Coupling of CO2 with epoxides is a green emerging alternative for the synthesis of cyclic organic carbonates (COC) and aliphatic polycarbonates (APC). The scope of this work is to provide a comprehensive overview of metal complexes having sulfur-containing ligands as homogeneous catalytic systems able to efficiently promote this transformation with a concise discussion of the most significant results. The crucial role of sulfur as the hemilabile ligand and its influence on the catalytic activity are highlighted as well.
- homogeneous catalysis
- sulfur
- carbon dioxide
- epoxides
- cyclic carbonates
- polycarbonates
1. Introduction
Homogeneous catalysis is one of the most fast-developing areas of chemistry because of the possibility to finely tune the course of a given reaction by rational design of the catalyst architecture. Indeed, compared to heterogeneous catalysis, mechanisms governing the activity and selectivity are easier to be understood and therefore the overall performances can be consequentially greatly improved. In particular, the use of soluble transition-metal complexes in catalysis opened unprecedented possibilities to control the product features by a judicious modification of the electronic and steric properties of the ancillary ligand. Inspired by this simple concept, chemists have unfolded countless stereoselective reactions encompassing all families of chemical transformations (oxidations, cross-coupling reactions, polymerizations, etc.) [1][2][3].
Parallel to this trend, in the last two decades, the use of renewable feedstocks has gained momentum because of the growing demand for more sustainable chemical processes. In particular, carbon dioxide utilization (CDU) has emerged as an important tool for the transition to a carbon-neutral society because of the possibility to close, through the use of renewable energies, an anthropogenic carbon cycle avoiding net emission of greenhouse gases. Besides, CO2 displays evident advantages in terms of toxicity, flammability, and the possibility of storage with respect to other C1 feedstocks (such as phosgene and carbon monoxide) that render the implementation of industrial processes based on this molecule quite promising [4][5][6][7][8][9].
Among the possible products obtainable from CO2, cyclic organic carbonates (COCs) and aliphatic polycarbonates (APCs) have received considerable attention because of their wide range of application and straightforward synthetic pathway starting from the corresponding epoxides [10][11][12][13][14][15][16][17][18][19]. The coupling between CO2 and an epoxide can result in the formation of the cyclic product or of the polymeric one depending on many factors such as the nature of the substrate, the reaction conditions, and the catalytic system. Notwithstanding the efforts to develop efficient metal-free catalysts, most of the active systems are based on the combination of a metal complex activated by a suitable nucleophile, often added as ammonium or phosphonium salt. The basic reaction mechanism can be exemplified following the steps depicted in Scheme 1: (i) The coordination of the epoxide to the metal center; (ii) the ring-opening of the activated epoxide by the nucleophilic attack and the formation of the metal-alkoxo bond; (iii) the insertion of CO2. At this stage, the intermediate can give an intramolecular ring-closing with the formation of the COC (path A) or the alternating insertion of epoxide and CO2 with the formation of the APC (path B). Since two consecutive insertions of the epoxide are possible as well (path C), often the resulting polymer also contains some polyether linkages [20].
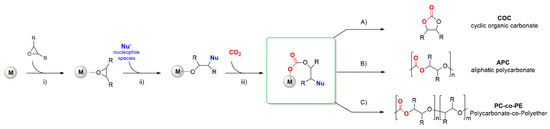
Scheme 1. Mechanism of the coupling of CO2 with epoxides [20].
The metal complexes successfully employed as catalysts in this reaction can be based both on main group metals such as Al, Mg, Ca [21][22][23] and on transition metals such as Ti, Cr, Fe, Co, Nb, La, and Zn [17][18][20][24][25][26][27][28]. Some examples of highly active catalysts for the formation of cyclic carbonates are given in Figure 1 [22][25][29][30].
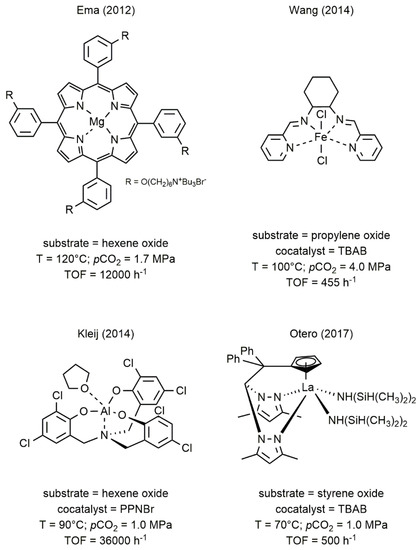
Figure 1. Selected catalytic systems for the synthesis of cyclic organic carbonates (COC) from CO2 and epoxides [22][25][29][30].
2. Metal Complexes Bearing Sulfur-Containing Ligands Active in the Coupling of CO2 and Epoxides
2.1. Chromium-Based Systems
Chromium Salen complexes are among the most studied coordination compounds in catalysis. Indeed, in the last years, their chemistry had been deeply investigated because of their unique properties in the oxidation, even in the enantioselective version, of various substrates [31]. The easy synthesis and the possibility to design chiral metal species sparked the interest in using these complexes also in the CO2/epoxide coupling. In this context, Darensbourg and Nguyen separately reported the use of chromium Salen complexes for the coupling of cyclohexene oxide (CHO) or propylene oxide (PO) with CO2 for the formation of polycyclohexene carbonate (PCHC) and propylene carbonate (PC), respectively (Figure 52) [32][33].
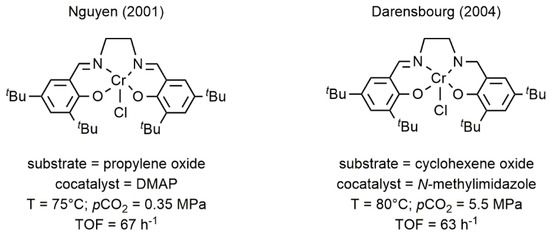
Figure 52. Chromium(III) Salen complexes for the reaction of CO2 with CHO or propylene oxide (PO) [32][33].
After these first reports, many mononuclear and dinuclear chromium Salen complexes have been developed for the copolymerization of epoxides with CO2 allowing the synthesis, with high activity, of new copolymer microstructures (Figure 63) [34][35].
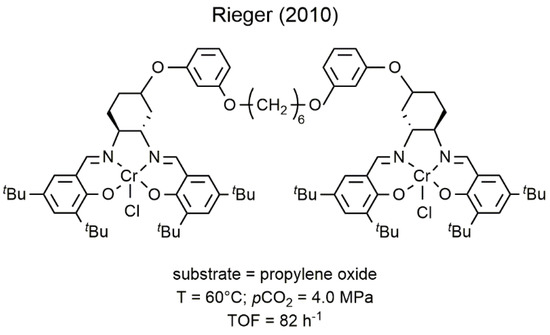
Figure 63. Bimetallic chromium(III) Salen complex developed by Rieger for the coupling of CO2 with PO [34].
In the search of alternative ligands for the CO2/epoxide chemistry, the first example of chromium catalysts bearing a soft donor atom in the ligand skeleton was reported by Duchateau and coworkers only in 2014 and regarded the utilization of two chromium(III) complexes having an aminophosphine bidentate ligand (Figure 74) [36]. These catalysts showed moderate catalytic activity in the copolymerization of CO2 with CHO affording low molecular weight PCHC (Mn up to 1930 g mol−1).
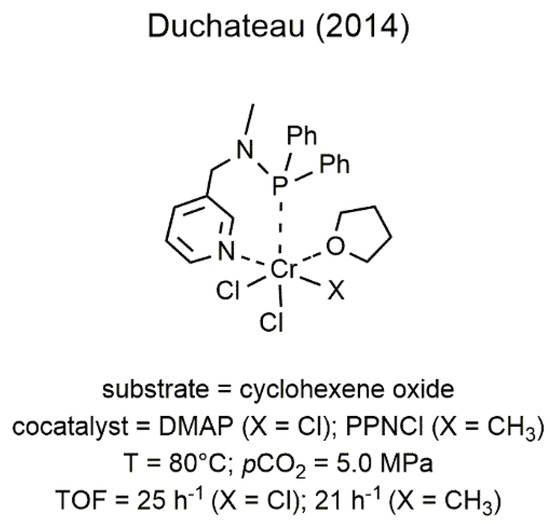
Figure 74. Chromium(III)-Aminophosphine complexes developed by Duchateau for the coupling of CO2 with CHO [36].
In this field, the choice of sulfur as the soft Lewis base is even more recent. In 2015, the tetradentate [OSSO]-type chromium complexes (1–4) were developed by Liu and coworkers for the copolymerization of various epoxides with phthalic anhydride and subsequently used for the copolymerization of CO2 with vinylcyclohexeneoxide (VCHO) (Figure 85) [37].
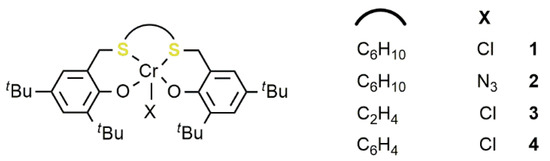
Figure 85. Chromium(III)-[OSSO] catalysts 1–4 [37].
Complexes 1–4, activated by bis(triphenylphosphoranylidene) ammonium chloride (PPNCl) are all active catalysts in the CO2/VCHO coupling by using a [VCHO]/[[OSSO]CrX]/[PPNCl] ratio of 1000/1/2 at 90 °C and high pressure (pCO2 = 3.0 MPa). Activity and chemoselectivity toward the APC vis a vis COC depended on the catalyst structure. Indeed, the complexes 1–3 with a cyclohexyl or an ethyl carbon bridge showed better performances compared to the phenylene analogue 4.
In addition, 4 also produced a low molecular weight polymer, in comparison to the other complexes, albeit with a narrow dispersity (Ð = 1.24–1.49). For the most active catalyst 1, the authors also explored the use of different co-catalysts, such as bis(triphenylphosphoranylidene)ammonium azide, (PPNN3), tetrabutylammonium chloride (TBAC), and 4-dimethylaminopyridine (DMAP) obtaining the best compromise between activity and selectivity toward the polymeric product by using 2 equivalents, with respect to the metal, of PPNCl. Notably, by adjusting the [VCHO]/[1]/[PPNCl] ratio to 500/1/2, the activity reached the value of 134 h−1 with 76% of selectivity for the polymeric product at 90 °C and with a CO2 pressure of 3.0 MPa. 13C NMR analysis showed that the resulting polymer is atactic in all cases. The reaction of terminal epoxides such as PO or epichlorohydrine (EPC) invariably resulted in the formation of the corresponding COC.
2.2. Iron-Based Systems
Despite the abundance of this metal on the Earth-crust, the use of iron in homogeneous catalysis has been longtime neglected and only in the last two decades emerged as a viable alternative to catalysts based on rare/precious metals. In this trend, the development of iron catalysts for the reaction of CO2 with epoxides was not an exception, with only few examples of complexes that showed significant activity in the cycloaddition of CO2 to epoxide or in the alternating copolymerization [18][38][39][40][41]. As for other metals, the structural motifs of choice were based on macrocyclic or polydentate ligands having oxygen and nitrogen as donor atoms. In particular, the complexes developed by Williams and Nozaki [38][39], depicted in Figure 116, were the first examples of iron catalysts able to promote the copolymerization of CO2 with CHO and PO, respectively. The iron complexes developed by Kleij [40][41] (Figure 116), efficiently produced cyclic carbonates from a variety of terminal and internal epoxides.
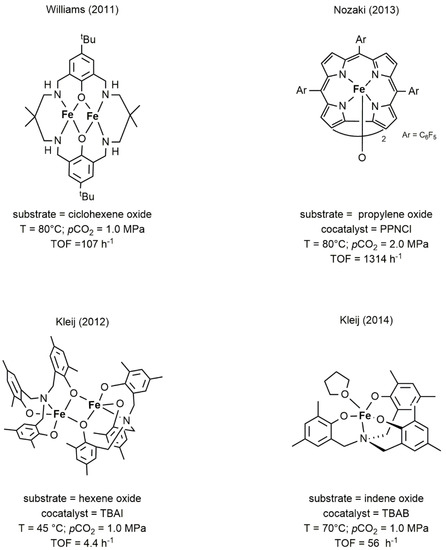
Figure 116. Representative examples of iron-based complexes active in the coupling of CO2 with epoxides [18][38][39][40][41].
The first example of sulfur-containing ligand for iron complexes active in the reaction of CO2 with epoxides was reported by Abu-Surrah and coworkers in 2013 (10, Figure 127) [42]. This thiophenaldimine Fe(II) complex, activated by TBAB, at 130 °C and 0.5 MPa of CO2 pressure, promoted the formation of styrene carbonate (SC) with low activity (TON = 799; TOF = 33.3 h−1).
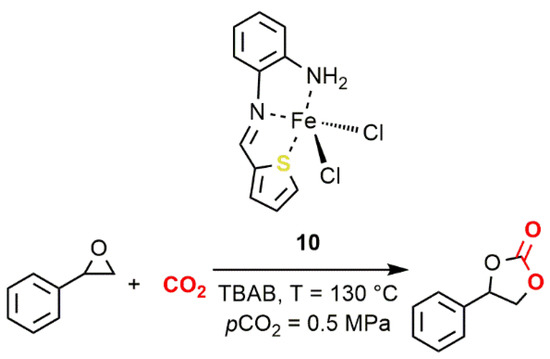
Figure 127. Coupling of styrene oxide (SO) and CO2 promoted by the iron(II) thiophenaldimine complex 10 [42].
In 2015, our group reported on the synthesis of a new air-stable dinuclear iron(III) complex (11, Figure 138) bearing a bis-thioether-triphenolate ligand [43][44][45]. These complexes were stable in solution, and when activated by TBAB (11/TBAB = 1/2), were able to promote the transformation of various terminal or internal epoxides to the corresponding COCs with good activity and selectivity. In particular, PO was converted to PC reaching a TOF of 580 h−1 at 100 °C with a CO2 pressure of 2.0 MPa, and with a [PO]/[11]/[TBAB] ratio of 4000/1/5. Furthermore, this catalyst showed to be also highly stereoselective affording exclusively the cis-COC by using CHO as the substrate regardless the TBAB/[11] ratio. Moreover, by using the enantiopure SO as the epoxide (ee = 94%) the corresponding SC was gained with a good degree of stereoretention (ee = 74%).
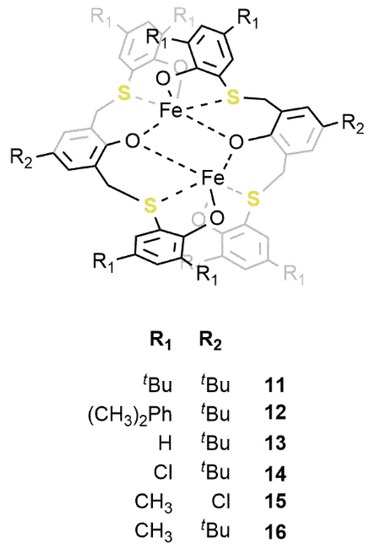
Figure 138. Dinuclear iron(III) complexes 11–16 containing a bis-thioether-triphenolate ligand [43][44].
An enlargement of the catalysts’ family and an optimization of the reaction conditions led to a further improvement of the catalytic activity. In particular, complex 16, bearing methyl substituents on the phenol ring, displayed the highest activity so far reported for an iron catalyst with a TOF of 5200 and 7000 h−1 in the conversion of PO and EPC, respectively, in the corresponding COCs at 120 °C with a CO2 pressure of 2.0 MPa and a [PO]/[16]/[TBAB] ratio of 10000/1/10. Conversely, the introduction of bulkier substituents in catalyst 12 and electron-withdrawing groups in catalyst 14 resulted in a lowering of the catalyst activity.
In order to shed more light on the reaction mechanism, DFT calculations were performed and highlighted three important features regarding the formation of COCs promoted by these catalysts: (i) The coordination of the epoxide to the iron(III) center requires the detachment of a sulfur atom from the coordination sphere; (ii) the insertion of CO2 is not mediated by the second iron center; (iii) the ring-closure of the COCs is the rate-limiting step (Figure 149). As a matter of fact, the hemilability of the sulfur atoms plays a fundamental role in determining the catalytic activity. This was also confirmed by the worse performances of catalyst 14, which were reasonably caused by a stronger sulfur-iron interaction which, in turn, was caused by the presence of electron-withdrawing substituents on the phenoxo rings.
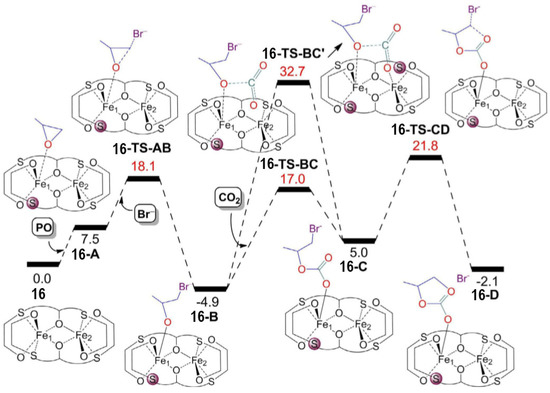
Figure 149. Free energy profile for the coupling of PO and CO2 promoted by complex 16 [44]. Reprinted with permission from ref. 68. Copyright (2020) John Wiley and Sons.
Later on, we moved to the development of iron(III) mononuclear complexes bearing [OSSO]-type ligands 17–20 (Figure 150) [46].
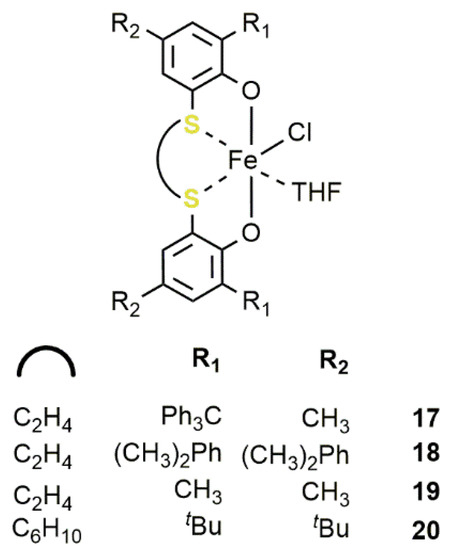
Figure 150. Iron(III)-[OSSO] complexes 17–20 [46].
Notably, when activated by TBAB, these catalysts efficiently performed in the cycloaddition of CO2 to terminal and internal epoxides giving the corresponding COC under mild reaction conditions (35 °C; pCO2 = 0.1 MPa) and, by using catalyst 20, reaching a TOF of 290 h−1 in the case of PO with a [PO]/[20]/[TBAB] ratio of 1000/1/5. Substituents on the ligand framework had a negligible influence on the catalytic activity. In analogy with the bis-thiother-triphenolate complexes 11–16, the reaction occurred with a high degree of stereoretention (98%) in the case of internal epoxides such as 2-buteneoxide. More importantly, when the substrate was CHO, only the alternating PCHC was observed. In this case, the best cocatalyst was TBAC and the activity order was 18 > 20 ≈ 17 > 19.
2.3. Bismuth-Based Systems
Among the possible Lewis acidic metal centers in catalysis, the last two decades have witnessed a rising interest in the use of bismuth. Indeed, bismuth is cheap and low-toxic and therefore the implementation of catalytic processes based on this metal are an active task of research in green chemistry [47][48][49][50].
Actually, the use in CO2/epoxide reactions was barely explored and only recently received more attention [51][52]. In this field, one of the most efficient catalytic systems was developed by Shimada e Yin in 2009 [53]. In this study, the synthesis of Bi(III) compounds 35 and 36, bearing a sulfur bridged bis(phenolato) ligand, was reported (Figure 2011).
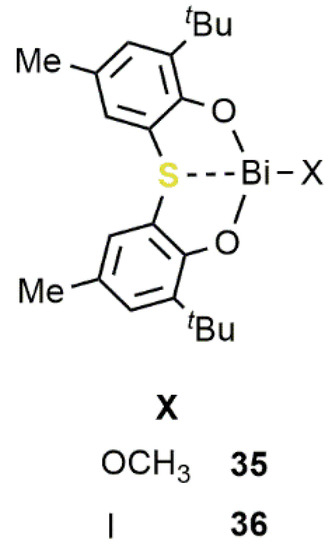
Figure 2011. Bi(III) complexes 35–36 containing an [OSO]-bisphenolate ligand [53].
In the presence of iodide as the nucleophile, by using sources such as Ph3PI, tetrabutylammonium iodide (TBAI), NaI or LiI, these compounds were found to be active catalysts for the conversion of PO to PC under mild reaction conditions (RT; pCO2 = 0.1 MPa; [PO]/[Bi]/[I−] = 820/1/4) reaching a TOF of 34 h−1. More recently, Wong, Au, and coworkers reported that the dinuclear sulfide-bridged complexes 37–40 (Figure 121) performed as active catalysts for the conversion of EPC to the corresponding carbonate at 140 °C and a CO2 pressure of 3.0 MPa ([EPC]/[Bi]/[TBAI] = 200/1/0.2) reaching TOFs up to 25 h−1 [54]. Complexes 37–39 are also among the few air-stable organobismuth compounds reported so far.
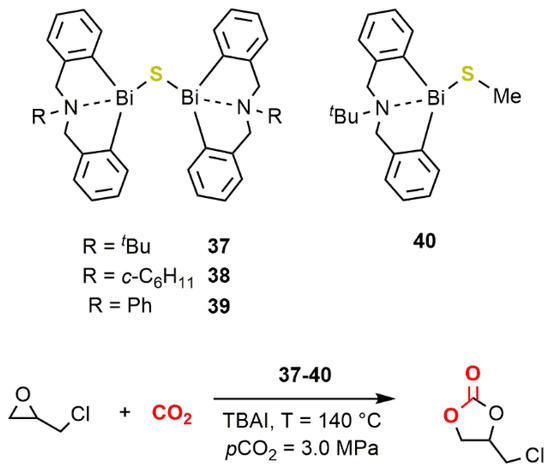
Figure 121. Dinuclear sulfide-bridged complexes 37–40 [54].
3. Conclusions
The use of sulfur-containing ligands to support metal ions in the reaction between CO2 and epoxides has attracted an increasing interest in the past years. In many cases, the presence of sulfur in the ligand backbone imparts unique properties in terms of activity, selectivity, and stability of the catalyst precursor. Furthermore, both cyclic carbonates and polycarbonates were obtained with good activity and selectivity for a wide range of substrates showing a high potential for further development. In particular, the use of these ligands can be extended to a major variety of metal centers, allowing an increase in the catalytic performances and expanding the portfolio of possible products. Finally, the design and the synthesis of new structural motifs containing sulfur atoms in the ligand backbone is another important objective for a possible expansion of the catalytic applications.
References
- Cornils, B.; Herrmann, W.A.; Beller, M.; Paciello, R. (Eds.) Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Four Volumes, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; ISBN 978-3-527-32897-0.
- Bhaduri, S.; Mukesh, D. Homogeneous Catalysis: Mechanisms and Industrial Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2014; ISBN 978-1-118-87251-2.
- van Leeuwen, P.W.N.M. Homogeneous Catalysis: Understanding the Art; Springer Netherlands: Dordrecht, The Netherlands, 2004; ISBN 978-1-4020-3176-2.
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 28, 2975–2992.
- Burkart, M.D.; Hazari, N.; Tway, C.L.; Zeitler, E.L. Opportunities and challenges for catalysis in carbon dioxide utilization. ACS Catal. 2019, 9, 7937–7956.
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799.
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2014, 114, 1709–1742.
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kühn, F.E. Transformation of carbon dioxide with homogeneous transition-metal catalysts: A molecular solution to a global challenge? Angew. Chem. Int. Ed. 2011, 50, 8510–8537.
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97.
- Dai, W.-L.; Luo, S.-L.; Yin, S.-F.; Au, C.-T. The direct transformation of carbon dioxide to organic carbonates over heterogeneous catalysts. Appl. Catal. A Gen. 2009, 366, 2–12.
- Decortes, A.; Castilla, A.M.; Kleij, A.W. Salen-complex-mediated formation of cyclic carbonates by cycloaddition of CO2 to epoxides. Angew. Chem. Int. Ed. 2010, 49, 9822–9837.
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514.
- Darensbourg, D.J.; Wilson, S.J. What’s new with CO2? Recent advances in its copolymerization with oxiranes. Green Chem. 2012, 14, 2665.
- Pescarmona, P.P.; Taherimehr, M. Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2. Catal. Sci. Technol. 2012, 2, 2169.
- Sakakura, T.; Kohno, K. The synthesis of organic carbonates from carbon dioxide. Chem. Commun. 2009, 1312.
- Coates, G.W.; Moore, D.R. Discrete Metal-based catalysts for the copolymerization of CO2 and epoxides: Discovery, reactivity, optimization, and mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618–6639.
- Lu, X.-B.; Darensbourg, D.J. Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem. Soc. Rev. 2012, 41, 1462–1484.
- Della Monica, F.; Buonerba, A.; Capacchione, C. Homogeneous iron catalysts in the reaction of epoxides with carbon dioxide. Adv. Synth. Catal. 2019, 361, 265–282.
- Poland, S.J.; Darensbourg, D.J. A quest for polycarbonates provided via sustainable epoxide/CO2 copolymerization processes. Green Chem. 2017, 19, 4990–5011.
- Della Monica, F.; Kleij, A.W. Mechanistic guidelines in nonreductive conversion of CO2: The case of cyclic carbonates. Catal. Sci. Technol. 2020, 10, 3483–3501.
- Whiteoak, C.J.; Kielland, N.; Laserna, V.; Castro-Gómez, F.; Martin, E.; Escudero-Adán, E.C.; Bo, C.; Kleij, A.W. Highly active aluminium catalysts for the formation of organic carbonates from CO2 and oxiranes. Chem. Eur. J. 2014, 20, 2264–2275.
- Maeda, C.; Miyazaki, Y.; Ema, T. Recent progress in catalytic conversions of carbon dioxide. Catal. Sci. Technol. 2014, 4, 1482.
- Liu, X.; Zhang, S.; Song, Q.-W.; Liu, X.-F.; Ma, R.; He, L.-N. Cooperative calcium-based catalysis with 1,8-diazabicyclo[5.4.0]-undec-7-ene for the cycloaddition of epoxides with CO2 at atmospheric pressure. Green Chem. 2016, 18, 2871–2876.
- Comerford, J.W.; Ingram, I.D.V.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987.
- Martínez, J.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Castro-Osma, J.A.; Lara-Sánchez, A.; Otero, A. An efficient and versatile lanthanum heteroscorpionate catalyst for carbon dioxide fixation into cyclic carbonates. ChemSusChem 2017, 10, 2886–2890.
- Chen, A.; Chen, C.; Xiu, Y.; Liu, X.; Chen, J.; Guo, L.; Zhang, R.; Hou, Z. Niobate salts of organic base catalyzed chemical fixation of carbon dioxide with epoxides to form cyclic carbonates. Green Chem. 2015, 17, 1842–1852.
- Chen, F.; Liu, N.; Dai, B. Iron(II) bis-cnn pincer complex-catalyzed cyclic carbonate synthesis at room temperature. ACS Sustain. Chem. Eng. 2017, 5, 9065–9075.
- Wang, Y.; Qin, Y.; Wang, X.; Wang, F. Coupling reaction between CO2 and cyclohexene oxide: Selective control from cyclic carbonate to polycarbonate by ligand design of salen/salalen titanium complexes. Catal. Sci. Technol. 2014, 4, 3964–3972.
- Sheng, X.; Qiao, L.; Qin, Y.; Wang, X.; Wang, F. Highly efficient and quantitative synthesis of a cyclic carbonate by iron complex catalysts. Polyhedron 2014, 74, 129–133.
- Whiteoak, C.J.; Kielland, N.; Laserna, V.; Escudero-Adán, E.C.; Martin, E.; Kleij, A.W. A powerful aluminum catalyst for the synthesis of highly functional organic carbonates. J. Am. Chem. Soc. 2013, 135, 1228–1231.
- Bellemin-Laponnaz, S.; Dagorne, S. Coordination chemistry and applications of salen, salan and salalen metal complexes. In PATAI’S Chemistry of Functional Groups; Rappoport, Z., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; p. pat0611. ISBN 978-0-470-68253-1.
- Darensbourg, D.J.; Mackiewicz, R.M.; Rodgers, J.L.; Fang, C.C.; Billodeaux, D.R.; Reibenspies, J.H. Cyclohexene Oxide/CO2 copolymerization catalyzed by chromium(iii) salen complexes and n-methylimidazole: Effects of varying salen ligand substituents and relative cocatalyst loading. Inorg. Chem. 2004, 43, 6024–6034.
- Paddock, R.L.; Nguyen, S.T. Chemical CO2 fixation: Cr(III) Salen complexes as highly efficient catalysts for the coupling of CO2 and epoxides. J. Am. Chem. Soc. 2001, 123, 11498–11499.
- Vagin, S.I.; Reichardt, R.; Klaus, S.; Rieger, B. Conformationally flexible dimeric salphen complexes for bifunctional catalysis. J. Am. Chem. Soc. 2010, 132, 14367–14369.
- Wang, Y.; Darensbourg, D.J. Carbon dioxide-based functional polycarbonates: Metal catalyzed copolymerization of CO2 and epoxides. Coord. Chem. Rev. 2018, 372, 85–100.
- Gurnham, J.; Gambarotta, S.; Korobkov, I.; Jasinska-Walc, L.; Duchateau, R. Chromium-catalyzed CO2–epoxide copolymerization. Organometallics 2014, 33, 4401–4409.
- Si, G.; Zhang, L.; Han, B.; Zhang, H.; Li, X.; Liu, B. Chromium complexes containing a tetradentate [OSSO]-type bisphenolate ligand as a novel family of catalysts for the copolymerization of carbon dioxide and 4-vinylcyclohexene oxide. RSC Adv. 2016, 6, 22821–22826.
- Nakano, K.; Kobayashi, K.; Ohkawara, T.; Imoto, H.; Nozaki, K. Copolymerization of epoxides with carbon dioxide catalyzed by iron–corrole complexes: Synthesis of a crystalline copolymer. J. Am. Chem. Soc. 2013, 135, 8456–8459.
- Buchard, A.; Kember, M.R.; Sandeman, K.G.; Williams, C.K. A bimetallic iron(III) catalyst for CO2/epoxide coupling. Chem. Commun. 2011, 47, 212–214.
- Laserna, V.; Fiorani, G.; Whiteoak, C.J.; Martin, E.; Escudero-Adán, E.; Kleij, A.W. Carbon dioxide as a protecting group: Highly efficient and selective catalytic access to cyclic cis-Diol scaffolds. Angew. Chem. Int. Ed. 2014, 53, 10416–10419.
- Whiteoak, C.J.; Martin, E.; Belmonte, M.M.; Benet-Buchholz, J.; Kleij, A.W. An Efficient iron catalyst for the synthesis of five- and six-membered organic carbonates under mild conditions. Adv. Synth. Catal. 2012, 354, 469–476.
- Sunjuk, M.; Abu-Surrah, A.S.; Al-Ramahi, E.; Qaroush, A.K.; Saleh, A. Selective coupling of carbon dioxide and epoxystyrene via salicylaldimine-, thiophenaldimine-, and quinolinaldimine-iron(II), iron(III), chromium(III), and cobalt(III)/Lewis base catalysts. Transit. Met. Chem. 2013, 38, 253–257.
- Buonerba, A.; De Nisi, A.; Grassi, A.; Milione, S.; Capacchione, C.; Vagin, S.; Rieger, B. Novel iron(III) catalyst for the efficient and selective coupling of carbon dioxide and epoxides to form cyclic carbonates. Catal. Sci. Technol. 2015, 5, 118–123.
- Della Monica, F.; Vummaleti, S.V.C.; Buonerba, A.; Nisi, A.D.; Monari, M.; Milione, S.; Grassi, A.; Cavallo, L.; Capacchione, C. Coupling of carbon dioxide with epoxides efficiently catalyzed by thioether-triphenolate bimetallic iron(III) complexes: Catalyst structure-reactivity relationship and mechanistic DFT study. Adv. Synth. Catal. 2016, 358, 3231–3243.
- Buonerba, A.; Della Monica, F.; De Nisi, A.; Luciano, E.; Milione, S.; Grassi, A.; Capacchione, C.; Rieger, B. Thioether-triphenolate bimetallic Iron(III) complexes as robust and highly efficient catalysts for cycloaddition of carbon dioxide to epoxides. Faraday Discuss. 2015, 183, 83–95.
- Della Monica, F.; Maity, B.; Pehl, T.; Buonerba, A.; De Nisi, A.; Monari, M.; Grassi, A.; Rieger, B.; Cavallo, L.; Capacchione, C. [OSSO]-type iron(III) complexes for the low-pressure reaction of carbon dioxide with epoxides: Catalytic activity, reaction kinetics, and computational study. ACS Catal. 2018, 8, 6882–6893.
- Ollevier, T. New trends in bismuth-catalyzed synthetic transformations. Org. Biomol. Chem. 2013, 11, 2740.
- Ollevier, T. (Ed.) Bismuth-Mediated Organic Reactions; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; Volume 311, ISBN 978-3-642-27238-7.
- Yamamoto, H.; Ishihara, K. (Eds.) Acid Catalysis in Modern Organic Synthesis; WILEY-VCH: Weinheim, Germany, 2008; ISBN 978-3-527-31724-0.
- Salvador, J.A.R.; Silvestre, S.M.; Pinto, R.M.A. Bismuth(III) reagents in steroid and terpene chemistry. Molecules 2011, 16, 2884–2913.
- Chen, Y.; Qiu, R.; Xu, X.; Au, C.-T.; Yin, S.-F. Organoantimony and organobismuth complexes for CO2 fixation. RSC Adv. 2014, 4, 11907–11918.
- Zhang, X.; Dai, W.; Yin, S.; Luo, S.; Au, C.-T. Cationic organobismuth complex as an effective catalyst for conversion of CO2 into cyclic carbonates. Front. Environ. Sci. Eng. China 2009, 3, 32–37.
- Yin, S.-F.; Shimada, S. Synthesis and structure of bismuth compounds bearing a sulfur-bridged bis(phenolato) ligand and their catalytic application to the solvent-free synthesis of propylene carbonate from CO2 and propylene oxide. Chem. Commun. 2009, 1136.
- Qiu, R.; Meng, Z.; Yin, S.; Song, X.; Tan, N.; Zhou, Y.; Yu, K.; Xu, X.; Luo, S.; Au, C.-T.; et al. Synthesis and structure of binuclear o/s-bridged organobismuth complexes and their cooperative catalytic effect on CO2 fixation. ChemPlusChem 2012, 77, 404–410.
