Malignant neoplasms are among the most common diseases and are responsible for the majority of deaths in the developed world. In contrast to men, available data show a clear upward trend in the incidence of lung cancer in women, making it almost as prevalent as breast cancer. Women might be more susceptible to the carcinogenic effect of tobacco smoke than men. Furthermore, available data indicate a much more frequent mutation of the tumor suppressor gene-
p53
in non-small cell lung cancer (NSCLC) female patients compared to males. Another important factor, however, might lie in the female sex hormones, whose mitogenic or carcinogenic effect is well known. Epidemiologic data show a correlation between hormone replacement therapy (HRT) or oral contraceptives (OCs), and increased mortality rates due to the increased incidence of malignant tumors, including lung cancer. Interestingly, two types of estrogen receptors have been detected in lung cancer cells: ERα and ERβ. The presence of ERα has been detected in tissues and non-small-cell lung carcinoma (NSCLC) cell lines. In contrast, overexpression of ERβ is a prognostic marker in NSCLC.
- estrogens
- lung cancer
- sex hormones
- lung adenocarcinoma
- estrogen receptor
- 17β-estradiol
- p53
- A549
- non-small cell lung cancer
- NSCLC
1. Introduction
In the developed countries, lung cancer is the most frequent malignancy and is responsible for about 1 million deaths annually. The overall survival rate involving this tumor is about 10%. The decisive trigger is long-term smoking. Available data indicate that only 20% of lung cancer cases develop in non-smokers [1,2]. Other environmental factors include pollution, exhaust fumes, ionizing radiation, mycotoxins, second hand smoke, occupational exposure to chemicals such as chromium, nickel, asbestos, polycyclic aromatic hydrocarbons, arsenic, vinyl chloride and radioactive gas—radon [1,2,3,4,5]. Susceptibility to the disease is also genetically determined [6,7]. The WHO classification distinguishes two main types of lung cancer: small cell carcinoma (SCLC) and non-small cell carcinoma (NSCLC) [6]. The latter is divided into subtypes including squamous cell carcinoma and adenocarcinoma [6].
In the developed countries, lung cancer is the most frequent malignancy and is responsible for about 1 million deaths annually. The overall survival rate involving this tumor is about 10%. The decisive trigger is long-term smoking. Available data indicate that only 20% of lung cancer cases develop in non-smokers [1][2]. Other environmental factors include pollution, exhaust fumes, ionizing radiation, mycotoxins, second hand smoke, occupational exposure to chemicals such as chromium, nickel, asbestos, polycyclic aromatic hydrocarbons, arsenic, vinyl chloride and radioactive gas—radon [1][2][3][4][5]. Susceptibility to the disease is also genetically determined [6][7]. The WHO classification distinguishes two main types of lung cancer: small cell carcinoma (SCLC) and non-small cell carcinoma (NSCLC) [6]. The latter is divided into subtypes including squamous cell carcinoma and adenocarcinoma [6].
2. Estrogens Short Review
Steroid hormones are endogenous estrogens that include estrone (E1), estriol (E3) and 17β-estradiol (E2) (
Figure 3). These structural and biogenic hormones are derived from cholesterol C17. LDL-cholesterol is the major reactant necessary for the synthesis of steroid hormones, which is called steroidogenesis [52,53,54].
). These structural and biogenic hormones are derived from cholesterol C17. LDL-cholesterol is the major reactant necessary for the synthesis of steroid hormones, which is called steroidogenesis [8][9][10].
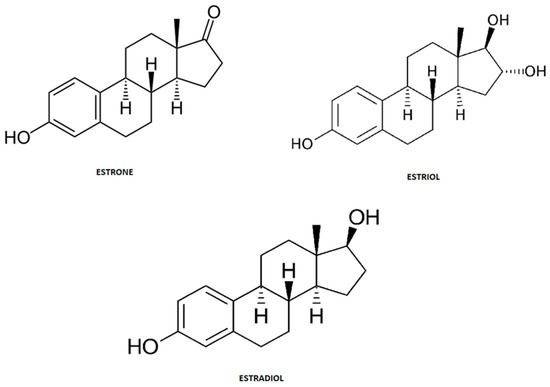
Figure 3.
Chemical structure of steroid hormones.
Cholesterol is metabolized in a number of enzymatic pathways [55,56]. The process of their creation depends on the aromatization of androgens [55,56]. In addition, they have the ability to bind to the protein receptor (ER) as well as to diffuse through the cell membrane. Direct penetration through the cell membrane into the cytosol occurs due to the properties of the lipophilic structure. In addition, estrogens are included in the group of pleiotropic hormones [55,56].
Cholesterol is metabolized in a number of enzymatic pathways [11][12]. The process of their creation depends on the aromatization of androgens [11][12]. In addition, they have the ability to bind to the protein receptor (ER) as well as to diffuse through the cell membrane. Direct penetration through the cell membrane into the cytosol occurs due to the properties of the lipophilic structure. In addition, estrogens are included in the group of pleiotropic hormones [11][12].
The two main estrogens also named “parent” estrogens—estrone, and estradiol—are low-molecular steroids of lipophilic nature acting as agonists of estrogen receptors ERα and ERβ [56]. However, estrogen-like activity should be also attributed (with varying extent) to a range of estrogen metabolites, usually referred to as EM [57,58]. The parent estrogens are irreversibly oxidized in the cytochrome P450 dependent pathway by hydroxylation at the C-2, C-4 and C-16 positions of the steroid ring forming hydroxylated metabolites. The main and mots studied metabolites include 2-hydroxyestrogen (2-OH-E), 4-hydroxyestrogen (4-OH-E) and 16-hydroxyestrogen (16α-OH-E) have significant estrogenic activity (
The two main estrogens also named “parent” estrogens—estrone, and estradiol—are low-molecular steroids of lipophilic nature acting as agonists of estrogen receptors ERα and ERβ [12]. However, estrogen-like activity should be also attributed (with varying extent) to a range of estrogen metabolites, usually referred to as EM [13][14]. The parent estrogens are irreversibly oxidized in the cytochrome P450 dependent pathway by hydroxylation at the C-2, C-4 and C-16 positions of the steroid ring forming hydroxylated metabolites. The main and mots studied metabolites include 2-hydroxyestrogen (2-OH-E), 4-hydroxyestrogen (4-OH-E) and 16-hydroxyestrogen (16α-OH-E) have significant estrogenic activity (
Figure 4). Those metabolites are further transformed by conjugation with a methyl group, glucuronic acid, and sulfuric acid (forming methoxy-metabolites, glucuronates and sulfates, respectively). Thus, many authors point out the necessity of studying a wide panel of estrogens, including minor metabolites in order to fully understand their influence on human physiology as well as the etiology and progression of various pathological states [59,60]. This relatively new approach needs easily accessible and reliable bioanalytical methods to determine their concentrations in human biofluids and tissues.
). Those metabolites are further transformed by conjugation with a methyl group, glucuronic acid, and sulfuric acid (forming methoxy-metabolites, glucuronates and sulfates, respectively). Thus, many authors point out the necessity of studying a wide panel of estrogens, including minor metabolites in order to fully understand their influence on human physiology as well as the etiology and progression of various pathological states [15][16]. This relatively new approach needs easily accessible and reliable bioanalytical methods to determine their concentrations in human biofluids and tissues.
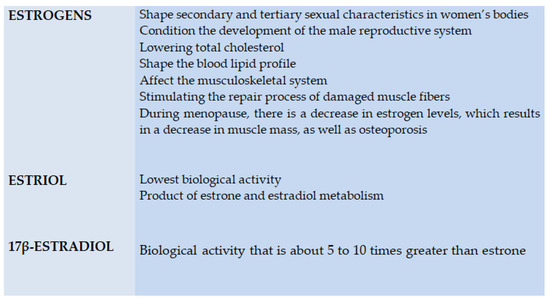
Figure 4.
Biological activity of steroid hormones.
From the analytical point of view, this is not a straightforward task for several main reasons (
Figure 5) Firstly, the analytical technique needs to have enough selectivity to differentiate between chemically similar compounds, so an efficient separation technique is required. To achieve that, chromatography is applied, with high performance liquid chromatography coupled to mass spectrometry as a method of choice [61,62]. Practically, it is not possible to separate lipophilic parent estrogens, their hydroxylated metabolites, and much more polar conjugates with glucuronic and sulfuric acids. In order to avoid the development validation of separate LC-MS methods for polar and nonpolar analytes, enzymatic hydrolysis is typically involved as a sample preparation step. β-glucuronidase/sulfatase from Helix pomatia has been proven to sufficiently hydrolyze estrogen metabolites [62]. The involvement of enzymatic cleavage enables us to gain detailed information about a wide range of estrogen metabolites. Secondly, due to the low levels of many of the above mentioned metabolites, the high sensitivity of the analysis is a critical issue. Sensitive quantification depends on the detection method and sample preparation. Mass spectrometry, despite being expensive, can detect estrogen compounds down to the pmol/L level [59]. On the other hand, extensive clean-up of a sample with simultaneous preconcentration of analytes is beneficial to improve sensitivity and avoid interfering compounds. Improvement at the sample preparation step in estrogen analysis is thus still required. The application of novel selective materials, including sorbents processed by using 3D-printing, can be a promising approach, especially in a high throughput format [63,64]. More selective extraction utilizing specific sorbent-analyte interactions can potentially further improve quantification of a wide range of estrogens. In particular, boronate affinity solid-phase microextraction, as was previously claimed to be useful for diol-containing compounds [65], seems to be an attractive approach.
) Firstly, the analytical technique needs to have enough selectivity to differentiate between chemically similar compounds, so an efficient separation technique is required. To achieve that, chromatography is applied, with high performance liquid chromatography coupled to mass spectrometry as a method of choice [17][18]. Practically, it is not possible to separate lipophilic parent estrogens, their hydroxylated metabolites, and much more polar conjugates with glucuronic and sulfuric acids. In order to avoid the development validation of separate LC-MS methods for polar and nonpolar analytes, enzymatic hydrolysis is typically involved as a sample preparation step. β-glucuronidase/sulfatase from Helix pomatia has been proven to sufficiently hydrolyze estrogen metabolites [18]. The involvement of enzymatic cleavage enables us to gain detailed information about a wide range of estrogen metabolites. Secondly, due to the low levels of many of the above mentioned metabolites, the high sensitivity of the analysis is a critical issue. Sensitive quantification depends on the detection method and sample preparation. Mass spectrometry, despite being expensive, can detect estrogen compounds down to the pmol/L level [15]. On the other hand, extensive clean-up of a sample with simultaneous preconcentration of analytes is beneficial to improve sensitivity and avoid interfering compounds. Improvement at the sample preparation step in estrogen analysis is thus still required. The application of novel selective materials, including sorbents processed by using 3D-printing, can be a promising approach, especially in a high throughput format [19][20]. More selective extraction utilizing specific sorbent-analyte interactions can potentially further improve quantification of a wide range of estrogens. In particular, boronate affinity solid-phase microextraction, as was previously claimed to be useful for diol-containing compounds [21], seems to be an attractive approach.
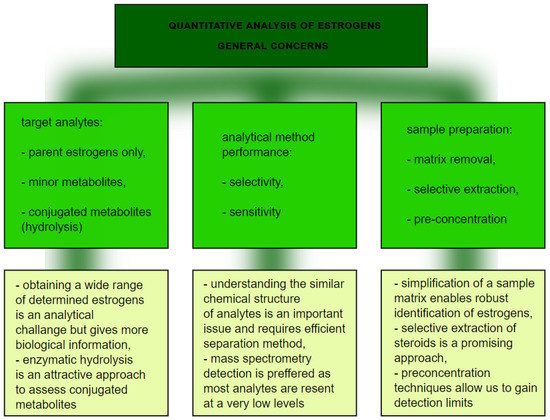
Figure 5.
The graph presents quantitative analysis of estrogens.
The main role of estrogens in a woman’s body is to shape secondary and tertiary sexual characteristics, which affects the development of external genitalia, as well as the fallopian tubes, uteri, vaginas and nipples. Estrogens also perform a key function in the male body—they condition the development of the male reproductive system [55,66,67,68,69,70,71,72].
The main role of estrogens in a woman’s body is to shape secondary and tertiary sexual characteristics, which affects the development of external genitalia, as well as the fallopian tubes, uteri, vaginas and nipples. Estrogens also perform a key function in the male body—they condition the development of the male reproductive system [11][22][23][24][25][26][27][28].
Estrogens also have a positive effect on the cardiovascular system, among other factors, by lowering total cholesterol [60], shaping the blood lipid profile and also affecting the musculoskeletal system by stimulating the repair process of damaged muscle fibers [56,71]. Notably, during menopause, there is a decrease in estrogen levels, which results in a decrease in muscle mass, as well as osteoporosis. The lowest biological activity is shown by estriol, which is considered to be the weakest of estrogens, being a product of estrone and estradiol metabolism. Estrone, in turn, exhibits a markedly higher biological activity [56,68,69,70,71]. Finally, the type of estrogen with the highest activity is a 17β-estradiol, with a potency of about 5 to 10 times greater than the former type. Both types of estrogen receptors—ERα and ERβ—have a relationship with the heat shock proteins (HSP) complex. In addition, estrogen receptors have the ability to form heterodimers and homodimers [47,68,69,70,71].
Estrogens also have a positive effect on the cardiovascular system, among other factors, by lowering total cholesterol [16], shaping the blood lipid profile and also affecting the musculoskeletal system by stimulating the repair process of damaged muscle fibers [12][27]. Notably, during menopause, there is a decrease in estrogen levels, which results in a decrease in muscle mass, as well as osteoporosis. The lowest biological activity is shown by estriol, which is considered to be the weakest of estrogens, being a product of estrone and estradiol metabolism. Estrone, in turn, exhibits a markedly higher biological activity [12][24][25][26][27]. Finally, the type of estrogen with the highest activity is a 17β-estradiol, with a potency of about 5 to 10 times greater than the former type. Both types of estrogen receptors—ERα and ERβ—have a relationship with the heat shock proteins (HSP) complex. In addition, estrogen receptors have the ability to form heterodimers and homodimers [29][24][25][26][27].
3. Estrogens in Etiopathogenesis and Therapy of Lung Cancer
It is well known that estrogens can cause carcinogenicity. The impact of estrogens is noted in female cancers, for example breast cancer, or in the case of modulation of genetic mutations. However, based on available clinical studies, it is also hypothesized that the mechanism of action of the estrogen pathway in lung cancer is similar to the one established for breast cancer [72,73,74].
It is well known that estrogens can cause carcinogenicity. The impact of estrogens is noted in female cancers, for example breast cancer, or in the case of modulation of genetic mutations. However, based on available clinical studies, it is also hypothesized that the mechanism of action of the estrogen pathway in lung cancer is similar to the one established for breast cancer [28][30][31].
Available studies indicate that estrogen affects lung carcinogenesis via non-genomic and genomic signaling. In genomic signaling, homodimers and heterodimers are formed acting as ligands, which bind to the ER nucleus. In contrast, non-genomic signaling works by means of the mitogen-activated protein kinase (MAPK1) pathways through the ER [71,75].
Available studies indicate that estrogen affects lung carcinogenesis via non-genomic and genomic signaling. In genomic signaling, homodimers and heterodimers are formed acting as ligands, which bind to the ER nucleus. In contrast, non-genomic signaling works by means of the mitogen-activated protein kinase (MAPK1) pathways through the ER [27][32].
The endogenous metabolite of 17β-estradiol (E2) resulting from the hydroxylation and methylation of the second-position is 2-methoxyestradiol (2ME, (17beta)-2-methoxyestra-1,3,5 (10)-triene-3,17-diol). This metabolite inhibits angiogenesis by reducing endothelial cell proliferation. In addition, 2ME is an antiproliferative and anti-angiogenic agent [76,77,78,79].
The endogenous metabolite of 17β-estradiol (E2) resulting from the hydroxylation and methylation of the second-position is 2-methoxyestradiol (2ME, (17beta)-2-methoxyestra-1,3,5 (10)-triene-3,17-diol). This metabolite inhibits angiogenesis by reducing endothelial cell proliferation. In addition, 2ME is an antiproliferative and anti-angiogenic agent [33][34][35][36].
The metabolite 2ME inhibits carcinogenic cell growth due to tubulin binding. In vitro studies show that 2-methoxyestradiol inhibits a wide range of non-cancer and cancer cell lines. It has also been shown in vitro that 2ME inhibits several stages of the andiogenic cascade, thereby inhibiting proliferation and inducing tumor cell apoptosis. Cell line growth inhibition was achieved in the lung cancer line of human origin A459 and H460
p53
wild-type. Minor changes after treatment with 2ME occurred in H322
p53
and H358 type
p53
cell lines. Western Blot analysis was performed, which resulted in a significant increase in
p53
protein after treatment with 2-ME. The main change observed during the study involving treatment with 2ME was an eight-fold increase in endogenous
p53
protein. The level of mutated
p53
protein remained unchanged. The
p53 protein is the major tumor suppressor responsible for regulating the cell’s life cycle and apoptosis [76,77].
protein is the major tumor suppressor responsible for regulating the cell’s life cycle and apoptosis [33][34].
The Charité University Clinic in Berlin conducted a study to confirm the inhibition of the growth of various cell lines with 2ME, including lung cancer. Orally administered 2-ME was combined with gene therapy and an adenovirus expressing the
p53 gene was administered intravenously. The results demonstrated that lung cancer cells that were resistant to cisplatin were particularly sensitive to 2ME [78].
gene was administered intravenously. The results demonstrated that lung cancer cells that were resistant to cisplatin were particularly sensitive to 2ME [35].
Several experiments have been devoted to the metabolite 4-hydroxyestrogen (4-OH-E), which is a CYP1B1 product, and has mutagenic and carcinogenic effects [78]. Studies indicate that tobacco smoke stimulates the metabolism of 17β-estradiol to the toxic metabolite 4-OH-E. In addition, 4-OH-E levels are elevated in patients with lung cancer as compared to healthy controls. It has been hypothesized that the 4-OH-E metabolite affects oncogene mutation in the lungs and also activates ER signaling, which increases the risk of lung cancer. Last year at the Research Institute of Fox Chase Cancer Center in Philadelphia, it was discovered that the human lung can metabolize estrogen to 4-hydroxyestrogen [79].
Several experiments have been devoted to the metabolite 4-hydroxyestrogen (4-OH-E), which is a CYP1B1 product, and has mutagenic and carcinogenic effects [35]. Studies indicate that tobacco smoke stimulates the metabolism of 17β-estradiol to the toxic metabolite 4-OH-E. In addition, 4-OH-E levels are elevated in patients with lung cancer as compared to healthy controls. It has been hypothesized that the 4-OH-E metabolite affects oncogene mutation in the lungs and also activates ER signaling, which increases the risk of lung cancer. Last year at the Research Institute of Fox Chase Cancer Center in Philadelphia, it was discovered that the human lung can metabolize estrogen to 4-hydroxyestrogen [36].
Studies show differential expression of nuclear ER-β in NSCLC [80]. Nuclear expression of ER-α and ER-β was determined by immunohistochemistry. The study identified ER-β nuclear expression in NSCLC tumor tissue and control tissue correctly, in both women and men. In men, nuclear ER-β expression was found to be more frequent in adenocarcinomas of the lung [80].
Studies show differential expression of nuclear ER-β in NSCLC [37]. Nuclear expression of ER-α and ER-β was determined by immunohistochemistry. The study identified ER-β nuclear expression in NSCLC tumor tissue and control tissue correctly, in both women and men. In men, nuclear ER-β expression was found to be more frequent in adenocarcinomas of the lung [37].
In the case of NSCLC, research indicates stimulation of tumor growth through the expression of ER forms that interact with the epidermal growth factor receptor (EGFR) [81]. Importantly, EGFR supports the growth of NSCLC and breast cancer. The available data indicate the responsibility of HER2 and EGFR for a number of states of endocrine immunity [66]. Moreover, in response to estrogen, the ERs proliferate as a result of their interaction with ER-containing vascular endothelial cells [81].
In the case of NSCLC, research indicates stimulation of tumor growth through the expression of ER forms that interact with the epidermal growth factor receptor (EGFR) [38]. Importantly, EGFR supports the growth of NSCLC and breast cancer. The available data indicate the responsibility of HER2 and EGFR for a number of states of endocrine immunity [22]. Moreover, in response to estrogen, the ERs proliferate as a result of their interaction with ER-containing vascular endothelial cells [38].
Estrogens regulate the expression of miRNAs, which are found in small non-coding RNAs containing about 21–25 nucleosites [82]. The miRNA finds application in distinguishing between different subtypes of lung cancer [82,83,84]. Studies have reported that miR-124a is characteristic of NSCLC lung adenocarcinoma and miR-205 for squamous cell carcinoma, while miR-375 and miR-21-5p are highly expressed in SCLC [82,83,84,85,86,87,88]. In addition, the histological patterns of growth of lung adenocarcinomas were analyzed, showing a significant influence of miRNA expression [89]. In tumors, the presence of solid components in tumors was demonstrated when miR-212, miR-27a and miR-132 were expressed [89]. However, in order to demonstrate the possible benefits of miRNA targeted therapy in lung cancer patients, more comprehensive studies should be conducted.
Estrogens regulate the expression of miRNAs, which are found in small non-coding RNAs containing about 21–25 nucleosites [39]. The miRNA finds application in distinguishing between different subtypes of lung cancer [39][40][41]. Studies have reported that miR-124a is characteristic of NSCLC lung adenocarcinoma and miR-205 for squamous cell carcinoma, while miR-375 and miR-21-5p are highly expressed in SCLC [39][40][41][42][43][44][45]. In addition, the histological patterns of growth of lung adenocarcinomas were analyzed, showing a significant influence of miRNA expression [46]. In tumors, the presence of solid components in tumors was demonstrated when miR-212, miR-27a and miR-132 were expressed [46]. However, in order to demonstrate the possible benefits of miRNA targeted therapy in lung cancer patients, more comprehensive studies should be conducted.
Research indicates that estradiol can be synthesized locally in NSCLC, analogous to breast cancer tissue [90]. Moreover, on the basis of the obtained results, it was proved that the concentration of estradiol in the NSCLC tissues was significantly 3.7 times higher in men than in women after menopause [90]. Researchers say that the essence of this phenomenon are the circulating androgens produced by aromatase, which in the case of NSCLC and estradiol production could be the leading substrates [90]. The study determined the estradiol concentration in 59 NSCLC cases, followed by in vitro A549 NSCLC cell cultures. Forty-three of the subjects showed an increase in the concentration of estradiol in the neoplastic tissues compared to the non-neoplastic lung tissues of the patients [90]. However, in the case of in vitro studies, the increase in the proliferation of cell cultures of both A549 + ER-α and A549 + ER-β was determined. Moreover, both cell cultures were found to express aromatase. Importantly, studies show an increase in A549 cell proliferation during testosterone use. Therefore, it is suggested on the basis of the obtained studies that if estrogens, and more specifically oestradiol occurring inside cancerous tumors by aromatase, including NSCLC, and favor their development, anti-estrogen therapy would be an effective therapy in the fight against cancer [90].
Research indicates that estradiol can be synthesized locally in NSCLC, analogous to breast cancer tissue [47]. Moreover, on the basis of the obtained results, it was proved that the concentration of estradiol in the NSCLC tissues was significantly 3.7 times higher in men than in women after menopause [47]. Researchers say that the essence of this phenomenon are the circulating androgens produced by aromatase, which in the case of NSCLC and estradiol production could be the leading substrates [47]. The study determined the estradiol concentration in 59 NSCLC cases, followed by in vitro A549 NSCLC cell cultures. Forty-three of the subjects showed an increase in the concentration of estradiol in the neoplastic tissues compared to the non-neoplastic lung tissues of the patients [47]. However, in the case of in vitro studies, the increase in the proliferation of cell cultures of both A549 + ER-α and A549 + ER-β was determined. Moreover, both cell cultures were found to express aromatase. Importantly, studies show an increase in A549 cell proliferation during testosterone use. Therefore, it is suggested on the basis of the obtained studies that if estrogens, and more specifically oestradiol occurring inside cancerous tumors by aromatase, including NSCLC, and favor their development, anti-estrogen therapy would be an effective therapy in the fight against cancer [47].
Both NSCLC and breast cancer are entities that frequently occur in everyday pathological diagnosis [91]. However, it should be remembered that both disease entities in the form of lung metastatic breast cancer and primary lung cancer are treated completely differently [91]. Typical immunohistochemical markers in the differential diagnosis of breast cancer are: HER2—tyrosine kinase receptor encoded by HER2—growth-promoting protein, ER, MAMG—mammaglobion, GATA3—GATA 3 binding protein (zinc finger transcription factor) and PgR—the steroid hormone progesterone receptor [91]. However, in the case of NSCLC, the most frequently used immunohistochemical markers are: TTF-1—thyroid transcription factor 1, Napsin A, CK7—cytokeratin 7,
Both NSCLC and breast cancer are entities that frequently occur in everyday pathological diagnosis [48]. However, it should be remembered that both disease entities in the form of lung metastatic breast cancer and primary lung cancer are treated completely differently [48]. Typical immunohistochemical markers in the differential diagnosis of breast cancer are: HER2—tyrosine kinase receptor encoded by HER2—growth-promoting protein, ER, MAMG—mammaglobion, GATA3—GATA 3 binding protein (zinc finger transcription factor) and PgR—the steroid hormone progesterone receptor [48]. However, in the case of NSCLC, the most frequently used immunohistochemical markers are: TTF-1—thyroid transcription factor 1, Napsin A, CK7—cytokeratin 7,
p63
,
p40 and CK5—cytokeratin 5 [91]. In addition, molecular tests for mutations are performed to diagnose NSCLC activators in the EGFR gene [91]. Due to the limited research on the immunohistological expression of NSCLC markers, a study was conducted using clinical variables and staining results for CK5/6,
and CK5—cytokeratin 5 [48]. In addition, molecular tests for mutations are performed to diagnose NSCLC activators in the EGFR gene [48]. Due to the limited research on the immunohistological expression of NSCLC markers, a study was conducted using clinical variables and staining results for CK5/6,
p40, TTF-1 and napsin A [91]. An analysis of 1291 samples of NSCLC patients with successively diagnosed adenocarcinoma (ADC) was performed—636 patient samples, squamous cell carcinoma (SqCC)—536 patient samples, large cell carcinoma (LLC)—65 patient samples, polymorphic carcinoma (PC)—34 patient samples, and large cell neuroendocrine carcinoma (LCNEC)—20 patient samples. Most of the patients had disease stages I to III [91]. In addition, 380 patients were women with more ADC than SqCC, while the remaining 911 patients were men. ER-positive tumors were found to be much more common in women than in men. On the basis of the conducted studies, the expression of all five markers was found in many patients, thus it can be concluded that the interpretation of tumor markers is important in the differential diagnosis [91].
, TTF-1 and napsin A [48]. An analysis of 1291 samples of NSCLC patients with successively diagnosed adenocarcinoma (ADC) was performed—636 patient samples, squamous cell carcinoma (SqCC)—536 patient samples, large cell carcinoma (LLC)—65 patient samples, polymorphic carcinoma (PC)—34 patient samples, and large cell neuroendocrine carcinoma (LCNEC)—20 patient samples. Most of the patients had disease stages I to III [48]. In addition, 380 patients were women with more ADC than SqCC, while the remaining 911 patients were men. ER-positive tumors were found to be much more common in women than in men. On the basis of the conducted studies, the expression of all five markers was found in many patients, thus it can be concluded that the interpretation of tumor markers is important in the differential diagnosis [48].
Estrogen is known to induce ERβ-mediated cell growth in NSCLC [92]. Moreover, high levels of circulating interleukins 6 (IL6) are associated with poor prognosis for NSCLC; however, the determination of the specific role of IL6 in NSCLC is not fully understood and requires a lot of research [92]. One of the studies assessed both the biological effects as well as the expression of interleukins in NSCLC cells after treatment with 17β-estradiol (E2) [92]. The expression of IL6/ERβ in 289 NSCLC samples was determined via immunohistochemistry [92]. The study included A549 and H1793 non-small cell lung cancer cells [92]. Cells were treated with E2. Their expression levels were determined sequentially by means of ELISA, western blotting and immunofluorescence staining [92]. The study also used an animal xenograft model to determine and observe differences in IL6 and ERβ expression in NSCLC tumor growth [92]. Research showed an increased increase in both ERβ and IL6, which was closely related, the researchers indicated, to either increased metastasis or decreased differentiation [92]. Indeed, the study showed ERβ mediated regulation of IL6 expression through the PI3K/AKT and MAPK/ERK pathways due to the use of E2 [92]. Importantly, an increase in malignancy of NSCLC cells was also found due to the regulation of E2 on IL6/ERβ [92].
Estrogen is known to induce ERβ-mediated cell growth in NSCLC [49]. Moreover, high levels of circulating interleukins 6 (IL6) are associated with poor prognosis for NSCLC; however, the determination of the specific role of IL6 in NSCLC is not fully understood and requires a lot of research [49]. One of the studies assessed both the biological effects as well as the expression of interleukins in NSCLC cells after treatment with 17β-estradiol (E2) [49]. The expression of IL6/ERβ in 289 NSCLC samples was determined via immunohistochemistry [49]. The study included A549 and H1793 non-small cell lung cancer cells [49]. Cells were treated with E2. Their expression levels were determined sequentially by means of ELISA, western blotting and immunofluorescence staining [49]. The study also used an animal xenograft model to determine and observe differences in IL6 and ERβ expression in NSCLC tumor growth [49]. Research showed an increased increase in both ERβ and IL6, which was closely related, the researchers indicated, to either increased metastasis or decreased differentiation [49]. Indeed, the study showed ERβ mediated regulation of IL6 expression through the PI3K/AKT and MAPK/ERK pathways due to the use of E2 [49]. Importantly, an increase in malignancy of NSCLC cells was also found due to the regulation of E2 on IL6/ERβ [49].
Experimental research indicate that the ER potentially promotes NSCLC progression via modulation of the membrane receptor signaling network, composed of the GSK3β/β-catenin, Notch1 and EGFR pathways [93]. Furthermore, one of the treatments for lung cancer may be anti-estrogen therapy. Additionally, 17-β-estradiol is produced by aromatase activity, which in turn influences the control of estrogen levels in the lung cancer microenvironment [94,95]. Clinical studies suggest that aromatase inhibitors are a good therapeutic option for lung adenocarcinoma [94,96]. Aromatase inhibitors are classified into classes I and II: (I) irreversible steroid inhibitors; (II) non-steroidal inhibitors [94,96]. Sulfotransferases activated by e.g., dexamethasone are also used in the treatment of hormone-dependent tumors [94,97]. Preclinical studies indicate inhibition of A549 cell tumor growth, while lowering estrogen levels [94,96]. Fulvestrant, an estrogen receptor degradator, is also used in NSCLC research [98]. According to the data, fulvestrant causes greater sensitization of the NSCLC tumor to chemotherapy and reduces the mesinochemical features [94,97]. Due to the fact that one of the leading elements of the patient’s immune profile are steroid hormones, next to chemotherapy, radiotherapy or surgery, immunotherapy is an effective lung cancer treatment strategy [94]. The development of personalized medicine is conditioned by numerous preclinical and clinical studies taking into account sex differences or the expression of hormonal markers, in which the response to therapy in patients with NSCLC, survival as well as pathological and clinical features are tested [94].
Experimental research indicate that the ER potentially promotes NSCLC progression via modulation of the membrane receptor signaling network, composed of the GSK3β/β-catenin, Notch1 and EGFR pathways [50]. Furthermore, one of the treatments for lung cancer may be anti-estrogen therapy. Additionally, 17-β-estradiol is produced by aromatase activity, which in turn influences the control of estrogen levels in the lung cancer microenvironment [51][52]. Clinical studies suggest that aromatase inhibitors are a good therapeutic option for lung adenocarcinoma [51][53]. Aromatase inhibitors are classified into classes I and II: (I) irreversible steroid inhibitors; (II) non-steroidal inhibitors [51][53]. Sulfotransferases activated by e.g., dexamethasone are also used in the treatment of hormone-dependent tumors [51][54]. Preclinical studies indicate inhibition of A549 cell tumor growth, while lowering estrogen levels [51][53]. Fulvestrant, an estrogen receptor degradator, is also used in NSCLC research [55]. According to the data, fulvestrant causes greater sensitization of the NSCLC tumor to chemotherapy and reduces the mesinochemical features [51][54]. Due to the fact that one of the leading elements of the patient’s immune profile are steroid hormones, next to chemotherapy, radiotherapy or surgery, immunotherapy is an effective lung cancer treatment strategy [51]. The development of personalized medicine is conditioned by numerous preclinical and clinical studies taking into account sex differences or the expression of hormonal markers, in which the response to therapy in patients with NSCLC, survival as well as pathological and clinical features are tested [51].
References
- Urman, A.; Hosgood, D. Lung Cancer Risk, Genetic Variation, and Air Pollution. EBioMedicine 2015, 2, 491–492.
- Alberg, A.J.; Samet, J.M. Epidemiology of lung cancer. Chest 2003, 123, 21–49.
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644.
- Hansen, H.H. Lung Cancer: European Commission: Series for General Practitioners; Springer: Berlin, Germany, 1990; pp. 1–48.
- Siegel, R.; Ward, E.; Brawley, O. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236.
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart; International Agency for Research on Cancer: Lyon, France, 2015.
- Addario, B.J. Lung cancer is a global epidemic and requires a global effort. Ann. Transl. Med. 2015, 3, 26.
- Kligerman, S.; White, C. Epidemiology of lung cancer in women: Risk factors, survival, and screening. Am. J. Roentgenol. 2011, 196, 287–295.
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung cancer in never smokers—A different disease. Nat. Rev. Cancer 2007, 7, 778–790.
- Mollerup, S.; Jorgensen, K.; Berge, G.; Haugen, A. Expression of estrogen receptors α and β in human lung tissue and cell lines. Lung Cancer 2002, 37, 153–159.
- Thomas, L.; Doyle, L.A.; Edelman, M.J. Lung cancer in women: Emerging differences in epidemiology, biology, and therapy. Chest 2005, 128, 370–381.
- Kalita, K.; Lewandowski, S.; Skrzypczak, M.; Szymczak, S.; Tkaczyk, M.; Kaczmarek, L. Receptory Strogenowe, Receptory i Mechanizmy Przekazywania Sygnału; PWN: Warszawa, Poland, 2004; pp. 604–616.
- Anstead, G.M.; Carlson, K.E.; Katzenellenbogen, J.A. The estradiol pharmacophore: Ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids 1997, 62, 268–303.
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870.
- Sampson, J.N.; Falk, R.T.; Schairer, C.; Moore, S.C.; Fuhrman, B.J.; Dallal, C.M.; Bauer, D.C.; Dorgan, J.F.; Shu, X.O.; Zheng, W.; et al. Association of Estrogen Metabolism with Breast Cancer Risk in Different Cohorts of Postmenopausal Women. Cancer Res. 2017, 77, 918–925.
- Fuhrman, B.J.; Xu, X.; Falk, R.T.; Dallal, C.M.; Veenstra, T.D.; Keefer, L.K.; Graubard, B.I.; Brinton, L.A.; Ziegler, R.G.; Gierach, G.L. Assay reproducibility and interindividual variation for 15 serum estrogens and estrogen metabolites measured by liquid chromatography-tandem mass spectrometry. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2649–2657.
- Xu, X.; Roman, J.M.; Issaq, H.J.; Keefer, L.K.; Veenstra, T.D.; Ziegler, R.G. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal. Chem. 2007, 79, 7813–7821.
- Xu, X.; Keefer, L.K.; Ziegler, R.G.; Veenstra, T.D. A liquid chromatography-mass spectrometry method for the quantitative analysis of urinary endogenous estrogen metabolites. Nat. Protoc. 2007, 2, 1350–1355.
- Konieczna, L.; Belka, M.; Okońska, M.; Pyszka, M.; Bączek, T. New 3D-printed sorbent for extraction of steroids from human plasma preceding LC–MS analysis. J. Chromatogr. A 2018, 1545, 1–11.
- Belka, M.; Konieczna, L.; Okońska, M.; Pyszka, M.; Ulenberg, S.; Bączek, T. Application of 3D-printed scabbard-like sorbent for sample preparation in bioanalysis expanded to 96-wellplate high-throughput format. Anal. Chim. Acta 2019, 1081, 1–5.
- He, J.; Liu, Z.; Ren, L.; Liu, Y.; Dou, P.; Qian, K.; Chen, H.Y. On-line coupling of in-tube boronate affinity solid phase microextraction with high performance liquid chromatography-electrospray ionization tandem mass spectrometry for the determination of cis-diol biomolecules. Talanta 2010, 82, 270–276.
- Kang, J.S.; Jung, N.J.; Kim, S.; Kim, D.J.; Jang, D.D.; Yang, K.H. Downregulation of estrogen receptor alpha and beta expression in carcinogen-induced mammary gland tumors of rats. Exp. Oncol. 2004, 26, 31–35.
- Corcoran, M.P.; Lichtenstein, A.H.; Meydani, M.; Dillard, A.; Schaefer, E.J.; Lamon-Fava, S. The effect of 17β-estradiol on cholesterol content in human macrophages is influenced by the lipoprotein milieu. J. Mol. Endocrinol. 2011, 47, 109–117.
- Price, R.H., Jr.; Handa, R.J. Expression of estrogen receptor-beta protein and mRNA in the cerebellum of the rat. Neurosci. Lett. 2000, 288, 115–118.
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209.
- Hewitt, S.C.; Winuthayanon, W.; Korach, K.S. What’s new in estrogen receptor action in the female reproductive tract. J. Mol. Endocrinol. 2016, 56, 55–71.
- Schulster, M.; Bernie, A.M.; Ramasamy, R. The role of estradiol in male reproductive function. Asian J. Androl. 2016, 18, 435–440.
- Van Pelt, R.E.; Gavin, K.M.; Kohrt, W.M. Regulation of Body Composition and Bioenergetics by Estrogens. Endocrinol. Metab. Clin. N. Am. 2015, 44, 663–676.
- Henschke, C.I.; Miettinen, O.S. Women’s susceptibility to tobacco carcinogens. Lung Cancer 2004, 43, 1–5.
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod. Med. Biol. 2016, 16, 4–20.
- Mukhopadhyay, T.; Roth, J.A. Induction of apoptosis in human lung cancer cells after wild-type p53 activation by methoxyestradiol. Oncogene 1997, 14, 379–384.
- LaVallee, T.M.; Zhan, X.H.; Herbstritt, C.J.; Kough, E.C.; Green, S.J.; Pribluda, V.S. 2-Methoxyestradiol Inhibits Proliferation and Induces Apoptosis Independently of Estrogen Receptors α and β. Cancer Res. 2002, 62, 3691–3697.
- Schumacher, G. 2-Methoxyestradiol als Neue Substanz zur Behandlung Solider Tumore; Medizinischen Fakultät Charité der Humboldt-Universität zu: Berlin, Germany, 2004; pp. 1–109.
- Marino, M.; Galluzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genom. 2006, 7, 497–508.
- Miao, S.; Yang, F.; Wang, Y.; Shao, C.; Zava, D.T.; Ding, Q.; Shi, Y.E. 4-Hydroxy estrogen metabolite, causing genomic instability by attenuating the function of spindle-assembly checkpoint, can serve as a biomarker for breast cancer. Am. J. Transl. Res. 2019, 11, 4992–5007.
- Peng, J.; Meireles, S.I.; Xu, X.; Smith, W.E.; Slifker, M.J.; Riel, S.L.; Zhai, S.; Zhang, G.; Ma, X.; Kurzer, M.S.; et al. Estrogen metabolism in the human lung: Impact of tumorigenesis, smoke, sex and race/ethnicity. Oncotarget 2017, 8, 106778–106789.
- Schwartz, A.G.; Prysak, G.M.; Murphy, V.; Lonardo, F.; Pass, H.; Schwartz, J.; Brooks, S. Nuclear Estrogen Receptor β in Lung Cancer: Expression and Survival Differences by Sex. Clin. Cancer Res. 2005, 11, 7280–7287.
- Pietras, R.J.; Márquez-Garbán, D.C. Membrane-Associated Estrogen Receptor Signaling Pathways in Human Cancers. Clin. Cancer Res. 2007, 13, 4672–4676.
- Wu, K.L.; Tsai, Y.M.; Lien, C.T.; Kuo, P.L.; Hung, A.J. The Roles of MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1611.
- Bishop, J.A.; Benjamin, H.; Cholakh, H.; Chajut, A.; Clark, D.P.; Westra, W.H. Accurate classification of non-small cell lung carcinoma using a novel microRNA-based approach. Clin. Cancer Res. 2010, 16, 610–619.
- Lebanony, D.; Benjamin, H.; Gilad, S.; Ezagouri, M.; Dov, A.; Ashkenazi, K.; Gefen, N.; Izraeli, S.; Rechavi, G.; Pass, H. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J. Clin. Oncol. 2009, 27, 2030–2037.
- Lujambio, A.; Ropero, S.; Ballestar, E.; Fraga, M.F.; Cerrato, C.; Setien, F.; Casado, S.; Suarez-Gauthier, A.; Sanchez-Cespedes, M.; Git, A. eGenetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007, 67, 1424–1429.
- Zhang, Y.K.; Zhu, W.Y.; He, J.Y.; Chen, D.D.; Huang, Y.Y.; Le, H.B.; Liu, X.G. miRNAs expression profiling to distinguish lung squamous-cell carcinoma from adenocarcinoma subtypes. J. Cancer Res. Clin. Oncol. 2012, 138, 1641–1650.
- Nishikawa, E.; Osada, H.; Okazaki, Y.; Arima, C.; Tomida, S.; Tatematsu, Y.; Taguchi, A.; Shimada, Y.; Yanagisawa, K.; Yatabe, Y. miR-375 Is Activated by ASH1 and Inhibits YAP1 in a Lineage-Dependent Manner in Lung Cancer. Cancer Res. 2011, 71, 6165–6173.
- Demes, M.; Aszyk, C.; Bartsch, H.; Schirren, J.; Fisseler-Eckhoff, A. Differential miRNA-Expression as an Adjunctive Diagnostic Tool in Neuroendocrine Tumors of the Lung. Cancers 2016, 8, 38.
- Nadal, E.; Zhong, J.; Lin, J.; Reddy, R.M.; Ramnath, N.; Orringer, M.B.; Chang, A.C.; Beer, D.G.; Chen, G. A MicroRNA Cluster at 14q32 Drives Aggressive Lung Adenocarcinoma. Clin. Cancer Res. 2014, 20, 3107–3117.
- Niikawa, H.; Suzuki, T.; Miki, Y.; Suzuki, S.; Nagasaki, S.; Akahira, J.; Honma, S.; Evans, D.B.; Hayash, S.; Kondo, T. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin. Cancer Res. 2008, 14, 4417–4426.
- Kriegsmann, K.; Zgorzelski, C.; Muley, T.; Christopoulos, P.; von Winterfeld, M.; Herpel, E.; Goeppert, B.; Mechtersheimer, G.; Sinn, P.; Stenzinger, A.; et al. Immunohistological expression of oestrogen receptor, progesterone receptor, mammaglobin, human epidermal growth factor receptor 2 and GATA-binding protein 3 in non-small-cell lung cancer. Histopathology 2020, 77, 900–914.
- Huang, Q.; Zhang, Z.; Liao, Y. 17β-estradiol upregulates IL6 expression through the ERβ pathway to promote lung adenocarcinoma progression. J. Exp. Clin. Cancer Res. 2018, 37, 133.
- Gao, X.; Cai, Y.; Wang, Z.; He, W.; Cao, S.; Xu, R.; Chen, H. Estrogen receptors promote NSCLC progression by modulating the membrane receptor signaling network: A systems biology perspective. J. Transl. Med. 2019, 17, 308.
- Rodriguez-Lara, V.; Hernandez-Martinez, J.M.; Arrieta, O. Influence of estrogen in non-small cell lung cancer and its clinical implications. J. Thorac. Dis. 2018, 10, 482–497.
- Smida, T.; Bruno, T.C.; Stabile, L.P. Influence of Estrogen on the NSCLC Microenvironment: A Comprehensive Picture and Clinical Implications. Front. Oncol. 2020, 10, 137.
- Koutras, A.; Giannopoulou, E.; Kritikou, I. Antiproliferative effect of exemestane in lung cancer cells. Mol. Cancer 2009, 8, 109.
- Wang, L.J.; Li, J.; Hao, F.R. Dexamethasone suppresses the growth of human non-small cell lung cancer via inducing estrogen sulfotransferase and inactivating estrogen. Acta Pharmacol. Sin. 2016, 37, 845–856.
- Tang, H.; Liao, Y.; Zhang, C. Fulvestrant-mediated inhibition of estrogen receptor signaling slows lung cancer progression. Oncol. Res. 2014, 22, 13–20.
