Gold nanoparticles (AuNPs) have gained high prominence in the biomedicine field, due to their own physico-chemical properties that are suitable for different imaging or therapeutic uses, versatile structural modification, including easy functionalization of their surface with different chemical entities (e.g., chelators, targeting biomolecules or cytotoxic drugs), favourable biological half-life, low toxicity and biocompatibility. In particular, the use of AuNPs in radiopharmaceutical development has provided various nanometric platforms for the delivery of medically relevant radioisotopes for SPECT/PET diagnosis and/or radionuclide therapy
- nanomedicine
- gold nanoparticles
- nuclear imaging
- radionuclide therapy
1. Introduction
Nanotechnology is a discipline of science and engineering that has led to innovative approaches in many areas of medicine based on the use of biocompatible nanoparticles. Its applications in the screening, diagnosis, and treatment of disease are collectively referred to as “nanomedicine”, an emerging field that has demonstrated great potential to revolutionize individual and population wide health in the future. It can be seen as a refinement of molecular medicine, integrating innovations in genomics and proteomics on the path to a more personalized medicine [1][2].
For biomedical applications, nanoparticles can be obtained with a wide variety of materials including inorganic compounds or organic polymers, among others. The use of different materials provides nanoparticles of different sizes and shapes with varied physico-chemical properties well-fitted for a specific use in biomedicine [3]. In this respect, it is important to have in mind the influence of surface and quantum effects that affect the chemical reactivity of nanosized materials, as well as their mechanical, optical, electric and magnetic properties [4][5].
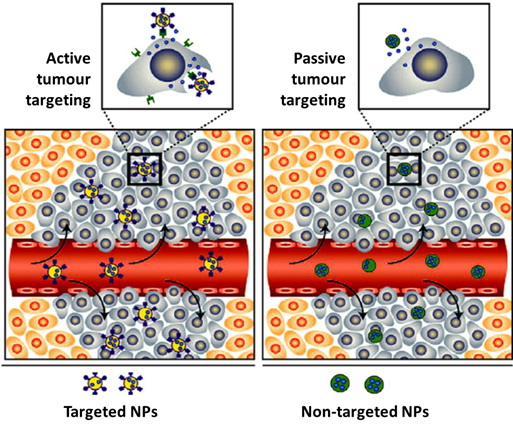
The biological fate and potential toxicity of nanoparticles are also crucial issues, which might restrict their use for medical applications. In fact, for some of them (e.g., quantum dots), their inherent toxicity is a potential drawback but for many others (e.g., iron oxide and AuNPs) toxicity issues are less relevant. Nanoparticle biodistribution can vary greatly depending on the type and size of the particle, as well as on their surface chemistry [6][7]. For imaging and/or therapy of cancer, the selective delivery of drugs or radionuclides into the tumour tissues is of paramount importance. For this purpose, nanoparticles offer unique advantages. In fact, many NPs undergo the enhanced permeability and retention (EPR) effect that is involved in the passive targeting of leaky tumour tissues. The EPR effect is a result of the leakiness of the newly forming blood vessels and poor lymphatic drainage in growing tumours. During the angiogenesis process, the endothelial cells from the blood vessel walls do not seal tightly against each other, leaving fenestrations of approximately 200–800 nm in diameter. These processes lead to a passive accumulation of nanoparticles in tumour tissues, as shown in Figure 1 [8]. On the other side, the versatile functionalization of the NPs surface with targeting biomolecules (e.g., a peptide or an antibody) allows the specific targeting of tumours through interaction with receptors overexpressed in the tumour cells or in the tumour microenvironment (Figure 1) [9][10][11].
Figure 1. Illustration of the accumulation of nanoparticles in tumour tissues: passive vs active targeting. Adapted from Mahmoudi et al. (2011) [12].
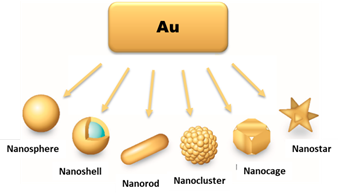
For biomedical applications, namely for cancer imaging and therapy, AuNPs offer the possibility of a versatile functionalization with targeting biomolecules for specific accumulation in tumour tissues, allowing more precise diagnostics and/or localized therapeutic effects. For instance, the combination of nuclear medicine modalities with nanotechnology offers unique opportunities to achieve this goal by allowing the easy and convenient merge of a variety of diagnostic and therapeutic capabilities into a single agent, within a theranostic approach of cancer. This requires the design of radiolabeled nanoconstructs that can be tailored ideally to the needs of every patient by selecting the appropriate nanoparticle, targeting biomolecule and imaging or therapeutic radionuclide [13][14][15]. Moreover, there are currently available different methods to manipulate the size and shape of gold nanoparticles, spanning from shapes like nanospheres (or nanoshells), nanorods, nanocages to nanostars (Figure 2), to obtain AuNPs tailored to the different biomedical uses [16][17].
Figure 2. Different types of AuNPs, according to their shape and morphology. Adapted from L. F. de Freitas et al. (2018) [18].
2. Synthesis of Gold Nanoparticles
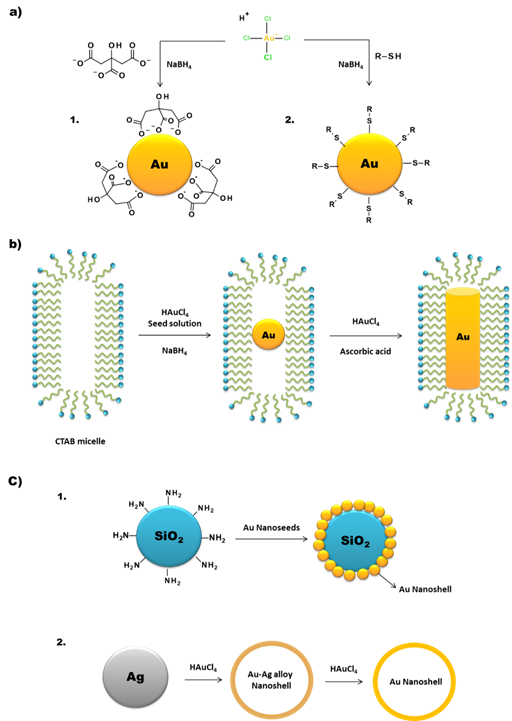
One of the most common methods of AuNP synthesis is by reduction of a gold precursor, generally the tetrachloroauric acid (HAuCl4), in the presence of a stabilizing agent (Figure 3a). In order to guarantee the reduction of the gold, strong to mild reducing agents are used, like NaBH4, hydrazine or citrate. In 1951, Turkevitch et al. developed one of the most conventional synthetic routes, still in use to this day, which consists on the reduction of Au(III) in HAuCl4 by citrate in water. It is known as the citrate reduction method, which allows the formation of citrate stabilized AuNPs and a controlled size of the particles by varying the citrate/gold ratio [19]. A few years later, in 1994, Brust et al. introduced a new procedure for the efficient synthesis of stable AuNPs with reduced dispersity and controlled size, which represented at the time an important breakthrough. This procedure is based on the use of thiolated ligands that strongly bind to gold due to the soft character of both Au and S. After addition of a reducing agent (NaBH4), the Au(III) is reduced to Au(I) and the AuNPs are formed [20]. This opened the opportunity to develop AuNPs using a great variety of thiolated ligands. This method allows the control of core nanoparticle size by shifting the ratio of thiol/Au in the reaction mixture; for instance, the use of larger thiol/Au ratios affords smaller core sizes with less polydispersity [21][22].
In recent years there has been an increased interest on green methodologies for the synthesis of AuNPs, using alternative reducing agents to NaBH4 or hydrazine that are environmentally toxic. In this regard, Katti et al. have developed extensive work with phytochemical agents extracted from various biological media (e.g soybeans, tea leaves) [23][24][25]. It was demonstrated that these phytochemical agents performed the dual function of reducing the gold salt to form the AuNPs and at the same time provide a protein coating that can stabilize the nanoparticle structure [24][25].
As mentioned above, AuNPs can be obtained in various forms, including nanospheres, nanorods, nanoshells or nanocages. The synthetic methods described above are commonly used to obtain AuNPs in spherical amorphous form. The synthesis of AuNPs with a more complex shape requires alternative methodologies [18][26][27]. Gold nanorods (AuNRs) are commonly synthesized through the seed-mediated approach, which involves a two-step process where initially a seed solution is prepared with tetrachloroauric acid in the presence of a strong reducing agent (e.g., NaBH4) (Figure 3b). The seed solution is then added to a mixture of cetyltrimethylammonium bromide (CTAB), a mild reducing agent (e.g., ascorbic acid) and tetrachloroauric acid. The elongated ellipsoidal shape of the CTAB micelles permits the growth of the AuNPs of the seed solution in an elongated manner, in order to obtain a rod shape [28][29][30]. Some variations on this procedure include the addition of AgNO3 prior to the growth phase, which allows a better control of the shape and increase the yield of AuNRs [28].
Besides the seed-mediated method, other methodologies have also been reported in literature for the synthesis of AuNRs. The template method is based on the electrochemical deposition of Au within nanoporous template membranes, which can be of different materials (e.g., polycarbonate or alumina). Ag or Cu is added to the template membrane to form a conductive film that allows for the electrodeposition of Au and growth of the nanoparticles within the membrane nanopores. The nanorods are then recovered by selective dissolution of the template membrane and Ag or Cu film. The diameter of the AuNRs is dependent of the nanopore diameter of the membrane, while the length can be controlled by the amount of Au deposited [31][32].
Electrochemical methods for AuNR synthesis are usually based on the use of a dual electrode electrochemical cell. A gold layer is used as the anode and a platinum layer as cathode. Both electrodes are immersed in a surfactant solution composed of the cationic surfactant CTAB and a more hydrophobic cationic surfactant like tetradodecylammonium bromide (TCAB), which are responsible for the formation of the rod-shaped nanoparticles. During the process of controlled current electrolysis, the gold layer releases Au ions that migrate to the cathode where reduction occurs and the AuNRs are formed [33].
Gold nanoshells (AuNSs) can be of two types, namely solid or with a hollow core (Figure 3c). The synthesis of core-containing AuNSs is based on the use of a seed nanoparticle, which will form the core of the nanoshell. Then, the addition of tetrachloroauric acid in the presence of a reducing agent leads to the deposition of gold seeds on the surface of the core. SiO2 is one of the most commonly used cores. These silica nanoparticles have a capping agent on their surface, like 3-aminopropyltriethoxysilane (APTES), which provides NH2 groups that can link to the gold [34].
Figure 3. Schematic synthesis of (a) AuNPs by the 1) Turkevitch and 2) Brust methodologies, (b) AuNRs by the seed-mediated method, and (c) 1) core and 2) hollow AuNSs.
For the synthesis of hollow AuNSs, one approach is to use the silica core to synthesize the gold nanoshells as described above and then use HF to remove the SiO2 core. Another method is the template galvanic replacement of silver. This methodology is based on the higher standard reduction potential of the AuCl4−/Au pair when compared with that of the Ag+/Ag pair. Silver is oxidized into Ag+ when silver nanostructures and HAuCl4 are mixed in an aqueous medium. By optimizing the ratio between the silver nanoparticles and HAuCl4, silver atoms can diffuse into the gold shell (or sheath) to form a seamless, hollow nanostructure with its wall made of Au-Ag alloys [34][35]. The further increasing of the HAuCl4 present in the medium triggers a dealloying process that selectively removes silver atoms from the alloyed wall. This induces morphological reconstruction that leads to the formation of pinholes in the walls, and the nanoparticles acquire a cage like structure. This is one of the common methodologies for the synthesis of gold nanocages (AuNCs). Temperature also plays an important role in the replacement reaction because the solubility constant of AgCl and the diffusion coefficients of Ag and Au atoms are both strongly dependent on this parameter [35].
Due to the inherent difficulties in analyzing nanoscale materials, in comparison with molecular or bulk materials, the characterization of NPs requires particular analytical techniques and methodologies. It is common to recur to various characterization techniques, in a complementary manner, to obtain reliable information on the NPs structure and their physico-chemical properties. Besides the techniques summarized below, there are various other methodologies available nowadays for NP characterization. The use of a single one of these characterization techniques cannot provide all the required data for a proper assessment of the NP structure, hence it is necessary to take into consideration the technique’s strengths and weaknesses, depending on the nature of the NP [36][37].
Microscopy techniques, like transmission electron microscopy (TEM) or scanning electron microscopy (SEM), can provide information regarding the size and shape of the nanoparticles. On the other hand, the study of the hydrodynamic size distribution relies on techniques like dynamic light scattering (DLS) or nanoparticle tracking analyses (NTA), which can also provide information on the agglomeration state of the NPs in solution. Other commonly used techniques are zeta-potential measurements for surface charge determination and UV-Vis spectroscopy for characterization of optical properties, namely to determine the surface plasmon resonance wavelength that can be correlated with the size and shape of the nanoparticles. In the particular case of metallic NPs, X-ray-based techniques, like X-ray diffraction (XRD), are used to assess the crystalline structure and elemental composition [36][37].
3. Radiolabelling of Gold Nanoparticles
To pursue with a stable radiolabeling of AuNPs it is commonly required to perform their functionalization with suitable molecular entities, which will allow for the coordination/conjugation of the radioisotopes [38]. In this regard, there are different synthetic pathways available to functionalize AuNPs: (i) using bifunctional molecules that can act as a capping/stabilizing agent during the synthesis of the AuNPs and that can bind to the radioisotopes [39][40]; (ii) direct conjugation of amino/thiolated molecules to the surface of preformed AuNPs [41][42]; (iii) ligand exchange, in which some/all of the capping/stabilizing molecules on the AuNPs are exchanged with a different molecule with gold bonding capabilities [43]; and (iv) chemical modification of molecules already present in the AuNP structure [44][45].
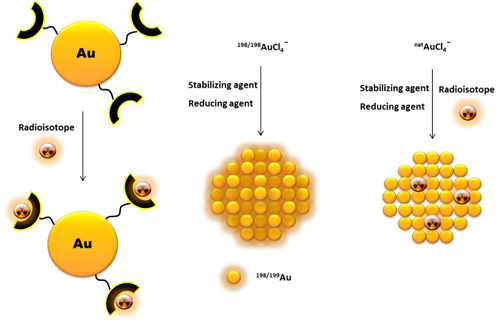
Another way to incorporate radionuclides into the AuNP structure, without their further chemical functionalization, is by directly introducing the radioisotopes in the nanoparticle core (Figure 4). This is commonly achieved by using a 198/199Au precursor in the synthesis of the nanoparticles [46][47]. Alternatively, it has also been reported the neutron irradiation of non-radioactive AuNPs to originate 198/199Au-containing nanoparticles through neutron capture reactions (197Au(n,γ)198Au and 198Au(n,γ)199Au) [48].
Figure 4. Schematic drawing of different pathways to incorporate radionuclides into AuNPs.
In some cases, it is possible to attach other radionuclides to the AuNPs without the need of extra chemical derivatization. This can be achieved by adsorption of the radionuclide to the AuNP surface, namely for 131I or 64Cu [49][50]. The incorporation of the radionuclide in the NPs core is another possibility, as reported by Liu et al. for 64Cu alloyed AuNPs modified with PEG. These 64Cu-labeled AuNPs were obtained starting from HAuCl4 and 64Cu(acac)2 and using oleylamine as reducing agent [51]. In the same way, Chen et al. have studied the integration of a 64Cu shell into PEG-stabilized AuNPs by reducing 64Cu(II) in the presence of hydrazine and polyacrylic acid [52].
References
- Abuzer Alp Yetisgin; Sibel Cetinel; Merve Zuvin; Ali Koşar; Ozlem Kutlu; Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193, 10.3390/molecules25092193.
- Hadis Daraee; Ali Eatemadi; Elham Abbasi; Sedigheh Fekri Aval; Mohammad Kouhi; Abolfazl Akbarzadeh; Application of gold nanoparticles in biomedical and drug delivery. Artificial Cells, Nanomedicine, and Biotechnology 2014, 44, 410-422, 10.3109/21691401.2014.955107.
- Mohammadrez Saboktakin; The biological and biomedical nanoparticles - synthesis and applications. Advanced Material Science 2017, 2, 0, 10.15761/AMS.1000127.
- Masoud Delfi; Matineh Ghomi; Ali Zarrabi; Reza Mohammadinejad; Zahra Baghban Taraghdari; Milad Ashrafizadeh; Ehsan Nazarzadeh Zare; Tarun Agarwal; Vinod V.T. Padil; Babak Mokhtari; et al.Filippo RossiGiuseppe PeraleMika SillanpääAssunta BorzacchielloTapas Kumar MaitiPooyan Makvandi Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis 2020, 2, 117-139, 10.3390/prosthesis2020012.
- Rukmani Thiruppathi; Sachin Mishra; Mathangi Ganapathy; Parasuraman Padmanabhan; Balázs Gulyás; Nanoparticle Functionalization and Its Potentials for Molecular Imaging. Advanced Science 2016, 4, 1600279, 10.1002/advs.201600279.
- Nazanin Hoshyar; Samantha Gray; HongBin Han; Gang Bao; The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673-692, 10.2217/nnm.16.5.
- Randa Zein; Wissam Sharrouf; Kim Selting; Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. Journal of Oncology 2020, 2020, 1-16, 10.1155/2020/5194780.
- Yang Shi; Roy Van Der Meel; Xiaoyuan Chen; Twan Lammers; The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921-7924, 10.7150/thno.49577.
- Karunanithi Rajamanickam; Multimodal Molecular Imaging Strategies using Functionalized Nano Probes. Journal of Nanotechnology Research 2019, 1, 119, 10.26502/jnr.2688-85210010.
- Joydeb Majumder; Tamara Minko; Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opinion on Drug Delivery 2020, 0, 0, 10.1080/17425247.2021.1828339.
- Lina Lu; Shuhe Kang; Chao Sun; Chufeng Sun; Zhong Guo; Jia Li; Taofeng Zhang; Xingping Luo; Bin Liu; Multifunctional Nanoparticles in Precise Cancer Treatment: Considerations in Design and Functionalization of Nanocarriers. Current Topics in Medicinal Chemistry 2020, 20, 2427-2441, 10.2174/1568026620666200825170030.
- Morteza Mahmoudi; Shilpa Sant; Ben Wang; Sophie Laurent; Tapas Sen; Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Advanced Drug Delivery Reviews 2011, 63, 24-46, 10.1016/j.addr.2010.05.006.
- Jongho Jeon; Review of Therapeutic Applications of Radiolabeled Functional Nanomaterials. International Journal of Molecular Sciences 2019, 20, 2323, 10.3390/ijms20092323.
- Jennifer R. Lamb; Jason P. Holland; Advanced Methods for Radiolabeling Multimodality Nanomedicines for SPECT/MRI and PET/MRI. Journal of Nuclear Medicine 2017, 59, 382-389, 10.2967/jnumed.116.187419.
- Jacek Koziorowski; Adina E Stanciu; Vanessa Gómez‐Vallejo; Jordi Llop; Radiolabeled Nanoparticles for Cancer Diagnosis and Therapy. Anti-Cancer Agents in Medicinal Chemistry 2017, 17, 333-354, 10.2174/1871520616666160219162902.
- Fatemeh Farjadian; Amir Ghasemi; Omid Gohari; Amir Roointan; Mahdi Karimi; Michael R. Hamblin; Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine 2019, 14, 93-126, 10.2217/nnm-2018-0120.
- Tyler D. Brown; Nahal Habibi; Debra Wu; Joerg Lahann; Samir Mitragotri; Effect of Nanoparticle Composition, Size, Shape, and Stiffness on Penetration Across the Blood–Brain Barrier. ACS Biomaterials Science & Engineering 2020, 6, 4916-4928, 10.1021/acsbiomaterials.0c00743.
- Lucas F. De Freitas; Gustavo H. C. Varca; Jorge Gabriel Dos Santos Batista; Ademar B. Lugão; An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939, 10.3390/nano8110939.
- John Turkevich; Peter Cooper Stevenson; James Hillier; A study of the nucleation and growth processes in the synthesis of colloidal gold. Discussions of the Faraday Society 1951, 11, 55-75, 10.1039/df9511100055.
- Mathias Brust; Merryl Walker; Donald Bethell; David J. Schiffrin; Robin Whyman; Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. Journal of the Chemical Society, Chemical Communications 1994, 0, 801-802, 10.1039/c39940000801.
- ShaoWei Chen; 4-Hydroxythiophenol-Protected Gold Nanoclusters in Aqueous Media. Langmuir 1999, 15, 7551-7557, 10.1021/la990398g.
- ShaoWei Chen; Royce W. Murray; Arenethiolate Monolayer-Protected Gold Clusters. Langmuir 1999, 15, 682-689, 10.1021/la980817u.
- Menka Khoobchandani; Kavita K Katti; Alice Raphael Karikachery; Velaphi C. Thipe; Deepak Srisrimal; Darsha Kumar Dhurvas Mohandoss; Rashmi Dhurvas Darshakumar; Chintamani M Joshi; Kattesh V Katti; New Approaches in Breast Cancer Therapy Through Green Nanotechnology and Nano-Ayurvedic Medicine – Pre-Clinical and Pilot Human Clinical Investigations. International Journal of Nanomedicine 2020, 15, 181-197, 10.2147/ijn.s219042.
- Satish K. Nune; Nripen Chanda; Ravi Shukla; Kavita K. Katti; Rajesh R. Kulkarni; Subramanian Thilakavathy; Swapna Mekapothula; Raghuraman Kannan; Kattesh V. Katti; Green nanotechnology from tea: phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. Journal of Materials Chemistry 2009, 19, 2912-2920, 10.1039/b822015h.
- Ravi Shukla; Satish K. Nune; Nripen Chanda; Kavita Katti; Swapna Mekapothula; Rajesh R. Kulkarni; Wade V. Welshons; Raghuraman Kannan; Kattesh V. Katti; Soybeans as a Phytochemical Reservoir for the Production and Stabilization of Biocompatible Gold Nanoparticles. Small 2008, 4, 1425-1436, 10.1002/smll.200800525.
- Daria Maccora; Valentina Dini; Chiara Battocchio; Ilaria Fratoddi; Antonella Cartoni; Dante Rotili; Massimo Castagnola; Riccardo Faccini; Isabella Bruno; Teresa Scotognella; et al.A. GiordanoIole Venditti Gold Nanoparticles and Nanorods in Nuclear Medicine: A Mini Review. Applied Sciences 2019, 9, 3232, 10.3390/app9163232.
- Carla D. Souza; Beatriz Ribeiro Nogueira; Maria Elisa C.M. Rostelato; Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. Journal of Alloys and Compounds 2019, 798, 714-740, 10.1016/j.jallcom.2019.05.153.
- Jorge Pérez-Juste; Isabel Pastoriza-Santos; Luis M. Liz-Marzán; Paul Mulvaney; Gold nanorods: Synthesis, characterization and applications. Coordination Chemistry Reviews 2005, 249, 1870-1901, 10.1016/j.ccr.2005.01.030.
- Lu An; Yuanyuan Wang; Qiwei Tian; Hong Yang; Small Gold Nanorods: Recent Advances in Synthesis, Biological Imaging, and Cancer Therapy. Materials 2017, 10, 1372, 10.3390/ma10121372.
- Waqqar Ahmed; Arshad Saleem Bhatti; Jan M. Van Ruitenbeek; Efficient seed-mediated method for the large-scale synthesis of Au nanorods. Journal of Nanoparticle Research 2017, 19, 115, 10.1007/s11051-017-3815-9.
- Chunxin Ji; Peter C. Searson; Synthesis and Characterization of Nanoporous Gold Nanowires. The Journal of Physical Chemistry B 2003, 107, 4494-4499, 10.1021/jp0222200.
- Jeong-Mi Moon; Alexander Wei; Uniform Gold Nanorod Arrays from Polyethylenimine-Coated Alumina Templates. The Journal of Physical Chemistry B 2005, 109, 23336-23341, 10.1021/jp054405n.
- Yu; Ser-Sing Chang; Chien-Liang Lee; C. R. Chris Wang; Gold Nanorods: Electrochemical Synthesis and Optical Properties. The Journal of Physical Chemistry B 1997, 101, 6661-6664, 10.1021/jp971656q.
- Yu-Chen Wang; Éric Rhéaume; Frederic Lesage; Ashok K Kakkar; Synthetic Methodologies to Gold Nanoshells: An Overview. Molecules 2018, 23, 2851, 10.3390/molecules23112851.
- Yugang Sun; Younan Xia; Mechanistic Study on the Replacement Reaction between Silver Nanostructures and Chloroauric Acid in Aqueous Medium. Journal of the American Chemical Society 2004, 126, 3892-3901, 10.1021/ja039734c.
- Stefanos Mourdikoudis; Roger M. Pallares; Nguyen Thi Kim Thanh; Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871-12934, 10.1039/c8nr02278j.
- Mario M. Modena; Bastian Rühle; Thomas P. Burg; Stefan Wuttke; Nanoparticle Characterization: What to Measure?. Advanced Materials 2019, 31, e1901556, 10.1002/adma.201901556.
- Pooja M. Tiwari; Komal Vig; Vida Dennis; Shree R. Singh; Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31-63, 10.3390/nano1010031.
- Francisco Silva; Ajit Zambre; Maria Paula Cabral Campello; Lurdes Gano; Isabel Santos; Ana Maria Ferraria; Mário Vale; Amolak Singh; Anandhi Upendran; António Paulo; et al.Raghuraman Kannan Interrogating the Role of Receptor-Mediated Mechanisms: Biological Fate of Peptide-Functionalized Radiolabeled Gold Nanoparticles in Tumor Mice. Bioconjugate Chemistry 2016, 27, 1153-1164, 10.1021/acs.bioconjchem.6b00102.
- Christophe Alric; Jacqueline Taleb; Géraldine Le Duc; Céline Mandon; Claire Billotey; Alice Le Meur-Herland; Thierry Brochard; Francis Vocanson; Marc Janier; Pascal Perriat; et al.Stéphane RouxOlivier Tillement Gadolinium Chelate Coated Gold Nanoparticles As Contrast Agents for Both X-ray Computed Tomography and Magnetic Resonance Imaging. Journal of the American Chemical Society 2008, 130, 5908-5915, 10.1021/ja078176p.
- Andrei N. Mendoza-Sánchez; Guillermina Ferro-Flores; Blanca E. Ocampo-García; Enrique Morales-Ávila; Flor De M. Ramírez; Luis Manuel M De Leon Rodriguez; Clara Santos-Cuevas; Luis Alberto Medina; Eva L. Rojas-Calderón; Marco A. Camacho-López; et al. Lys3-bombesin conjugated to 99mTc-labelled gold nanoparticles for in vivo gastrin releasing peptide-receptor imaging.. Journal of Biomedical Nanotechnology 2010, 6, 375-384, 10.1166/jbn.2010.1132.
- Alena Reznickova; N. Slavikova; Z. Kolska; K. Kolarova; T. Belinova; Marie Hubalek Kalbacova; M. Cieslar; V. Svorcik; PEGylated gold nanoparticles: Stability, cytotoxicity and antibacterial activity. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2019, 560, 26-34, 10.1016/j.colsurfa.2018.09.083.
- Martin Kluenker; Mihail Mondeshki; Muhammad Nawaz Tahir; Wolfgang Tremel; Martin Klünker; Monitoring Thiol–Ligand Exchange on Au Nanoparticle Surfaces. Langmuir 2018, 34, 1700-1710, 10.1021/acs.langmuir.7b04015.
- Jiangjiang Zhang; Lei Mou; Xingyu Jiang; Surface chemistry of gold nanoparticles for health-related applications. Chemical Science 2020, 11, 923-936, 10.1039/c9sc06497d.
- M. U. Farooq; Valentyn Novosad; Elena A. Rozhkova; Hussain Wali; Asghar Ali; Ahmed A. Fateh; Purnima B. Neogi; Arup Neogi; Zhiming Wang; Gold Nanoparticles-enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells. Scientific Reports 2018, 8, 1-12, 10.1038/s41598-018-21331-y.
- A. Y. Al-Yasiri; M. Khoobchandani; C. S. Cutler; L. Watkinson; T. Carmack; C. J. Smith; M. Kuchuk; Sudarshan K. Loyalka; A. B. Lugão; Kattesh V. Katti; et al. Mangiferin functionalized radioactive gold nanoparticles (MGF- 198 AuNPs) in prostate tumor therapy: green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalton Transactions 2017, 46, 14561-14571, 10.1039/C7DT00383H.
- Rubel Chakravarty; Sudipta Chakraborty; Apurav Guleria; Rakesh Shukla; Chandan Kumar; K.V. Vimalnath Nair; Haladhar Dev Sarma; Avesh Kumar Tyagi; Ashutosh Dash; Facile One-Pot Synthesis of Intrinsically Radiolabeled and Cyclic RGD Conjugated 199Au Nanoparticles for Potential Use in Nanoscale Brachytherapy. Industrial & Engineering Chemistry Research 2018, 57, 14337-14346, 10.1021/acs.iecr.8b02526.
- Seyedeh Fatemeh Hosseini; M. Sadeghi; Mohammad Reza Aboudzadeh; Morteza Mohseni; Production and modeling of radioactive gold nanoparticles in Tehran research reactor. Applied Radiation and Isotopes 2016, 118, 361-365, 10.1016/j.apradiso.2016.10.004.
- Yingying Zhang; Yongxue Zhang; Lianglan Yin; Xiaotian Xia; Fan Hu; Qingyao Liu; Chunxia Qin; Xiaoli Lan; Synthesis and Bioevaluation of Iodine-131 Directly Labeled Cyclic RGD-PEGylated Gold Nanorods for Tumor-Targeted Imaging. Contrast Media & Molecular Imaging 2017, 2017, 1-10, 10.1155/2017/6081724.
- Anders F. Frellsen; Anders Elias Hansen; Rasmus I. Jølck; Paul Joseph Kempen; Gregory W. Severin; Palle H. Rasmussen; Andreas Kjær; Andreas T. I. Jensen; Thomas Lars Andresen; Andreas Kjær; et al. Mouse Positron Emission Tomography Study of the Biodistribution of Gold Nanoparticles with Different Surface Coatings Using Embedded Copper-64. ACS Nano 2016, 10, 9887-9898, 10.1021/acsnano.6b03144.
- Yongfeng Zhao; Deborah Sultan; Lisa Detering; Sangho Cho; Guorong Sun; Richard Pierce; Karen L. Wooley; Yongjian Liu; Copper-64-Alloyed Gold Nanoparticles for Cancer Imaging: Improved Radiolabel Stability and Diagnostic Accuracy. Angewandte Chemie International Edition 2013, 53, 156-159, 10.1002/anie.201308494.
- Xiaolian Sun; Xinglu Huang; Xuefeng Yan; Yu Wang; Jinxia Guo; Orit Jacobson; Dingbin Liu; Lawrence P. Szajek; Wenlei Zhu; Gang Niu; et al.Dale O. KiesewetterShouheng SunXiaoyuan Chen Chelator-Free 64Cu-Integrated Gold Nanomaterials for Positron Emission Tomography Imaging Guided Photothermal Cancer Therapy. ACS Nano 2014, 8, 8438-8446, 10.1021/nn502950t.
- Jacek Koziorowski; Adina E Stanciu; Vanessa Gómez‐Vallejo; Jordi Llop; Radiolabeled Nanoparticles for Cancer Diagnosis and Therapy. Anti-Cancer Agents in Medicinal Chemistry 2017, 17, 333-354, 10.2174/1871520616666160219162902.