Flavonoids are common plant natural products able to suppress ROS-related damage and alleviate oxidative stress. One of key mechanisms, involved in this phenomenon is chelation of transition metal ions. From a physiological perspective, iron is the most significant transition metal, because of its abundance in living organisms and ubiquitous involvement in redox processes. The chemical, pharmaceutical, and biological properties of flavonoids can be significantly affected by their interaction with transition metal ions, mainly iron.
- Iron complexation
- Flavonoids
- oxidative stress
- iron homeostasis
1. Introduction
Flavonoids represent a group of secondary (specialized) metabolites (approx. up to 10,000) that are widely distributed in the plant kingdom. Collectively, they are a subclass of phenolics and remain the most intensively studied group of polyphenols, responsible for many health benefits attributable to high vegetable consumption and using polyphenol-rich herbs.
They perform a variety of functions, mainly ecological (giving a characteristic colour to plant organs, especially flowers and fruits), and they also participate in the regulation of plant development and growth, as well as plant–microbe and plant–animal interactions (signalling functions) [1]. Due to strong antioxidant properties, they are involved in UV protection. They are intensively discussed and studied thank their positive influence on human health. Flavonoids occur both in free state and as glycosides (O- or C-). The structure of flavonoids consists of a diphenylpropane (C6–C3–C6, benzo-γ-pyrone) skeleton [2], but biosynthetically, they originate from the general phenylpropanoid pathway complemented with malonyl-CoA to form the final core structure of flavane. Further, when the B ring is moved from position 2 of the C-ring to the carbon atom in position 3, the isoflavones are formed, whereas those in which the B ring is linked in position 4 are called neoflavonoids. However, B ring remains in position 2 of the basic flavane skeleton which can be subdivided into different subgroups depending the degree of unsaturation and oxidation of the C ring. These subgroups are: flavones, flavonols, flavanones, flavanonols, flavanols or catechins, and chalcones (you can see in Figure 1) [3].
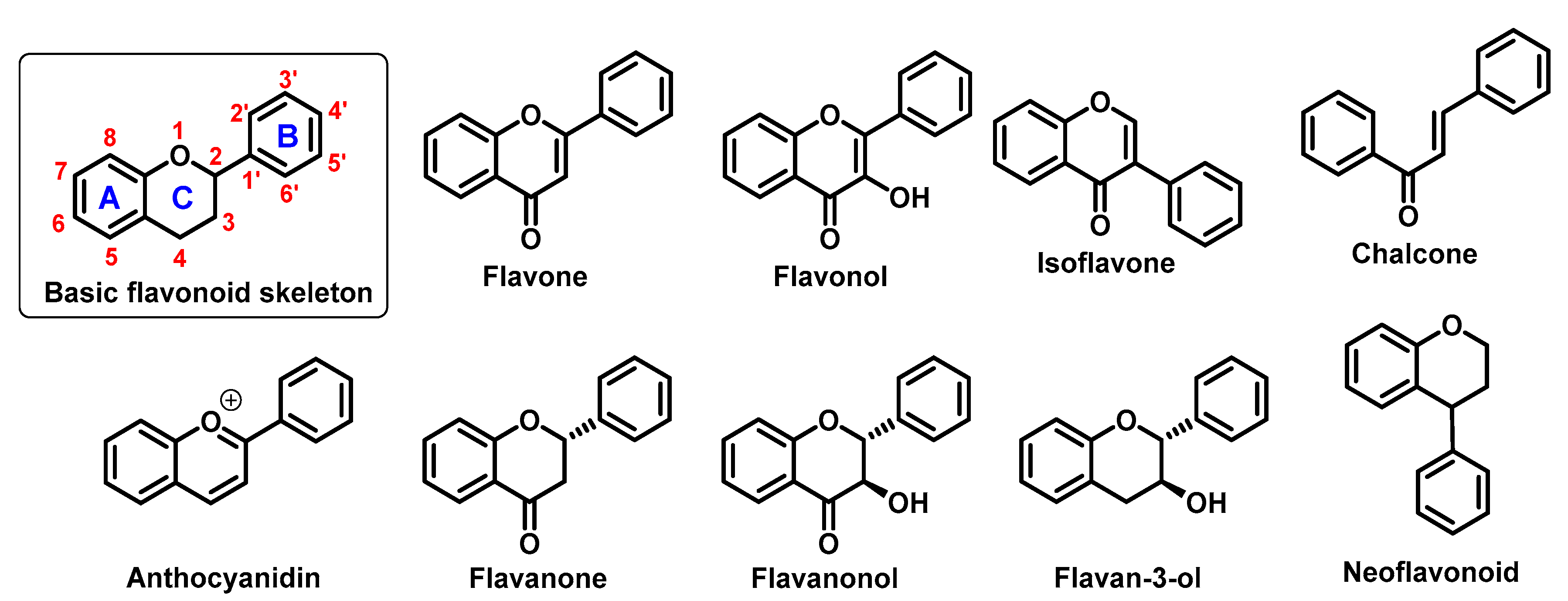
Specific flavonoids form can be represent by anthocyanidins and anthocyanins (their glycosylated form), derivates of 2-phenylbenzopyrylium cation [4]. Unlike other flavonoids, they are intensive pigments, and are responsible for the red, blue and purple color of many plant parts. The most common anthocyanidins are cyanidin, delphinidin, peonidin, malvidin, petunidin, and pelargonidin.
It has been repeatedly postulated that these compounds can be beneficial in the prevention of numerous diseases, particularly oncological, cardiovascular, metabolic and neurodegenerative diseases [5][6][7][8][9][10][11][5,6,7,8,9,10,11].
Numerous works proved various anticancer effects of flavonoids. For example, quercetin can reduce can activity of a nuclear factor kappa B and expression of the P-glycoprotein and thereby angiogenesis and multidrug resistance and cell migration [12][13][14][12,13,14]. Similarly, Gates et al. publish, that kaempferol can significantly decrease a risk of ovarian cancer [15]. Other important benefit of flavonoid application could be the reduction of drug toxicity. Mojžíšová et al. found, that quercetin decrease daunorubicin-induced toxicity for cardiomyoblasts [16].
In this case of cardiovascular diseases, therapeutic effects of flavonoids (e.g., quercetin, or kaempferol) include antihypertensive properties and improved endothelial functions [17][18][17,18]. Hang et al. report that patients with rheumatoid arthritis after bajcalin treatment have decreased levels of apolipoproteins, triglycerides, as well as total- and low density lipoprotein cholesterol, and a lower risk of the coronary artery disease [19]. Bakker et al. found that Montmorency cherry supplement (235 mg/per day anthocyanins) enhanced the recovery of an endothelium-dependent vasodilatation after ischemia-reperfusion injury [20].
Flavonoids are also promising agents for the treatment and prevention of metabolic diseases. Vital et al. found that cyanidin and delphinidin glucosides are potent inductors of insulin secretion [9]. It was observed that the consumption of anthocyanidin-rich extracts prevents obesity in healthy subjects, and helps to reduce the body weight of obese subjects [10]. Silveira et al. report that the daily intake of red orange juice (cyanidin glucosides as major active factors) [21] leads to an increase in serum antioxidant activity and reduction in the levels of C-reactive protein as well as total- and low-density lipoprotein cholesterol [22].
Flavonoids are intensively studied for the treatment of neurodegenerative diseases. Several longitudinal studies showed that habitual consumption of tea (source of catechin, epicatechin, and epigallocatechin gallate) inversely correlate with the onset of PD [23][24][23,24]. Catechins, such as epigallocatechin gallate displayed also numerous therapeutic effects against Alzheimer’s disease [25]. Their molecular targets include amyloid beta peptides and α-synuclein, inflammation, and elevated expression of pro-apoptotic proteins. Similarly, Fan et al. publish that the daily uptake of blackcurrant anthocyanins could be beneficial for Parkinson patients [26].
The above diseases are deeply associated with oxidative stress [27][28][29][30][27,28,29,30] and flavonoids are known as strong antioxidants due to the presence of several phenolic hydroxyl groups, which can be easily oxidized [31]. Structure–activity studies have demonstrated that the antiradical/antioxidant activities are related to structural criteria, such as the presence of an ortho-hydroxyl on the B-ring, presence of one or more free hydroxyl groups, C2–C3 double bond in the C-ring, or the presence of a 3-hydroxyl group [32]. As the result, they can effectively protect cells against oxidative damage caused by excess reactive oxygen (ROS) as well as reactive nitrogen species (RNS). A balance in ROS/RNS (e.g., hydroxyl, superoxide, nitric oxide, nitrogen dioxide, or peroxyl radicals together with non-radical hydrogen peroxide and peroxynitrite) is closely connected with the redox status of the cell that is influenced also by sulphur compounds (both sulphur-rich proteins and low-molecular thiols) and metal ions with chelating properties. Although most of results have been obtained from in vitro studies, increasing evidence indicates that flavonoids may display antioxidant functions also in vivo [33][34][33,34]. Due to the fact that they can be obtained from food in significant amounts, after ingestion of flavonoid-rich food, their blood levels can reach approximately micromolar concentrations, even if most of them are not absorbed or decomposed by gastrointestinal tract inhabiting microorganisms [35]. It implies, that their antioxidant properties can have some degree of importance in the therapy and prevention oxidative-stress related diseases. These properties are, at least partially, associated with chelation of free transition metals ions, mainly iron, which may contribute to oxidative stress exacerbation. Moreover, flavonoids can directly supress ROS itself and chelated metal ions, mainly iron ones can be significantly changed upon interaction with chelated metals.
Iron is one of the essential elements of every living organism and the most widespread transition metal. Iron participates in oxygen transport, (mainly in hem form) and is necessary for correct function of many enzymes involved in electron transfer and oxidation-reduction reactions [36]. Their various functions require two different oxidation states: Fe(II) or Fe(III), that determines their affinity to a number of biological ligands, such as amino acids, thiols, phenols and porphyrins. Most of the body iron is sequestered in the hem moiety of erythrocyte haemoglobin and muscle myoglobin (73%). Other 15% represent so called iron-labile-pool that is requested for many important ongoing biochemical reactions. The remaining 12% is stored in cellular ferritin.
A number of recent works showed that flavonoids (e.g., quercetin, baicalein, and baicalin) can form high-affinity complexes with transition metal ions, such as iron and copper [37][38][39][40][37,38,39,40]. This phenomenon is considered as the key mechanism of their biological activity (e.g., radical scavenging) [39][41][42][39,41,42]. Furthermore, metallocomplexes formed by iron chelation may display their own unique biological activities. Iron complexation affects their biochemical properties such as lipophilicity, membrane transport, or interactions with biomolecules [43][44][45][46][47][48][49][50][51][52][53][54][55][56][43,44,45,46,47,48,49,50,51,52,53,54,55,56].
2. Flavonoid Metallocomplexes in the Living Systems and Biological Effect of Their Interactions with Iron Ions
Iron-flavonoid complexes can interact with various biomolecules, hence the importance of research aimed at their potential biological and medicinal applications. For example, Yang et al. found that flavonoid affinity for HSA is significantly improved in the presence metal ions (e.g., Fe(II) and Co(II)) [52]. Also, part of the hemoglobin complexation with an isoflavonoid such as genistein is its interaction with hem iron [51].
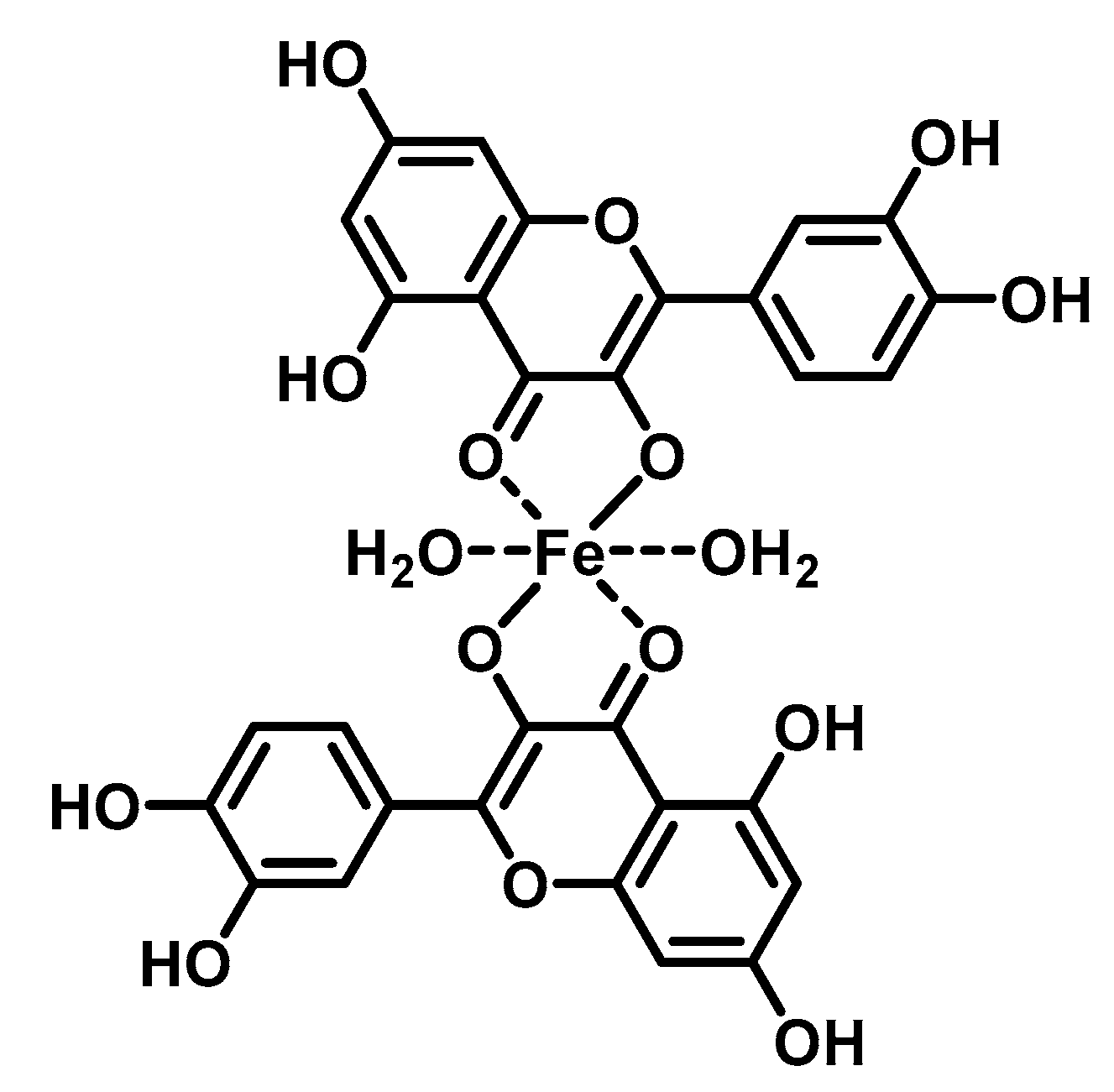
The DNA-flavonoid metallocomplexes displayed significantly higher affinity than free flavonoids. For some of them such as quercetin-zinc complex (2:1, ligand:metal), intercalation into DNA was observed, resulting in significant improvement of cytotoxicity to cancer cell lines (HepG2, SMMC-7721, and A549) [57][94]. Raza et al. showed that an enhanced antibacterial effect (e.g., Staphylococcus aureus) of quercetin-iron complex (2:1, ligand:metal; Figure 2Figure 4) could be associated with DNA intercalation and hydrolysis [49].
On the other hand, that metal complexes of flavonoids (rutin, dihydroquercetin, epigallocatechin gallate and epigallocatechin) displayed strong in vitro protective effect against chrysotile asbestos-induced hemolysis [56]. For example, combination of FeSO4 with rutin or dihydroquercetin unharmed red cells from oxidative stress an order of magnitude higher than free flavonoids. According to the authors, this effect could be related to the enhanced membrane uptake of these metallocomplexes.
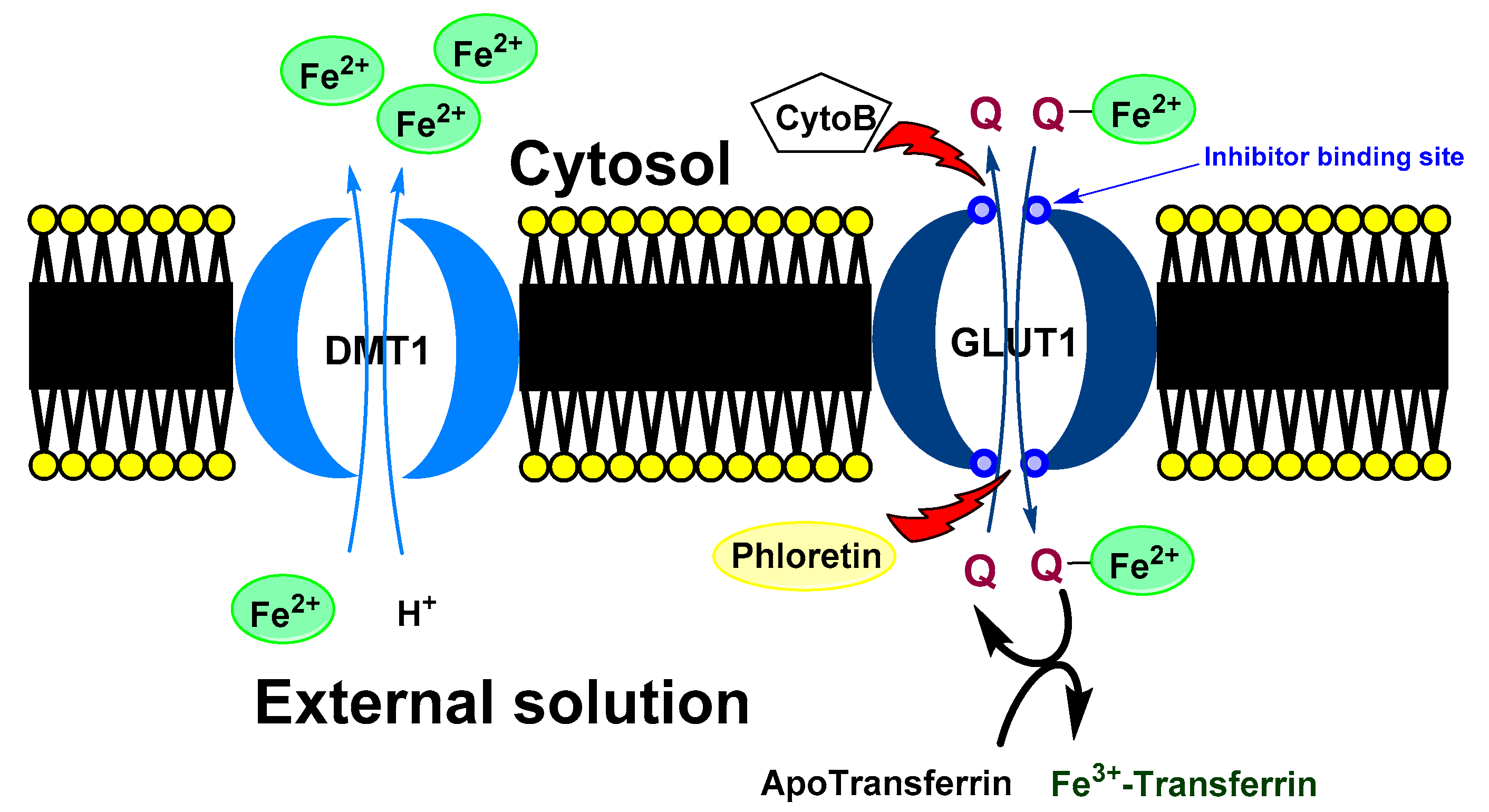
Baccan et al. reported that quercetin iron complexes can cross cell membrane of iron-overloaded HeLa cells (10 μM (NH4)2Fe(SO4)2 and 100 μM ascorbate) and transport the ions to transferrin [43]. A possible explanation of this phenomenon could be a formation of hydrophobic Fe(II) metallocomplex with more quercetin ligands. On the other hand, Horniblow et al. observed that formation of quercetin-iron complex hindered iron uptake into the RKO cells (human colon carcinoma cell line) [38]. However, preincubation with quercetin (12 h before FeSO4 application) resulted in ion higher labile intracellular Fe(II) level than without quercetin. This effect was coupled with significant reduction of ferritin expression both with quercetin and rutin and increased TfR1 level (transferrin receptor protein 1; required for iron import from transferrin into cells). This observation phenomena could be explained by higher cellular transport and lover storage capacity. Nevertheless, simultaneous application of FeSO4 with small amount of the quercetin (2 μmol/L) lead to higher concentration of Fe(II) ions, against alone FeSO4 but when rutin was used, the effect was observed dose-dependently at 2, 20 and 200 µmol/L. A plausible mechanism was suggested by Vlachodimitropoulou et al [58][91]. who found that GLUT1 (glucose transporter) can transport quercetin–Fe(II) (in this case small quercetin concentration; 1 μmol/L and less) from cytosol to extracellular medium. At the same time, quercetin, but not rutin, is also a GLUT1 inhibitor by binding to its exofacial site that would explain the irregular dose-response [59][95].
A proposed model of quercetin effect on the intracellular iron homeostasis is shown in Figure 3Figure 5.
It implies that their biological effect can be significantly different from both components (a free flavonoid and free iron ions). For example, in the study by Farid at al [46], the application of iron-quercetin complex lead to significant increase of catalase activity and reduction of protein carbonylation and level of thiobarbituric acid reactive substances in the adipose tissue of diabetic rats. The effect of only free Fe(II) ions in the form of iron sulphate or alone quercetin was opposite. It is plausible that some physiological effects attributed to quercetin are actually caused by its complexes with metal ions, such as Fe(II). For example, quercetin-iron complex facilitated the formation of NO from nitrite both in vitro and in vivo. Although, the quercetin itself can react with nitrite to form NO in vitro [60][61][96,97], this process can be limited in vivo. Raza et al. found that application of nitrite along with quercetin and FeSO4 increased formation of NO-hem complexes in rat erythrocyte haemoglobin, whereas the effect of FeSO4 and quercetin alone was significantly lower or negligible [48].
The evidence summarized above suggests that investigating biological effect of flavonoids should consider not only the original and intact molecular structure. Besides metabolic transformation (e.g., demethylation, deglycosylation and sulfonation of phenyl groups) [62][63][64][65][66][98,99,100,101,102], formation of flavonoid-metal complexes, in particular with iron and other transition biometals significantly modifies their properties. Full understanding of these interactions would certainly lead to their adequate application in prevention and treatment of many health disorders.
