ling, M.; Lv, Z.; Lin, S. Salmonella enteritidis Hcp distribute in the cytoplasm and regulate TNF signaling pathway in BHK-21 cells. 3 Biotech 2020, 10, 301.