Forward osmosis (FO), the most common osmotically driven membrane process, stands out as the most promising alternative for RO processes due to its inherently low fouling tendency, easier fouling removal, and energy efficiency when compared to pressure-driven–type membrane processes.
- thin film composite
- FO substrate
- desalination
- wastewater treatment
1. Introduction
In many parts of the world, rapid human development and economic growth combined with worsening climate change are creating negative pressures on water demands [1][2]. The limited access of clean water in some arid areas has resulted in long-term ecosystem damage and has threatened human security through waterborne-related diseases. These overarching global crises take priority over everything, further pushing researchers towards developing innovative, advanced, and affordable water treatment technologies to address the challenges. As the world continues to navigate the pandemic of coronavirus diseases 2019 (COVID-19), now more than ever the access to clean and safe water is critical to maintain a healthy lifestyle. Improving water and wastewater treatment systems is especially important in ensuring continual supply of clean and safe water for ourselves, our families, and our surroundings. Amidst the global water scarcity, various water/wastewater treatment technologies such as solvent extraction, membrane filtration [3][4][5], adsorption [6][7][8][9], chlorination [10][11], and electrocoagulation– flocculation [12][13][14] have been developed. The implementation of water reclamations through these technologies imposes a positive impact on treating water and decreasing health complications. Among the existing technologies, membrane-based separation has steadily gained increasing acceptance over the years and has often become the first choice for a reliable performance. Principally, membrane-based separations involve the selective filtration of solutes through pores of different sizes while allowing only water molecules to pass through. Membrane technology is crowned by sustainability criteria in terms of handy design programs, an easy scaling up process, minimal environmental impact, flexibility, and adaptability [15][16][17]. In addition to this, helpful literature and improved knowledge have led to this technology becoming much more familiar. Membrane technology has started to become a favorable treatment process since the development of cellulosic reverse osmosis (RO) membrane via phase inversion developed by Loeb and Sourirajan in the 1960s [18]. This innovation has reached maturity and has grown in line with the increasing acceptance of membrane-based wastewater treatment and desalination technology to deal with increasing water demands and stringent regulations [19][20]. The engineered applications of the four most common pressure-driven membrane processes, i.e., RO, nanofiltration (NF), ultrafiltration (UF), and microfiltration (MF) include water desalination, reclamation, purification, and wastewater recycling.[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110]
In particular, RO, with the finest degree of separation, has achieved huge commercial success due to its high efficiency in removing dissolved ions, particles, and bacteria in the water, as well as having broad tolerance with feed stream of different qualities. The operation of RO requires high hydraulic pressure as a driving force. This has imparted negative consequences to the cost, energy consumption, and fouling propensity of the processes. As a strategy to address these limitations, attention has been switched to the application of osmotically driven processes as an alternative technology for desalination and wastewater treatment. Forward osmosis (FO), the most common osmotically driven membrane process, stands out as the most promising alternative for RO processes due to its inherently low fouling tendency, easier fouling removal, and energy efficiency when compared to pressure-driven–type membrane processes [16][17][18][19]. Owing to these attractive inherent features, FO has been used as a concentration and dilution process in diverse areas including food processing [21][22][23][24], wastewater treatment [25][26][27], desalination [28][29][30][31], and power generation [3][32]. In medical application, FO assists in controlling the release of drugs with low solubility [33][34] and in preserving properties of feed (nutrition, taste, quality, etc.) when it comes to the pharmaceutical industry [35]. FO is also known as an ideal pretreatment step in many integrated membrane processes for desalination/wastewater treatment [36][37][38]. However, the high potential shown by the FO process to unravel the present-day water shortage has been hindered to some extent by some practical challenges. The frequently encountered issues of FO include concentration polarization (CP), fouling, weak membrane mechanical strength, low membrane flux, and high expense to regenerate draw solution and recover water from draw solution, which is behind the unfavorable separation performance of the FO membrane [39]. These factors have triggered more developmental and implementation research into a sustainable FO membrane separation process for a proper acknowledgement and understanding of the FO process. Various improvement attempts have been made to further advance FO technology in order to seize the foothold in mainstream water/wastewater industries.
The dominant factors affecting FO performance along with potential implications on the overall process have been investigated in numerous studies [40][41][42][43]. Some of the important factors are membrane properties, operating conditions, and types of draw and feed solutions [44]. Many studies have mentioned the crucial role of membrane properties such as charge, roughness, and pore size on the transport behavior and hence the overall FO performance [43][44][45][46][47][48]. An ideal draw solution should be capable of generating high osmotic pressure, reducing reverse diffusion, and easily re-concentrating and recovering in order to enhance driving force for efficient separation and water transport [49][50]. Low molecular weight salts, especially NaCl, are widely applied as draw solution due to their high solubility and re-concentration simplicity. It is also notable that characteristics and properties of draw solution have profound effects on the degree of membrane fouling as well as water flux [51]. In addition, operating conditions including solution chemistry (pH and temperature) and membrane orientations can be altered to reduce the effects of fouling. Membrane surface charge varies with pH of feed solution, where high charge facilitates diffusion of the draw solutes in the substrate [52]. Meanwhile, osmotic pressure, fluid viscosity, mass transfer, and solubility are dependent on solution temperature. It is essential to keep solution temperature constant so that the membrane performance is not altered [53]. Zhao and Zou [54] observed that water and salt permeabilities increased at a higher temperature as viscosity decreased and water diffusivity increased. In FO operations, membrane can be oriented to FO mode or AL-FS (active layer facing the feed solution), which provides a more stable and higher water flux than that in the alternative membrane orientation, i.e., pressure-retarded osmosis (PRO) mode or AL-DS (active layer facing the draw solution). Practically, the AL-FS orientation is more favorable when the membrane is employed in wastewater treatment since AL-DS orientation would lead to more severe and irreversible membrane fouling [55].
A large step in heightening the performance of the FO process is through the upgrading of the FO membrane. Unceasing pursuit of fabrication strategies for low CP and antifouling membrane remains a major theme in terms of this topic. Thin film composite (TFC) membranes are currently popularly used membranes owing to their superior performances in terms of water flux and salt rejection [56]. TFC membrane is composed of a substrate layer and a selective layer on top of the substrate [57][58]. The layers are fabricated and tailored separately towards each optimum structure and property. The produced membrane can be with or without the thin nonwoven layer underneath the substrate layer. To date, numerous studies related to TFC-FO membranes have been devoted to polyamide (PA) active layer and substrate layer modification to combat membrane fouling, alleviate CP, and achieve high flux performance [59][60][61][62][63]. Previous investigations revealed that surface roughness, porosity, pore size, and hydrophilicity of the substrate have substantial influence on the microstructure of the PA layer formed and the membrane performance [16][56][64]. The high porosity and low tortuosity membrane collectively lower the CP phenomena, especially internal concentration polarization (ICP). This has hence attracted researchers to fabricate and modify the substrate of the FO membrane to transform it into a valuable finished membrane. For instance, current studies are focusing on the selection of additives with a proper modification technique as they affect substrate characteristics and eventually determine the ultimate performance in the FO process [65]. Introduction of a suitable additive/nanofiller to the casting solution would enhance pore interconnectivity and/or hydrophilicity [66]. Many endeavors have been made by worldwide researchers on the use of additives, including hydrophilic polymer, nanomaterials, pore former via blending, surface coating, template-assisted, and electrospinning, all being among the most popular fabrication routes that promote construction of desired substrate properties [65].
The advancement has been rapidly made in the preparation and modification of FO membranes all the while. There is a need to review these developments in order to pave the way towards further studies in the future. Recently, Akther et al. [67] provided a state-of-the-art summary of the nanomaterial-modified PA layer, substrate layer, and the surface of the PA TFC-FO membranes. Suwaileh and colleagues [68] presented a review on the advancement of synthetic polymer and the substrate, focusing on the fabrication and chemical modifications. Aquaporin-based biomimetic FO membranes, which are different in fabrication technique and behavior, were also included. Their reported progress, however, is limited to research studies until 2017. Meanwhile, a comprehensive review by Goh et al. [69] outlined up-to-date strategies used in membrane designs and fabrications, also highlighting fouling mitigating strategies, particularly for wastewater treatment. However, the progress of substrate fabrication and modification to deal with ICP and fouling specifically for FO desalination and wastewater treatment based on more recent progress has not been reviewed.
2. Overview of Forward Osmosis Membranes
2.1. Forward Osmosis Membrane
Like for other membrane processes, the high-performance semipermeable membrane is the key to achieving a highly efficient FO process. Technically, a membrane that is comprised of dense, non-porous, and selectively permeable materials can be used for the FO process. On the basis of their fabrication materials, these available membranes would be categorized as cellulosic membranes, thin film composite (TFC) membranes, and chemically modified membranes [1]. Polymeric membranes account for the largest proportion of the currently installed membranes. In a period of history, typical cellulose acetate (CA)-RO membranes and TFC-RO membranes were feasibly explored for the FO process, as no membrane was designed specifically for the process. CA membrane profits from its comparative hydrophilicity, good mechanical strength, and wide availability balanced with cost-effectiveness [70][71]. This cellulosic membrane is commonly applied for nonsolvent-induced phase separation methods for both active and substrate layers, which is relatively easy to scale up and has good hydrophilicity and mechanical strength [58]. Despite favorable characteristics of CA membranes, coordinating trade-off between water permeability and rejection is challenging due to pH sensitivity, temperature resistance, and biofouling mitigation [39][72]. During the FO process, cellulosic membranes are usually susceptible to chemical hydrolysis, low selectivity, and biological attack [14]. Although innovation has been implemented on the CA-FO membrane, some its membrane performances are generally inferior to the TFC membrane, which is primarily correlated to the structural compaction and high operating pressures [57][73][74][75]. Thus, continuing research is needed on the TFC-FO membrane for the FO process because of its porous substrate and thin active layer that make it capable of promoting high water permeability and selectivity, respectively.
Later, Hydration Technologies, Inc. (HTI) developed the first generation of commercial FO membranes, one of which has a characteristic structure of cellulose triacetate (CTA) embedded with thin polyester mesh support. The flux of the TFC-FO spiral element was twice of that existing CTA membranes [76]. This achievement has provided a new benchmark in the development of FO membranes. Nevertheless, developing a FO membrane with superior water permeability and salt rejection is still one of the biggest challenges for the practical application of FO. The TFC-FO membranes have been designed differently from the TFC-RO membranes, especially with regard to the substrate (polymeric support layer), which for FO membranes is considerably more porous, more hydrophilic, and thinner. The most common polymers used for the preparation of TFC-FO membrane substrate are polysulfone (PSf), polyethersulfone (PES), polyacrylonitrile (PAN), polyvinylidene fluoride (PVDF), and cellulose derivatives. TFC membranes are the predominant kind of membranes as of now, thanks to their flexibility in design, as both the active and substrate layers can be tailored for specific needs [77]. Moreover, they provide higher selectivity and productivity with less energy consumption as compared to typical asymmetric membranes [47]. The topmost active layer is designed as a barrier that blocks feed solutes or contaminants while allowing only water molecules to be permitted. Differently, the substrate serves as the foundation of the composite membrane, providing mechanical strength and flow pathway. Beyond the basics, it also lays a versatile platform for growth of the PA layer. This membrane structure imposes less resistance to mass transport and improves the overall membrane productivity. The transport of components, namely, water and solute(s), depends on several parameters such as water permeability (A), solute permeability (B), and the structural parameter of the substrate layer (S), which are intrinsic membrane parameters [47].
As generally recognized, the fouling behavior in the FO processes has a strong dependence on the membrane-selective layer, while ICP is an inevitable key issue in the substrate layer causing a reduction in water flux [56][78][79]. As such, improvement of FO semi-permeable membranes necessitates further research and new thoughts in order to achieve great performance. On the basis of the data in the literature, the desired criteria of ideal membranes for FO would be (1) a dense and ultra-thin active layer for high solute rejection; (2) having a porous, thin, and low-tortuosity substrate to minimize ICP effects, thereby increasing flux and reducing membrane fouling; (3) hydrophilic substrate increasing the wetting of small pores for high flux and low fouling propensity; (4) and a robust mechanical, chemical, and thermal stability substrate to sustain both long-term operation and hydraulic pressure [16][80][81]. Therefore, alongside production of the commercial HTI-TFC membrane, there has been a rise in the attempt to modify and fabricate TFC membranes with essential properties suitable for FO applications. A wide range of TFC membranes have been implemented for the FO process in both flat sheet and hollow fiber configurations. In parallel to the advances of TFC flat sheet [40][61][82][83][84][85], hollow fiber membranes have been widely fabricated for FO application, owing to their self-supported structure and high packing density [86][87][88][89][90]. Membranes are usually provided as flat sheets for water treatment applications dealing with high concentration of fouling agents or solutions with high viscosities [91]. Meanwhile, large-volume water treatment commonly applies hollow fiber FO membranes [86][92][93].
2.2. Challenges Confronted by FO Membrane
Although facing some critical issues that limit the applications, research on FO membranes is still being conducted, even though the osmotically driven membrane processes have been extensively investigated with some noticeable results being reported. As mentioned earlier, ICP and fouling are the major hiccups in terms of the development of a TFC-FO membrane as they restrict the overall performance [57].
2.2.1. Concentration Polarization
Concerted research efforts have been dedicated in terms of better understanding and mitigating polarization effects imposed on all asymmetric membranes—pressure-driven and osmotically driven. The polarization issue relates especially to ICP, which occurs inside the pores of the porous substrate, remaining as a main constraint in FO. In FO, the differential osmotic pressure and solvent flow has been effectively reduced as feed solution is more concentrated on one side of the membrane and the draw solution is more diluted at the other. An unsatisfying value of water flux and reverse salt flux is obtained due to the severe mass transfer resistance built up both inside and around membranes during the osmosis process (CP phenomena) [50][96][97]. The magnitude of the effects depends on the membrane nature and mode of orientation, as illustrated in Figure 1a. Conceptually, CP falls into two main categories that occur concurrently, i.e., external concentration polarization (ECP) and ICP [73]. The orientation of AL-FS giving rise to concentrative ECP and dilutive ICP simultaneously. AL-DS on the other hand, would experience dilutive ECP and concentrative ICP.
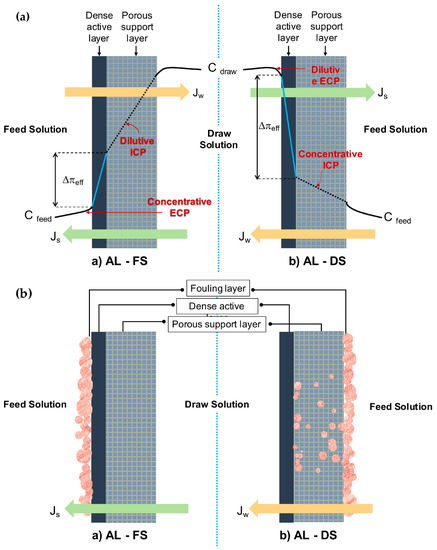
Figure 1. A conceptual illustration of the (a) membrane orientations AL-FS (active layer facing the feed) and AL-DS (substrate layer facing the feed) with a concentration polarization (CP) profile and (b) membrane fouling in forward osmosis (FO) membrane at different orientations.
ECP occurs outside of membrane due to solute accumulation within the surface of the dense active layer, which could possibly be counteracted by optimizing flow conditions and hydrodynamics design [98]. Another implemented solution to this problem is topping the porous substrate with a highly selective active layer [99][100]. Meanwhile, the difficult solute diffusion through the porous substrates mainly results in serious ICP. The occurrence is induced by the thick substrate layer and high S value that contributes to a massive decline in the effective osmotic driving force and thus the flux. [73]. The ICP problem is always more pronounced and burdensome than ECP [100]. ICP residing in the membrane structure cannot be easily controlled, neither by stirring nor spacer design [98]. Therefore, the key to solve this problem is to construct the substrate with its interior pores highly interconnected by understanding that the mechanism on ICP is essential in order to innovate membrane design and synthesis. The breakthrough for FO came with the innovation of tailored FO membranes, generating higher fluxes to ascertain the adverse effect of ICP [63][73]. For instance, it is known that a hydrophilic substrate with a smaller membrane S parameter are effective against ICP. The hydrophilic substrate allows a complete wetting throughout the structure. A hydrophilic substrate with improved wettability is beneficial with regards to water, and solute molecules facilitate transportation, decrease the effective tortuosity, and increase the porosity to reduce the air entrapping in the membrane pores. A combination of the effects led to remission of ICP and improvement of the performance while maintaining strength or flexibility of the membrane.
An insight to the degree of ICP is quantified using the S parameter and is primarily influenced by three intrinsic properties of substrate: wall thickness, porosity, and tortuosity, as defined in Equation (1):
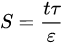
where t is the thickness, τ is the tortuosity, and ε is the porosity of the substrate layer. The S parameter is inversely proportional to membrane porosity. Less air entrapped within the membrane pores would increase the porosity, make the flow path less tortuous, and provide a direct path from draw solution to the PA layer. These structural characteristics reduce the value of the S parameter to increase effective osmotic difference across the membrane. The swift transport of water and solute molecules together with smaller value of S parameter contributes to IC suppression and higher water flux [56][73]. However, the value of the S parameter can never be lower than the wall thickness of the substrate layer. Analysis has shown that the thickness of substrate affects the S parameter by a factor of 10 compared to porosity and tortuosity, as their values only change within a limited range [101]. Membranes that suffer from S value that is too high would possibly produce high permeance but not the fluxes. Only at lower S values can the membrane permeance substantially contribute to the higher water flux. Other substrate parameters to be considered besides those three are the pore size and morphology hydrophilicity, and charge. To effectively implement this platform technology, the substrate structure needs to be engineered to achieve minimal thickness and tortuosity, as well as high porosity and hydrophilicity, without the mechanical fragility of the latter.
2.2.2. Membrane Fouling
Besides ICP, FO membranes also suffer from fouling, a long-standing problem shared by other membrane processes [1][102][103][104]. Fouling arises from a variety of factors associated with surface chemistry, membrane morphology, and structural properties. In general, solute particles that accumulate or adsorb either on the surface of a membrane or are entrapped within its pores causes a fouling [105]. Application of hydrophobic polymers i.e., PES, PVDF, PSf, and polypropylene (PP) used to fabricate the membrane do not swell in water, but they are likely to adsorb foulants. Physical and chemical interaction of foulants and the membrane results in poor membrane productivity by reducing the quality and quantity of permeate, i.e., pure water fluxes, as well as shortening membrane lifespan depending on how pronounced it is [55][106]. After comparing the fouling of FO with RO, Holloway et al. reported that the flux decline rate was greater with RO [107]. The authors speculated that both the lower extent of FO fouling and its reversibility (enabling easy cleaning) was due to the effects of hydraulic pressure upon the foulants on the membrane surface, which occurs rapidly in RO [69][107]. Still, a membrane with a long-term antifouling ability needs to be developed so as to have the prominent advantage of keeping flux decline caused by fouling to a minimum extent. Through this way, the FO membrane could be highly known for efficiently treating fouling/saline wastewater and thus outmaneuvering pressure-driven salt-rejecting membranes such as RO and NF membranes.
Like the fouling phenomenon in pressure-driven membranes, fouling for the FO membrane also experiences different types of fouling, namely, biofouling, colloidal fouling, inorganic scaling, and organic fouling [1]. Biofouling is a complex form that results from adhered microorganisms (from feed) on the membrane surface that subsequently form a biofilm, increasing its resistance to water [108][109]. Meanwhile the deposition of suspended particles (e.g., clay, silica) and organic matter (e.g., humic substances, proteins, and amino acids) on the membranes develop other types of fouling called colloidal and organic fouling, respectively [69]. Colloidal fouling on the surface leads to less porous fouling layers, hence reducing the flux. Scaling arises from the high concentration of some inorganic ions such as metal sulfates and carbonates in the water, leading to precipitation near/on the membrane surface [110]. In real-application conditions, different types of foulants almost always coexist in natural waters, resulting in simultaneous occurrence of different types of fouling that can influence each other. It is essential to determine the degree and nature of the fouling so as to select the appropriate cleaning and modification strategy.
As schematically diagrammed in Figure 1b, fouling in FO tends to occur internally and externally, at either on the active layer, on the surface, or inside the substrate layer, which is dependent on the type of operation modes. The literature claims that external fouling occurs in AL-FS mode as the deposition of foulants take place on the active layer, forming a cake-type layer. Surface properties such as surface roughness have a greater effect on this type of fouling than other properties (e.g., surface hydrophilicity), therefore enabling easier removal and cleaning. Differently, under AL-DS mode, the constriction of pores due to the deposited foulants on the active layer that is trapped within the membrane leads to internal fouling, which is very hard to clean up [31][80]. The fouling was dominated over structural properties of the support layer, which is more intense compared to AL-FS mode. Additionally, entrapment of foulants in the support layer would reduce porosity and enhance the effects of ICP in membrane, degrading the performance with a consequent increase of energy and membrane replacement costs.
3. Motivations of FO Membrane Modification
Despite the fact that ICP and fouling cannot be completely avoided in the osmosis process, upgrading of the properties of surface and structural properties of substrate is an appreciable perspective that ought to be considered in order to achieve excellent substrate properties without compromising flux performance. Recent literature has shown that modification focused on the substrate layer is much less studied in comparison with the selective active layer. For this reason, ongoing experiments and modeling on substrate construction are increasing in order to tackle afforested issues and meet the requirements of practical applications. On the basis of the analysis, it is clear that the porous nature (pore size, size distribution, and porosity) and the surface properties (hydrophilicity and roughness) of the substrate layer significantly affects the crosslinking degree during interfacial polymerization and correspondingly the thickness and morphology of the PA layers formed on top of the substrates [58]. This consequently leads to variation of the performances of the FO membranes including water permeability, salt rejection, and fouling resistance.
It would be of benefit to produce a promising TFC-FO membrane where the substrate layer adequately supports the active layer during both formation and operation. Another important aspect is long-term chemical and mechanical stability, as well as an efficient route for large-scale fabrication. It was found that the effect of ICP can be alleviated by minimizing the S value of the membrane substrates. The S parameter proportionally decreases as the porosity increases, while increasing with the thickness and tortuosity of the substrates. Such characteristics increase the mass transfer and reduce the ICP. As a main step in controlling ICP, the substrate layer hence needs to be resigned to incorporate a combination of characteristics: thinness, high porosity, excellent hydrophilicity, and less pore tortuosity to minimize ICP and maximize water flux in FO. Similarly, considerable efforts have been made in PA layer modification in recent years as an improvement in the aspects related to antifouling and anti-biofouling properties. However, as substrate has a synergic effect on the active layer, substrate modifications are needed so that the membrane has lower tendencies to fouling and so the performance will not deplete rapidly; hence, small maintenance is needed.
4. Overview of Fabrication and Modification Techniques
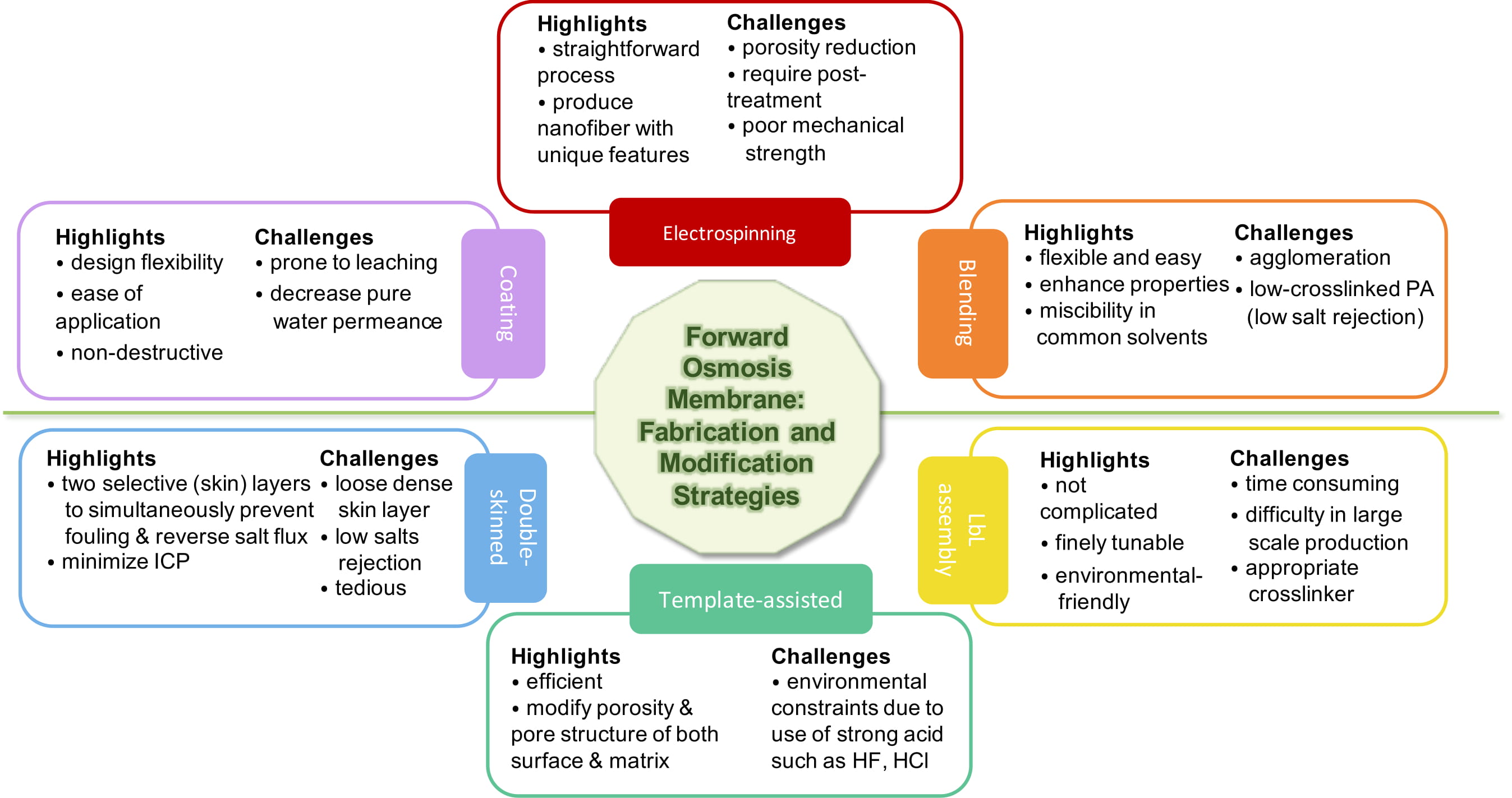
Close attention is paid to the role of the membrane substrate layer in terms of providing exciting avenues for FO in desalination and water reclamation processes. The application of asymmetric membranes in osmotically driven membrane processes require effective properties in terms of porosity, thickness, hydrophilicity, and surface charge in order to be able to improve its performance. As reported, high hydrophilicity and fully wet substrate allow for effective water transport, otherwise the trapped vapor or air may further block the water flux, reducing the effective porosity and dramatically exacerbating ICP [78]. Fabrication via electrospinning, modification through bulk modification, or surface modification are usually applied to the substrate to increase hydrophilicity, reduce thickness, and adjust porosity [58]. This could be achieved through various methods, particularly by incorporating additives/nanofillers via plasma treatment, grafting, blending, and coating [58,112], or redesigning the FO membrane structure, e.g., double-skinned membrane using layer-by-layer (LbL) assembly [113]. Figure 2 provides a pros and cons summary of the substrate fabrication and modification that is based on the studies reviewed in this contribution.
Figure 2. Summary of the pro and cons of substrate modifications of on polyamide thin film composite (TFC) membranes
5. Perspectives and Conclusions
The exponential increase of publications and patterns on this technology have witnessed the fundamental understanding of and major challenges in ICP and fouling modelling of FO membrane according to the growth of research activities in many applications, particularly wastewater treatment and desalination. This review is an attempt to provide a basis for the rational selection and modification protocols of the substrate layer on the basis of previous works, inasmuch as the literature has pointed out that a wide range of physical and chemical strategies have been explored on the substrate layer in achieving a favourable membrane with respect to structural properties and performance. Most of the modifications reviewed have successfully shown superiority in implementing hydrophilicity, functionality, selectivity, long-term durability, and antifouling nature to eliminate the intrinsic bottleneck ICP and fouling problem.
However, the real-in field applications of TFC-FO membranes for water treatment is still in its infancy. To facilitate knowledge transfer, more pilot studies on FO systems relying on more robust practical and large-scale operations should be established and studied with continuing monitoring and analysis. More pilot-scale studies in this direction are desired in order to look into possible hiccups and aspects to be improved before the deployment of FO for large-scale commercial applications. Such up-scaling studies should be performed on a case-by-case basis with a full consideration of the source water quality and application environments. Conducting more research in these areas through focusing on membrane replacement costs and reducing pretreatment requirement should be possible in order to establish FO as a treatment technology in manufacturing industries.
References
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Goh, P.S.; Matsuura, T.; Ismail, A.; Ng, B.C. The Water-Energy Nexus: Solutions towards Energy-Efficient Desalination. Energy Technol. 2017, 5, 1136–1155. [Google Scholar] [CrossRef]
- Altaee, A.; Sharif, A.; Zaragoza, G.; Ismail, A. Evaluation of FO-RO and PRO-RO designs for power generation and seawater desalination using impaired water feeds. Desalination 2015, 368, 27–35. [Google Scholar] [CrossRef]
- Ihsanullah, I. Carbon nanotube membranes for water purification: Developments, challenges, and prospects for the future. Sep. Purif. Technol. 2019, 209, 307–337. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Membrane technology for water production in agriculture: Desalination and wastewater reuse. Desalination 2015, 364, 17–32. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.-H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Sakulpaisan, S.; Vongsetskul, T.; Reamouppaturm, S.; Luangkachao, J.; Tantirungrotechai, J.; Tangboriboonrat, P. Titania-functionalized graphene oxide for an efficient adsorptive removal of phosphate ions. J. Environ. Manag. 2016, 167, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Suzaimi, N.D.; Goh, P.S.; Malek, N.A.N.N.; Lim, J.-W.; Ismail, A.F. Performance of branched polyethyleneimine grafted porous rice husk silica in treating nitrate-rich wastewater via adsorption. J. Environ. Chem. Eng. 2019, 7, 103235. [Google Scholar] [CrossRef]
- He, X.; Yang, D.-P.; Zhang, X.; Liu, M.; Kang, Z.; Lin, C.; Jia, N.; Luque, R. Waste eggshell membrane-templated CuO-ZnO nanocomposites with enhanced adsorption, catalysis and antibacterial properties for water purification. Chem. Eng. J. 2019, 369, 621–633. [Google Scholar] [CrossRef]
- Bao, X.; Wu, Q.; Shi, W.; Wang, W.; Yu, H.; Zhu, Z.; Zhang, X.; Zhang, Z.; Zhang, R.; Cui, F. Polyamidoamine dendrimer grafted forward osmosis membrane with superior ammonia selectivity and robust antifouling capacity for domestic wastewater concentration. Water Res. 2019, 153, 1–10. [Google Scholar] [CrossRef]
- Boyd, G.R.; Zhang, S.; Grimm, D.A. Naproxen removal from water by chlorination and biofilm processes. Water Res. 2005, 39, 668–676. [Google Scholar] [CrossRef]
- Koparal, A.S.; Öğütveren, Ü.B. Removal of nitrate from water by electroreduction and electrocoagulation. J. Hazard. Mater. 2002, 89, 83–94. [Google Scholar] [CrossRef]
- Akyol, A. Treatment of paint manufacturing wastewater by electrocoagulation. Desalination 2012, 285, 91–99. [Google Scholar] [CrossRef]
- Yang, T.; Qiao, B.; Li, G.-C.; Yang, Q.-Y. Improving performance of dynamic membrane assisted by electrocoagulation for treatment of oily wastewater: Effect of electrolytic conditions. Desalination 2015, 363, 134–143. [Google Scholar] [CrossRef]
- Goh, P.; Matsuura, T.; Ismail, A.; Hilal, N. Recent trends in membranes and membrane processes for desalination. Desalination 2016, 391, 43–60. [Google Scholar] [CrossRef]
- Long, Q.; Jia, Y.; Li, J.; Yang, J.; Liu, F.; Zheng, J.; Yu, B. Recent Advance on Draw Solutes Development in Forward Osmosis. Processes 2018, 6, 165. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.; Comas, J.; Rodriguez-Roda, I.; Le-Clech, P. Efficiently Combining Water Reuse and Desalination through Forward Osmosis—Reverse Osmosis (FO-RO) Hybrids: A Critical Review. Membranes 2016, 6, 37. [Google Scholar] [CrossRef]
- Loeb, S. Sea Water Demineralization by Means of a Semipermeable Membrane: Progress Report July 1, 1962–December 31, 1962; Contribution (California Water Resources Center); Department of Engineering, University of California: Oakland, CA, USA, 1963. [Google Scholar]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A.F. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Wan, C.F.; Chung, N.T.-S. Energy recovery by pressure retarded osmosis (PRO) in SWRO–PRO integrated processes. Appl. Energy 2016, 162, 687–698. [Google Scholar] [CrossRef]
- An, X.; Hu, Y.; Wang, N.; Zhou, Z.; Liu, Z. Continuous juice concentration by integrating forward osmosis with membrane distillation using potassium sorbate preservative as a draw solute. J. Membr. Sci. 2019, 573, 192–199. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, R.; Li, W.; Tang, C.Y. Whey recovery using forward osmosis—Evaluating the factors limiting the flux performance. J. Membr. Sci. 2017, 533, 179–189. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Saxena, V.; Dutta, S. Fruit juice processing using membrane technology: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 136–153. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; McCutcheon, J.R.; Elimelech, M. Performance evaluation of sucrose concentration using forward osmosis. J. Membr. Sci. 2009, 338, 61–66. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, S.; Zhang, B.; Wang, L.; He, Z. Forward osmosis promoted in-situ formation of struvite with simultaneous water recovery from digested swine wastewater. Chem. Eng. J. 2018, 342, 274–280. [Google Scholar] [CrossRef]
- Zhang, J.; She, Q.; Chang, V.W.C.; Tang, C.Y.; Webster, R.D. Mining Nutrients (N, K, P) from Urban Source-Separated Urine by Forward Osmosis Dewatering. Environ. Sci. Technol. 2014, 48, 3386–3394. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, W.-H.; Ge, Q. A cage-like supramolecular draw solute that promotes forward osmosis for wastewater remediation and source recovery. J. Membr. Sci. 2020, 600, 117862. [Google Scholar] [CrossRef]
- Thabit, M.S.; Hawari, A.H.; Ammar, M.H.; Zaidi, S.; Zaragoza, G.; Altaee, A. Evaluation of forward osmosis as a pretreatment process for multi stage flash seawater desalination. Desalination 2019, 461, 22–29. [Google Scholar] [CrossRef]
- Dabaghian, Z.; Rahimpour, A. Carboxylated carbon nanofibers as hydrophilic porous material to modification of cellulosic membranes for forward osmosis desalination. Chem. Eng. Res. Des. 2015, 104, 647–657. [Google Scholar] [CrossRef]
- Giagnorio, M.; Ricceri, F.; Tiraferri, A. Desalination of brackish groundwater and reuse of wastewater by forward osmosis coupled with nanofiltration for draw solution recovery. Water Res. 2019, 153, 134–143. [Google Scholar] [CrossRef]
- Deng, L.; Wang, Q.; An, X.; Li, Z.; Hu, Y. Towards enhanced antifouling and flux performances of thin-film composite forward osmosis membrane via constructing a sandwich-like carbon nanotubes-coated support. Desalination 2020, 479, 114311. [Google Scholar] [CrossRef]
- Mermier, N.-D.; Borges, C.P. Direct osmosis process for power generation using salinity gradient: FO/PRO pilot plant investigation using hollow fiber modules. Chem. Eng. Process. Process. Intensif. 2016, 103, 27–36. [Google Scholar] [CrossRef]
- Stamatialis, D.F.; Papenburg, B.J.; Gironés, M.; Saiful, S.; Bettahalli, S.N.; Schmitmeier, S.; Wessling, M. Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. J. Membr. Sci. 2008, 308, 1–34. [Google Scholar] [CrossRef]
- Shokri, J.; Ahmadi, P.; Rashidi, P.; Shahsavari, M.; Rajabi-Siahboomi, A.; Nokhodchi, A. Swellable elementary osmotic pump (SEOP): An effective device for delivery of poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2008, 68, 289–297. [Google Scholar] [CrossRef]
- Wang, K.Y.; Teoh, M.M.; Nugroho, A.; Chung, T.-S. Integrated forward osmosis-membrane distillation (FO–MD) hybrid system for the concentration of protein solutions. Chem. Eng. Sci. 2011, 66, 2421–2430. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Li, X.; Chung, T.-S. The forward osmosis-pressure retarded osmosis (FO-PRO) hybrid system: A new process to mitigate membrane fouling for sustainable osmotic power generation. J. Membr. Sci. 2018, 559, 63–74. [Google Scholar] [CrossRef]
- Li, J.; Hou, D.; Li, K.; Zhang, Y.; Wang, J.; Zhang, X. Domestic wastewater treatment by forward osmosis-membrane distillation (FO-MD) integrated system. Water Sci. Technol. 2018, 77, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Sardar, M.; Pal, M.; Chakrabortty, S.; Nayak, J. Modelling forward osmosis-nanofiltration integrated process for treatment and recirculation of leather industry wastewater. Comput. Chem. Eng. 2019, 127, 99–110. [Google Scholar] [CrossRef]
- Sreedhar, I.; Khaitan, S.; Gupta, R.; Reddy, B.M.; Venugopal, A. An odyssey of process and engineering trends in forward osmosis. Environ. Sci. Water Res. Technol. 2018, 4, 129–168. [Google Scholar] [CrossRef]
- Singh, N.; Dhiman, S.; Basu, S.; Balakrishnan, M.; Petrinic, I.; Helix-Nielsen, C. Dewatering of sewage for nutrients and water recovery by Forward Osmosis (FO) using divalent draw solution. J. Water Process. Eng. 2019, 31, 100853. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, M.; Yang, Z.; Wu, H.-C.; Fang, C.; Bai, L.; Fang, L.-F.; Yoshioka, T.; Matsuyama, H. Antifouling Double-Skinned Forward Osmosis Membranes by Constructing Zwitterionic Brush-Decorated MWCNT Ultrathin Films. ACS Appl. Mater. Interfaces 2019, 11, 19462–19471. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Kim, J.E.; Phuntsho, S.; Jang, A.; Choi, J.Y.; Shon, H.K. Forward osmosis system analysis for optimum design and operating conditions. Water Res. 2018, 145, 429–441. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, T.; Huang, M.; Chen, G.; Cai, T.; Chen, H.; Meng, L.; Luo, X. Nanofiber composite forward osmosis (NCFO) membranes for enhanced antibiotics rejection: Fabrication, performance, mechanism, and simulation. J. Membr. Sci. 2020, 595, 117425. [Google Scholar] [CrossRef]
- Xiao, P.; Li, J.; Ren, Y.; Wang, X. A comprehensive study of factors affecting fouling behavior in forward osmosis. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 499, 163–172. [Google Scholar] [CrossRef]
- Suwaileh, W.; Johnson, D.; Khodabakhshi, S.; Hilal, N. Superior cross-linking assisted layer by layer modification of forward osmosis membranes for brackish water desalination. Desalination 2019, 463, 1–12. [Google Scholar] [CrossRef]
- Liu, C.; Lee, J.; Ma, J.; Elimelech, M. Antifouling Thin-Film Composite Membranes by Controlled Architecture of Zwitterionic Polymer Brush Layer. Environ. Sci. Technol. 2017, 51, 2161–2169. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Impact of support layer pore size on performance of thin film composite membranes for forward osmosis. J. Membr. Sci. 2015, 483, 25–33. [Google Scholar] [CrossRef]
- Widjojo, N.; Chung, T.-S.; Weber, M.; Maletzko, C.; Warzelhan, V. The role of sulphonated polymer and macrovoid-free structure in the support layer for thin-film composite (TFC) forward osmosis (FO) membranes. J. Membr. Sci. 2011, 383, 214–223. [Google Scholar] [CrossRef]
- Johnson, D.J.; Suwaileh, W.A.; Mohammed, A.W.; Hilal, N. Osmotic’s potential: An overview of draw solutes for forward osmosis. Desalination 2018, 434, 100–120. [Google Scholar] [CrossRef]
- Cath, T.; Childress, A.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Shokrollahzadeh, S.; Tajik, S. Fabrication of thin film composite forward osmosis membrane using electrospun polysulfone/polyacrylonitrile blend nanofibers as porous substrate. Desalination 2018, 425, 68–76. [Google Scholar] [CrossRef]
- Zuo, H.-R.; Fu, J.-B.; Cao, G.-P.; Hu, N.; Lu, H.; Liu, H.-Q.; Chen, P.-P.; Yu, J. The effects of surface-charged submicron polystyrene particles on the structure and performance of PSF forward osmosis membrane. Appl. Surf. Sci. 2018, 436, 1181–1192. [Google Scholar] [CrossRef]
- Chollom, M.N.; Rathilal, S. Fouling and Cleaning in Osmotically Driven Membranes. In Osmotically Driven Membrane Processes—Approach, Development and Current Status; Du, H., Thompson, A., Wang, X., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Zhao, S.; Zou, L. Effects of working temperature on separation performance, membrane scaling and cleaning in forward osmosis desalination. Desalination 2011, 278, 157–164. [Google Scholar] [CrossRef]
- Tang, C.Y.; She, Q.; Lay, W.C.; Wang, R.; Fane, A.G. Coupled effects of internal concentration polarization and fouling on flux behavior of forward osmosis membranes during humic acid filtration. J. Membr. Sci. 2010, 354, 123–133. [Google Scholar] [CrossRef]
- Tiraferri, A.; Yip, N.Y.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. Relating performance of thin-film composite forward osmosis membranes to support layer formation and structure. J. Membr. Sci. 2011, 367, 340–352. [Google Scholar] [CrossRef]
- Phillip, W.A.; Yong, J.S.; Elimelech, M. Reverse Draw Solute Permeation in Forward Osmosis: Modeling and Experiments. Environ. Sci. Technol. 2010, 44, 5170–5176. [Google Scholar] [CrossRef]
- Suwaileh, W.A.; Johnson, D.J.; Sarp, S.; Hilal, N. Advances in forward osmosis membranes: Altering the sub-layer structure via recent fabrication and chemical modification approaches. Desalination 2018, 436, 176–201. [Google Scholar] [CrossRef]
- Lim, S.; Park, M.J.; Phuntsho, S.; Tijing, L.D.; Nisola, G.M.; Shim, W.-G.; Chung, W.-J.; Shon, H.K. Dual-layered nanocomposite substrate membrane based on polysulfone/graphene oxide for mitigating internal concentration polarization in forward osmosis. Polymer 2017, 110, 36–48. [Google Scholar] [CrossRef]
- Liu, X.; Ong, S.L.; Ng, H.Y. Fabrication of mesh-embedded double-skinned substrate membrane and enhancement of its surface hydrophilicity to improve anti-fouling performance of resultant thin-film composite forward osmosis membrane. J. Membr. Sci. 2016, 511, 40–53. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, X.; Tian, L.; Li, Z.; Ding, C.; Yi, M.; Han, C.; Yu, X.; Wang, Y. Constructing substrate of low structural parameter by salt induction for high-performance TFC-FO membranes. J. Membr. Sci. 2020, 600, 117866. [Google Scholar] [CrossRef]
- Heikkinen, J.; Kyllönen, H.; Järvelä, E.; Grönroos, A.; Tang, C.Y. Ultrasound-assisted forward osmosis for mitigating internal concentration polarization. J. Membr. Sci. 2017, 528, 147–154. [Google Scholar] [CrossRef]
- Tan, C.H.; Ng, H.Y. Revised external and internal concentration polarization models to improve flux prediction in forward osmosis process. Desalination 2013, 309, 125–140. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Lang, W.-Z.; Wang, Y. Improved performance of thin-film composite membrane with PVDF/PFSA substrate for forward osmosis process. J. Membr. Sci. 2017, 535, 188–199. [Google Scholar] [CrossRef]
- Bin Darwish, N.; Alkhudhiri, A.; Alromaih, H.; Alalawi, A.; Leaper, M.C.; Hilal, N. Effect of lithium chloride additive on forward osmosis membranes performance. J. Water Process. Eng. 2020, 33, 101049. [Google Scholar] [CrossRef]
- Yeo, H.-T.; Lee, S.-T.; Han, M.-J. Role of a Polymer Additive in Casting Solution in Preparation of Phase Inversion Polysulfone Membranes. J. Chem. Eng. Jpn. 2000, 33, 180–184. [Google Scholar] [CrossRef]
- Akther, N.; Phuntsho, S.; Chen, Y.; Ghaffour, N.; Shon, H.K. Recent advances in nanomaterial-modified polyamide thin-film composite membranes for forward osmosis processes. J. Membr. Sci. 2019, 584, 20–45. [Google Scholar] [CrossRef]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward osmosis membranes and processes: A comprehensive review of research trends and future outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Ng, B.C.; Abdullah, M.S. Recent Progresses of Forward Osmosis Membranes Formulation and Design for Wastewater Treatment. Water 2019, 11, 2043. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Lu, J.; Shan, B.; Gao, C. Enhanced performance of cellulose triacetate membranes using binary mixed additives for forward osmosis desalination. Desalination 2017, 405, 68–75. [Google Scholar] [CrossRef]
- Syed Ibrahim, G.P.; Isloor, A.M.; Yuliwati, E. A Review: Desalination by Forward Osmosis. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Ghasemzadeh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 199–214. ISBN 9780128135518. [Google Scholar]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, K.Y.; Chung, T.S.N.; Chen, H.; Jean, Y.; Amy, G.L. Well-constructed cellulose acetate membranes for forward osmosis: Minimized internal concentration polarization with an ultra-thin selective layer. J. Membr. Sci. 2010, 360, 522–535. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Gebreslase, G.A.; Van Der Bruggen, B. Incorporation of Al2O3 into cellulose triacetate membranes to enhance the performance of pervaporation for desalination of hypersaline solutions. Desalination 2020, 474, 114198. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Yuan, B.; Wang, Z.; Li, X.; Ren, Y. Comparison of biofouling mechanisms between cellulose triacetate (CTA) and thin-film composite (TFC) polyamide forward osmosis membranes in osmotic membrane bioreactors. Bioresour. Technol. 2016, 202, 50–58. [Google Scholar] [CrossRef]
- Ismail, A.F.; Padaki, M.; Hilal, N.; Matsuura, T.; Lau, W.J. Thin film composite membrane — Recent development and future potential. Desalination 2015, 356, 140–148. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of membrane support layer hydrophobicity on water flux in osmotically driven membrane processes. J. Membr. Sci. 2008, 318, 458–466. [Google Scholar] [CrossRef]
- Bao, X.; Wu, Q.; Tian, J.; Shi, W.; Wang, W.; Zhang, Z.; Zhang, R.; Zhang, B.; Guo, Y.; Shu, S.; et al. Fouling mechanism of forward osmosis membrane in domestic wastewater concentration: Role of substrate structures. Chem. Eng. J. 2019, 370, 262–273. [Google Scholar] [CrossRef]
- Chun, Y.; Mulcahy, D.; Chun, Y.; Kim, I.S. A Short Review of Membrane Fouling in Forward Osmosis Processes. Membranes 2017, 7, 30. [Google Scholar] [CrossRef]
- Chekli, L.; Phuntsho, S.; Kim, J.E.; Kim, J.; Choi, J.Y.; Choi, J.-S.; Kim, S.; Kim, J.H.; Hong, S.; Sohn, J.; et al. A comprehensive review of hybrid forward osmosis systems: Performance, applications and future prospects. J. Membr. Sci. 2016, 497, 430–449. [Google Scholar] [CrossRef]
- Choi, H.-G.; Son, M.; Choi, H. Integrating seawater desalination and wastewater reclamation forward osmosis process using thin-film composite mixed matrix membrane with functionalized carbon nanotube blended polyethersulfone support layer. Chemosphere 2017, 185, 1181–1188. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Park, M.J.; Tijing, L.; Han, D.S.; Phuntsho, S.; Shon, H.K. Modification of Nanofiber Support Layer for Thin Film Composite forward Osmosis Membranes via Layer-by-Layer Polyelectrolyte Deposition. Membranes 2018, 8, 70. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, J.; He, C. A stable and hydrophilic substrate for thin-film composite forward osmosis membrane revealed by in-situ cross-linked polymerization. Desalination 2018, 433, 1–9. [Google Scholar] [CrossRef]
- Ren, J.; O’Grady, B.; DeJesus, G.; McCutcheon, J.R. Sulfonated polysulfone supported high performance thin film composite membranes for forward osmosis. Polymer 2016, 103, 486–497. [Google Scholar] [CrossRef]
- Lotfi, F.; Phuntsho, S.; Majeed, T.; Kim, K.; Han, D.S.; Abdel-Wahab, A.; Shon, H.K. Thin film composite hollow fibre forward osmosis membrane module for the desalination of brackish groundwater for fertigation. Desalination 2015, 364, 108–118. [Google Scholar] [CrossRef]
- Han, G.; Cheng, Z.L.; Chung, T.-S. Thin-film composite (TFC) hollow fiber membrane with double-polyamide active layers for internal concentration polarization and fouling mitigation in osmotic processes. J. Membr. Sci. 2017, 523, 497–504. [Google Scholar] [CrossRef]
- Shibuya, M.; Yasukawa, M.; Mishima, S.; Tanaka, Y.; Takahashi, T.; Matsuyama, H. A thin-film composite-hollow fiber forward osmosis membrane with a polyketone hollow fiber membrane as a support. Desalination 2017, 402, 33–41. [Google Scholar] [CrossRef]
- Han, G.; De Wit, J.S.; Chung, T.-S. Water reclamation from emulsified oily wastewater via effective forward osmosis hollow fiber membranes under the PRO mode. Water Res. 2015, 81, 54–63. [Google Scholar] [CrossRef]
- Lim, J.; Kim, C.-M.; Lee, J.; Choi, C.; Yang, E.; Jung, B.; Kim, I.S. Effect of the support layer morphological structure on the performance of forward osmosis hollow fiber membranes. J. Membr. Sci. 2020, 608, 118196. [Google Scholar] [CrossRef]
- Yadav, S.; Saleem, H.; Ibrar, I.; Naji, O.; Hawari, A.A.; AlAnezi, A.A.; Zaidi, S.J.; Altaee, A.; Zhou, J. Recent developments in forward osmosis membranes using carbon-based nanomaterials. Desalination 2020, 482, 114375. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. The feasibility of kaolin as main material for low cost porous ceramic hollow fibre membrane prepared using combined phase inversion and sintering technique. J. Teknol. 2017, 79, 35–39. [Google Scholar] [CrossRef]
- Yusof, M.S.M.; Jaafar, J.; Mustafa, A.; Rahman, M.A.; Jaafar, J.; Ismail, A. Feasibility study of cadmium adsorption by palm oil fuel ash (POFA)-based low-cost hollow fibre zeolitic membrane. Environ. Sci. Pollut. Res. 2018, 25, 21644–21655. [Google Scholar] [CrossRef]
- Zahid, M.; Rashid, A.; Akram, S.; Rehan, Z.A.; Razzaq, W. A Comprehensive Review on Polymeric Nano-Composite Membranes for Water Treatment. J. Membr. Sci. Technol. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Beh, J.J.; Ng, E.P.; Sum, J.Y.; Ooi, B.S. Thermodynamics and kinetics control of support layer synthesis for enhanced thin film composite membrane performance. J. Appl. Polym. Sci. 2017, 135, 45802. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zhou, Z.; Xie, J.; Lee, J.Y. Hydrophilic Mineral Coating of Membrane Substrate for Reducing Internal Concentration Polarization (ICP) in Forward Osmosis. Sci. Rep. 2016, 6, 19593. [Google Scholar] [CrossRef]
- Choi, Y.; Shin, Y.; Cho, H.; Jang, Y.; Hwang, T.-M.; Lee, S. Economic evaluation of the reverse osmosis and pressure retarded osmosis hybrid desalination process. Desalin. Water Treat. 2016, 57, 26680–26691. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.Y. Relating solution physicochemical properties to internal concentration polarization in forward osmosis. J. Membr. Sci. 2011, 379, 459–467. [Google Scholar] [CrossRef]
- Amini, M.; Jahanshahi, M.; Rahimpour, A. Synthesis of novel thin film nanocomposite (TFN) forward osmosis membranes using functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2013, 435, 233–241. [Google Scholar] [CrossRef]
- Khorshidi, B.; Bhinder, A.; Thundat, T.; Pernitsky, D.; Sadrzadeh, M. Developing high throughput thin film composite polyamide membranes for forward osmosis treatment of SAGD produced water. J. Membr. Sci. 2016, 511, 29–39. [Google Scholar] [CrossRef]
- Mohammadifakhr, M.; De Grooth, J.; Roesink, H.D.W.; Kemperman, A.J.B. Forward Osmosis: A Critical Review. Processes 2020, 8, 404. [Google Scholar] [CrossRef]
- Lee, W.; Ng, Z.; Hubadillah, S.; Goh, P.; Lau, W.; Othman, M.; Ismail, A.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Malaeb, L.; Ayoub, G.M. Reverse osmosis technology for water treatment: State of the art review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Ab Wahid, Z.; Ismail, A.; Sakinah, M. Application and Challenges of Membrane in Surface Water Treatment. J. Appl. Sci. 2010, 10, 380–390. [Google Scholar] [CrossRef]
- Chun, Y.; Zaviska, F.; Kim, S.-J.; Mulcahy, D.; Yang, E.; Kim, I.S.; Zou, L. Fouling characteristics and their implications on cleaning of a FO-RO pilot process for treating brackish surface water. Desalination 2016, 394, 91–100. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, R.; Liu, Y.; He, M.; Su, Y.; Gao, C.; Jiang, Z. Antifouling membrane surface construction: Chemistry plays a critical role. J. Membr. Sci. 2018, 551, 145–171. [Google Scholar] [CrossRef]
- Holloway, R.W.; Childress, A.; Dennett, K.; Cath, T.Y. Forward osmosis for concentration of anaerobic digester centrate. Water Res. 2007, 41, 4005–4014. [Google Scholar] [CrossRef]
- Faria, A.F.; Liu, C.; Xie, M.; Perreault, F.; Nghiem, L.D.; Ma, J.; Elimelech, M. Thin-film composite forward osmosis membranes functionalized with graphene oxide–silver nanocomposites for biofouling control. J. Membr. Sci. 2017, 525, 146–156. [Google Scholar] [CrossRef]
- Johnson, D.; Hilal, N. Characterisation and quantification of membrane surface properties using atomic force microscopy: A comprehensive review. Desalination 2015, 356, 149–164. [Google Scholar] [CrossRef]
- Majeed, T.; Phuntsho, S.; Jeong, S.; Zhao, Y.; Gao, B.; Shon, H.K. Understanding the risk of scaling and fouling in hollow fiber forward osmosis membrane application. Process. Saf. Environ. Prot. 2016, 104, 452–464.