Peroxosolvates - adducts of hydrogen peroxide and molecules or salts formed by hydrogen bonding.
- hydrogen peroxide
- two-component crystals
- periodic DFT computations
- hydrogen bond
- hydrogen bond enthalpy and energy
- peroxosolvate
- mixed pharmaceutical forms
- perhydrate
- adduct
1. Introduction
Crystalline peroxosolvates, adducts of hydrogen peroxide, were first introduced by Tanatar who synthesized sodium percarbonate Na2CO3·1.5H2O2 [1] and urea perhydrate (percarbamide) CH4N2O·H2O2 [2]. These compounds are the two most widely used solid peroxocompounds with annual production in the millions of tons[3]. Sustainable, nontoxic, and minimal hazard processing trends combine to intensify the use of hydrogen peroxide in diverse fields and the same trends are responsible for the perpetually growing use of peroxosolvates[3][4][5]. Peroxosolvates are now used for bleaching, disinfection, and oxidation; as chemical reagents in household commodities, cosmetics, pharmaceuticals, and washing powders; in industrial environmental processes such as remediation, bioremediation, and oxygen production; and as explosive ingredients and reagents for chemical synthesis [3][4][5][6]. In general, hydrogen peroxide release from peroxosolvates tends to lower the pH, whereas hydroperoxo- and peroxo-complexes tend to increase the pH [6][7][8][9][10]. Thus, peroxosolvates are considered safer and more economic as aqueous hydrogen peroxide decomposes at high pH.
Cambridge Structural Database (CSD)[11] and the Inorganic Crystal Structure Database (ICSD)[12] contain information on 134 peroxosolvates. This is several orders of magnitude less than the number of crystalline hydrates that exist in these databases[11][13]. A significant number of peroxosolvates (44 adducts) were synthesized and structurally characterized at the Kurnakov Institute of General and Inorganic Chemistry of Russian Academy of Sciences (IGIS RAS).
2. Chemical Composition of Crystalline Peroxosolvates
All 134 crystalline peroxosolvates known to date can be divided into three main groups depending on the chemical nature of the coformer (Figure 1):
1) salts of inorganic and carboxylic acids,
2) amino acids, peptides and related zwitterions, and
3) molecular compounds with a lone electron pair on nitrogen and/or oxygen atoms.
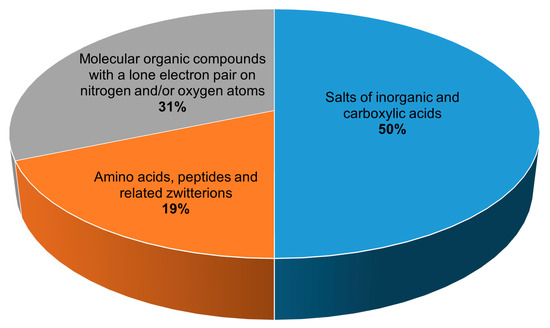
Figure 1.
Distribution of peroxosolvates by the chemical nature of the coformer.
Intermolecular H-bonds play a structure-directing role in the considered crystals.
The largest number of structurally characterized peroxosolvates, 67 compounds, are adducts of hydrogen peroxide and salts of inorganic and carboxylic acids with various cations. Among the salts of carboxylic acids that form stable peroxosolvates [14][15][16][17][18], oxalates can be distinguished in the composition of six compounds. The salts of inorganic acids that form adducts with hydrogen peroxide are very diverse. In addition to fluorides [19][20][21], chlorides, and bromides[22][23], a number of peroxosolvates of alkali metal and ammonium carbonates are known[24][25][26]. The latter include the commercially demanded sodium peroxocarbonate synthesized by Tanatar[1]. This class of compounds should include peroxosolvates of complex anions, which can formally be attributed to the salts of the corresponding complex acids, for example, peroxovanadates[27][28][29][30][31][32][33][34], peroxoniobates[35][36][37], peroxotantalates[38], uranyl peroxo complexes[39], peroxotellurates[40], and platinum complexes[41][42][43]. Peroxosolvates of metal peroxides[44][45][46][47] can formally belong to the specified class of peroxosolvates of salts of inorganic acids if hydrogen peroxide is considered as a diacid.
The next group in terms of the number of compounds (42 compounds) are peroxosolvates formed by molecular organic compounds with a lone electron pair(s) on the nitrogen and/or oxygen atom(s). The main representatives of this group of crystalline hydrogen peroxide adducts are organophosphorus compounds containing the P=O functional group [48][49][50][51][52][53][54][55] and nitrogen-containing heterocyclic compounds[56][57][58][59][60][61][62][63][64][65], in particular N-oxides[66][67][68][69][70][71][72][73], obtained as a result of the oxidation reaction of the corresponding compounds with hydrogen peroxide. Urea peroxosolvate[74] is used as a solid source of hydrogen peroxide, and, along with 1,4-diazabicyclo[2.2.2]octane (DABCO) peroxosolvate[75], is used in organic syntheses to obtain anhydrous hydrogen peroxide solutions.
Separately, it is worth highlighting the third group, which includes amino acids, peptides, and related zwitterions (25 peroxosolvates). Peroxosolvates of a number of proteionogenic l-amino acids (serine, threonine, leucine, isoleucine, tyrosine, glycine, and phenylalanine)[76][77] and non-proteinogenic amino acids (gamma-aminobutyric acid, beta-alanine, and sarcosine) were obtained and structurally characterized at the Kurnakov Institute of General and Inorganic Chemistry RAS[77][78]. The class of zwitterions related to amino acids that form peroxosolvates includes pyridine carboxylic acids: nicotinic, isonicotinic, and picolinic acids[79], as well as 2-aminonicotinic acid[66]. Peroxosolvates of cyclic dipeptides—diglycine, disarcosine, and dialanine—are an example of the nonoxidative interaction of concentrated hydrogen peroxide and a peptide fragment[80].
The CSD analysis revealed the necessary properties of co-former peroxosolvates, which are promising compounds for the synthesis of new crystalline peroxosolvates[56]:
1) Hydrogen peroxide should not participate in redox reactions with coformers.
2) They must be sufficiently soluble in protic solvents to carry out the crystallization process.
3) Coformers should have the ability to form H-bonds, primarily as proton acceptors.
4) Compounds with pronounced acidic properties do not form peroxosolvates [14], since in such compounds the proton-acceptor groups are protonated.
5) Coformers should exhibit amphoteric or basic properties. Strong bases deprotonate hydrogen peroxide and form peroxide or hydroperoxide as ionic or complex moieties (ZnO2 [81], NH4+OOH− [82], or [Sn(OOH)6]2− [7]).
A significant part of the 134 peroxosolvates available in the structural databases, namely 40 structures, contain incomplete or erroneous data. Some crystal structures contain unlocalized hydrogen atoms and errors in the O-O bond lengths and H-O-O-H and O-O-H angles in the hydrogen peroxide molecule. This is due to the fact that during the preparation of these compounds, H2O2 was used as an oxidizing agent or ligand to obtain the corresponding peroxo complexes; therefore, the mass content of hydrogen peroxide in the obtained crystals is low. The chemical composition of 94 crystal structures of peroxosolvates with objectively localized protons and free of structural errors is presented in Supplementary Materials Table S1. These 94 crystal structures are the subject of the review, as they allow one to analyze the topology of H2O2 hydrogen-bonded networks.
References
- S. Tanatar; Percarbonate. Berichte der deutschen chemischen Gesellschaft 1899, 32, 1544-1546, 10.1002/cber.18990320233.
- Tanatar, S.; Double Compounds of Hydrogen Peroxide with Organic Substances. Journal of Russian PhysChem Society 1906, 40L, 376-380.
- Jakob, H.; Leininger, S.; Lehmann, T.; Jacobi, S.; Gutewort, S. . Peroxo Compounds, Inorganic; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; pp. 1-33.
- Goti, A.; Cardona, F.. Hydrogen Peroxide in Green Oxidation Reactions: Recent Catalytic Processes; Springer: Dordrecht, The Netherlands, 2008; pp. 191-212.
- Jones, C.W. . Applications of Hydrogen Peroxide and Derivatives; Royal Society of Chemistry: Cambridge, UK, 1999; pp. 1-264.
- Schumb, W.C.; Satterfield, C.N.; Wentworth, R.L. . Hydrogen peroxide; Reinhold Publishing Corporation: New York, NY, USA, 1955; pp. 1-759.
- Andrei V. Churakov; Sergey Sladkevich; Ovadia Lev; Tatiana A. Tripol'skaya; Petr V. Prikhodchenko; Cesium Hydroperoxostannate: First Complete Structural Characterization of a Homoleptic Hydroperoxocomplex. Inorganic Chemistry 2010, 49, 4762-4764, 10.1021/ic100554u.
- Alexey A. Mikhaylov; Alexander G. Medvedev; Tatiana A. Tripol'skaya; Victor Popov; Artem S. Mokrushin; D. P. Krut"ko; Petr V. Prikhodchenko; Ovadia Lev; H2O2induced formation of graded composition sodium-doped tin dioxide and template-free synthesis of yolk–shell SnO2particles and their sensing application. Dalton Transactions 2017, 46, 16171-16179, 10.1039/c7dt03104a.
- Yitzhak Wolanov; Ovadia Lev; Andrei V. Churakov; Alexander G. Medvedev; Vladimir M. Novotortsev; Petr V. Prikhodchenko; Preparation of pure hydrogen peroxide and anhydrous peroxide solutions from crystalline serine perhydrate. Tetrahedron 2010, 66, 5130-5133, 10.1016/j.tet.2010.04.109.
- Alexander G. Medvedev; Alexey A. Mikhaylov; Andrei V. Churakov; Mikhail V. Vener; Tatiana A. Tripol'skaya; Shmuel Cohen; Ovadia Lev; Petr V. Prikhodchenko; Potassium, Cesium, and Ammonium Peroxogermanates with Inorganic Hexanuclear Peroxo Bridged Germanium Anion Isolated from Aqueous Solution. Inorganic Chemistry 2015, 54, 8058-8065, 10.1021/acs.inorgchem.5b01293.
- Colin R. Groom; Ian J. Bruno; Matthew P. Lightfoot; Suzanna C. Ward; The Cambridge Structural Database. Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials 2016, 72, 171-179, 10.1107/s2052520616003954.
- Alec Belsky; Mariette Hellenbrandt; Vicky Lynn Karen; Peter Luksch; New developments in the Inorganic Crystal Structure Database (ICSD): accessibility in support of materials research and design. Acta Crystallographica Section B Structural Science 2002, 58, 364-369, 10.1107/s0108768102006948.
- Frank H. Allen; The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Crystallographica Section B Structural Science 2002, 58, 380-388, 10.1107/s0108768102003890.
- B. F. Pedersen; The crystal structure of ammonium oxalate monoperhydrate. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 1972, 28, 746-754, 10.1107/s0567740872003139.
- Berit F. Pedersen; T. Kindt Larsen; H. Soling; Lena Torbjörnsson; Per-Erik Werner; Ulf Junggren; Bo Lamm; Benny Samuelsson; The Crystal Structure of Lithium Oxalate Monoperhydrate, Li2C2O4.H2O2.. Acta Chemica Scandinavica 2008, 23, 1871-1877, 10.3891/acta.chem.scand.23-1871.
- B. F. Pedersen; Å. Kvick; Neutron diffraction study of potassium oxalate monoperhydrate at 123 K. Acta Crystallographica Section C Crystal Structure Communications 1990, 46, 21-23, 10.1107/s010827018900332x.
- Berit F. Pedersen; Hans M. Seip; Johan Santesson; Pär Holmberg; G. Eriksson; R. Blinc; S. Pausak; L. Ehrenberg; J. Dumanović; The Crystal Structure of Potassium and Rubidium Oxalate Monoperhydrates, K2C2O4.H2O2 and Rb2C2O4.H2O2.. Acta Chemica Scandinavica 1967, 21, 779-790, 10.3891/acta.chem.scand.21-0779.
- J. M. Adams; R. G. Pritchard; The crystal structure of guanidinium oxalate dihydrate monoperhydrate. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 1976, 32, 2438-2440, 10.1107/s0567740876007929.
- Sarin, V.A.; Dudarev, V.Y.; Dobrynina, T.A.; Fykin, L.E.; Zavodnik, V.E. X-Ray and Neutron-diffraction Study of KF·2H2O2. Kristallografiya 1976, 21, 929–936.
- Sarin, V.A.; Dudarev, V.Y.; Dobrynina, T.A.; Fykin, L.E.; Zavodnik, V.E. X-Ray and Neutron-diffraction Studies of RbF·H2O2 Crystals. Kristallografiya 1977, 22, 982–987.
- Sarin, V.A.; Dudarev, V.Y.; Dobrynina, T.A.; Zavodnik, V.E. X-Ray structural Investigation of NH4F·H2O2 Crystals. Kristallografiya 1979, 24, 824–825.
- Robin G. Pritchard; Zubeda Begum; Yuf Fai Lau; Jonothan Austin; Structures of Na9[SO4]4 X·2H2O2, where X = Cl or Br, in which the halide anions orchestrate extended orientation sequences of H2O2 solvate molecules. Acta Crystallographica Section B Structural Science 2005, 61, 663-668, 10.1107/s010876810503212x.
- Andrei V. Churakov; Petr V. Prikhodchenko; Judith A. K. Howard; The preparation and crystal structures of novel perhydrates Ph4X+Hal–·nH2O2: anionic hydrogen-bonded chains containing hydrogen peroxide. CrystEngComm 2005, 7, 664-669, 10.1039/b511834d.
- Maria Arménia Carrondo; William P. Griffith; D. Philip Jones; Andrzej C. Skapski; X-Ray crystal structure of the industrial bleaching agent ‘sodium percarbonate’[sodium carbonate–hydrogen peroxide (2/3)]. J. Chem. Soc., Dalton Trans. 1977, -, 2323-2327, 10.1039/dt9770002323.
- Robin Gavin Pritchard; Emran Islam; Sodium percarbonate between 293 and 100 K. Acta Crystallographica Section B Structural Science 2003, 59, 596-605, 10.1107/s0108768103012291.
- Alexander G. Medvedev; Alexey A. Mikhaylov; Andrei V. Churakov; Petr V. Prikhodchenko; Ovadia Lev; Ammonium and caesium carbonate peroxosolvates: supramolecular networks formed by hydrogen bonds. Acta Crystallographica Section C Crystal Structure Communications 2012, 68, i20-i24, 10.1107/s0108270112006701.
- Thierbach, D.; Huber, F.; Preut, H. Structure of Triphenylphosphine Oxide Hemiperhydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 974–977, doi:10.1107/S0567740880005067.
- Stomberg, R.; Klemets, R.; Lundström, I.; Fontell, K.; Nielsen, C.J.; Urso, F.; Weidlein, J.; Zingaro, R.A. The Crystal Structures of Potassium Bis(oxalato)oxoperoxovanadate(V) Hemihydrate, K3[VO(O2)(C2O4)2]·½H2O, and Potassium Bis(oxalato)dioxovanadate(V) Trihydrate, K3[VO2(C2O4)2]·3H2O. Acta Chem. Scand. 1986, 40a, 168–176, doi:10.3891/acta.chem.scand.40a-0168.
- Won, T.-J.; Barnes, C.L.; Schlemper, E.O.; Thompson, R.C. Two Crystal Structures Featuring the Tetraperoxovanadate(V) Anion and a Brief Reinvestigation of Peroxovanadate Equilibria in Neutral and Basic Solutions. Inorg. Chem. 1995, 34, 4499–4503, doi:10.1021/ic00121a031.
- Szentivanyi, H.; Stomberg, R.; Hämäläinen, R.; Kohl, F.X.; Seip, R. The Crystal Structure of 2,2′-Bipyridinium(1+) mu-Hydrogen-bis[(2,2′-bipyridine)oxodiperoxovanadate](1-)-x-hydrogen peroxide-(6-x)-water, (Hbipy)[H{VO(O2)2bipy}2]·xH2O2·(6-x)H2O, x ~= 0.5, at −100 degrees C. Acta Chem. Scand. 1984, 38, 101–107, doi:10.3891/acta.chem.scand.38a-0101.
- Campbell, N.J.; Capparelli, M.V.; Griffith, W.P.; Skapski, A.C. On the Existence of Triperoxo Vanadium Complexes. X-ray Crystal Structures of K3[VO(O2)2(C2O4]·H2O2 and of (NH4)[VO(O2)2(bipy)]·4H2O. Inorg. Chim. Acta 1983, 77, L215–L216, doi:10.1016/S0020-1693(00)82620-1.
- Schwendt, P.; Ahmed, M.; Marek, J. Complexation between Vanadium (V) and Phenyllactate: Synthesis, spectral Studies and Crystal Structure of (NEt4)(NH4)3[V2O2(O2)2(R-3-phlact)2][V2O2(O2)2(S-3-phlact)2]·6H2O, [3-phlact=3-phenyllactato(2−)]. Inorg. Chim. Acta 2005, 358, 3572–3580, doi:10.1016/j.ica.2005.06.039.
- Shao, M.; Dong, X.U.N.; Tang, Y. Crystal Structure Investigation of Vanadyl Complexes of Tridentate Ligand. (II)—Synthese and Crystal Structure of 1-(2-pyridylazo)-2-naphtholato-dioxovanadium(V) Dimer [VO2(C15)H10N3O)]2(H2O2)(CHCl3)2 and Pyridine-(1-(2-pyridylazo)-2-naphtolato)peroxo Oxovanadium(V) VO(O2)(C15H10N3O)(C5H5N). Sci. Sin. Ser. B (Engl. Ed.) 1988, 31, 789–799, doi:10.1360/yb1988-31-7-789.
- Šimuneková, M.; Šimunek, J.; Chrappová, J.; Schwendt, P.; Žák, Z.; Pavelčík, F. Dinucleating Role of a Strong Hydrogen Bond in Crystal Structure of [N(C4H9)4]{[VO(HO2)(O2)(phen)][VO(O2)2(phen)]}·3H2O2·H2O. Inorg. Chem. Commun. 2012, 24, 125–128, doi:10.1016/j.inoche.2012.08.003.
- Mathern, G.; Weiss, R. Structure des Complexes Peroxydiques des Métaux de Transition. II. Structure Cristalline du Triperoxo-(o-phénanthroline)niobate de Potassium à Trois Molécules d’Eau et de son Perhydrate KNb(O2)3(C12H8N2)·3H2O et KNb(O2)3(C12H8N2)·3H2O·H2O2. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1971, 27, 1582–1597, doi:10.1107/S0567740871004400.
- Bayot, D.; Tinant, B.; Mathieu, B.; Declercq, J.-P.; Devillers, M. Spectroscopic and Structural Characterizations of Novel Water-Soluble Peroxo[polyaminocarboxylato bis(N-oxido)]niobate(V) Complexes. Eur. J. Inorg. Chem. 2003, 2003, 737–743, doi:10.1002/ejic.200390102.
- Bayot, D.; Tinant, B.; Devillers, M. Homo- and Heterobimetallic Niobium(V) and Tantalum(V) Peroxo-tartrate Complexes and Their Use as Molecular Precursors for Nb−Ta Mixed Oxides. Inorg. Chem. 2005, 44, 1554–1562, doi:10.1021/ic0484250.
- Daisy Bayot; Bernard Tinant; Michel Devillers; Spectroscopic and Structural Characterizations of Novel Water-Soluble Tetraperoxo and Diperoxo[polyaminocarboxylato bis(N-oxido)]tantalate(V) Complexes. Inorganic Chemistry 2004, 43, 5999-6005, 10.1021/ic049639k.
- Jie Qiu; Bess Vlaisavljevich; Laurent Jouffret; Kevin Nguyen; Jennifer E. S. Szymanowski; Laura Gagliardi; Peter C. Burns; Cation Templating and Electronic Structure Effects in Uranyl Cage Clusters Probed by the Isolation of Peroxide-Bridged Uranyl Dimers. Inorganic Chemistry 2015, 54, 4445-4455, 10.1021/acs.inorgchem.5b00248.
- Alexey A. Mikhaylov; Alexander G. Medvedev; Andrei V. Churakov; Dmitry A. Grishanov; Petr V. Prikhodchenko; Ovadia Lev; Peroxide Coordination of Tellurium in Aqueous Solutions. Chemistry – A European Journal 2016, 22, 2980-2986, 10.1002/chem.201503614.
- Mühle, C.; Peters, E.-M.; Jansen, M. New Hydrogen Peroxide Adducts of Alkali Metal Tetracyanoplatinates A2[Pt(CN)4]·H2O2 (A = K, Rb, Cs). Z. Naturforsch. B 2009, 64, 111–115, doi:10.1515/znb-2009-0115.
- Khodadad, P.; Rodier, N. Trans-Diammine-trans-dichloro-trans-dihydroxoplatine(IV) di(peroxyde d`hydrogène). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 2219–2220, doi:10.1107/S0108270187088383.
- Barnard, C.F.J.; Hydes, P.C.; Griffiths, W.P.; Mills, O.S. A Stable Platinum Complex Perhydrate Adduct: Crystal Stucture of cis,trans-[PtCl2(OH)2(2-NH2Pr)2]·0.5H2O2 and water and N,N-dimethylacetamide adducts. J. Chem. Res. Synop. 1983, 302–303.
- Vannerberg, N.G. On the System SrO2-H2O-H2O2. I. The Crystal Structure of α -SrO2·2H2O2 and β -SrO2·2H2O2. Ark. Kemi 1958, 13, 29–41.
- Vannerberg, N.G. On the System BaO2-H2O-H2O2. I. Investigation of the Existing Phases and their Preparation. Ark. Kemi 1959, 14, 147–149.
- Vannerberg, N.G. On the System BaO2-H2O-H2O2. II The Structure of BaO2·H2O2. Ark. Kemi 1959, 14, 149–159.
- Vannerberg, N.G. On the System BaO2-H2O-H2O2. III. The Crystal Structure of α-BaO2, β-BaO2, and γ-BaO2·2H2O2 and BaO2·H2O2·2H2O. Ark. Kemi 1959, 14, 125–145.
- D. Thierbach; F. Huber; H. Preut; Structure of triphenylphosphine oxide hemiperhydrate. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 1980, 36, 974-977, 10.1107/s0567740880005067.
- Arp, F.F.; Ahn, S.H.; Bhuvanesh, N.; Blümel, J. Selective Synthesis and Stabilization of Peroxides via Phosphine Oxides. New J. Chem. 2019, 43, 17174–17181, doi:10.1039/C9NJ04858H.
- Arp, F.F.; Bhuvanesh, N.; Blümel, J. Hydrogen Peroxide Adducts of Triarylphosphine Oxides. Dalton Trans. 2019, 48, 14312–14325, doi:10.1039/C9DT03070K.
- Ahn, S.H.; Cluff, K.J.; Bhuvanesh, N.; Blümel, J. Hydrogen Peroxide and Di(hydroperoxy)propane Adducts of Phosphine Oxides as Stoichiometric and Soluble Oxidizing Agents. Angew. Chem. Int. Ed. 2015, 54, 13341–13345, doi:10.1002/anie.201505291.
- Hilliard, C.R.; Bhuvanesh, N.; Gladysz, J.A.; Blümel, J. Synthesis, Purification, and Characterization of Phosphine Oxides and their Hydrogen Peroxide Adducts. Dalton Trans. 2012, 41, 1742–1754, doi:10.1039/C1DT11863C.
- Čermák, J.; Kvı́čalová, M.; Šabata, S.; Blechta, V.; Vojtı́šek, P.; Podlaha, J.; Shaw, B.L. Diphosphinoazines (Z,Z)-R2PCH2C(But)=NN=C(But)CH2PR2 with R Groups of Various Sizes and Complexes {[(Z,Z)-R2PCH2C(But)=NN=C(But)CH2PR2]-[η3-CH2C(CH3)=CH2PdCl]2}. Inorg. Chim. Acta 2001, 313, 77–86, doi:10.1016/S0020-1693(00)00376-5.
- Neda, I.; Kaukorat, T.; Fischer, A.; Jones, P.G.; Schmutzler, R. Oxidationsreaktionen an 2-[2-(N,N-Dimethylamino)ethyl-methylamino]-1,3,5-trimethyl-1,3,5-triaza-2λ3-phosphorinan-4,6-dion; Hydrolyse und Thermolyse eines Perfluorpinakolylsubstituierten Spirophosphorans. J. Fluor. Chem. 1994, 69, 35–40, doi:10.1016/0022-1139(93)03036-L.
- Sevcik, R.; Necas, M.; Novosad, J. The Synthesis and Characterization of Three Oxidized Derivatives of bis(diphenylphosphino)pyridine and their Sn(IV) Complexes. Polyhedron 2003, 22, 1585–1593, doi:10.1016/S0277-5387(03)00291-2.
- Chernyshov, I.Y.; Vener, M.V.; Prikhodchenko, P.V.; Medvedev, A.G.; Lev, O.; Churakov, A.V. Peroxosolvates: Formation Criteria, H2O2 Hydrogen Bonding, and Isomorphism with the Corresponding Hydrates. Cryst. Growth Des. 2017, 17, 214–220, doi:10.1021/acs.cgd.6b01449.
- Churakov, A.V.; Grishanov, D.A.; Medvedev, A.G.; Mikhaylov, A.A.; Vener, M.V.; Navasardyan, M.A.; Tripol’skaya, T.A.; Lev, O.; Prikhodchenko, P.V. Stabilization of Hydrogen Peroxide by Hydrogen Bonding in the Crystal Structure of 2-aminobenzimidazole Perhydrate. CrystEngComm 2020, 22, 2866–2872, doi:10.1039/D0CE00096E.
- Wiscons, R.A.; Bellas, M.K.; Bennion, J.C.; Matzger, A.J. Detonation Performance of Ten Forms of 5,5′-Dinitro-2H,2H′-3,3′-bi-1,2,4-triazole (DNBT). Cryst. Growth Des. 2018, 18, 7701–7707, doi:10.1021/acs.cgd.8b01583.
- Laus, G.; Schwärzler, A.; Bentivoglio, G.; Hummel, M.; Kahlenberg, V.; Wurst, K.; Kristeva, E.; Schütz, J.; Kopacka, H.; Kreutz, C.; et al. Synthesis and Crystal Structures of 1-Alkoxy-3-alkylimidazolium Salts Including Ionic Liquids, 1-Alkylimidazole 3-oxides and 1-Alkylimidazole Perhydrates. Z. Naturforsch. B 2008, 63, 447–464, doi:10.1515/znb-2008-0411.
- Jakob, F.; Herdtweck, E.; Bach, T. Synthesis and Properties of Chiral Pyrazolidines Derived from (+)-Pulegone. Chem. Eur. J. 2010, 16, 7537–7546, doi:10.1002/chem.201000219.
- Navasardyan, M.A.; Bezzubov, S.I.; Kuz’mina, L.G.; Prikhodchenko, P.V.; Churakov, A.V. Crystal Structure of 2,3,5,6-tetrakis(pyridin-2-yl)pyrazine Hydrogen Peroxide 4.75-solvate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 1793–1796, doi:10.1107/S2056989017015328.
- Churakov, A.V.; Chetina, O.V.; Howard, J.A.K. Dicyclohexylamine Hydrogen Peroxide Hemisolvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, o3503–o3505, doi:10.1107/S1600536806028030.
- Serra, M.A.; Dorner, B.K.; Silver, M.E. Structure of an Adenine-hydrogen Peroxide Adduct. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1992, 48, 1957–1960, doi:10.1107/S0108270192002294.
- Kersten, K.M.; Breen, M.E.; Mapp, A.K.; Matzger, A.J. Pharmaceutical Solvate Formation for the Incorporation of the Antimicrobial Agent Hydrogen Peroxide. Chem. Commun. 2018, 54, 9286–9289, doi:10.1039/C8CC04530E.
- Bennion, J.C.; Chowdhury, N.; Kampf, J.W.; Matzger, A.J. Hydrogen Peroxide Solvates of 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane. Angew. Chem. Int. Ed. 2016, 55, 13118–13121, doi:10.1002/anie.201607130.
- Grishanov, D.A.; Navasardyan, M.A.; Medvedev, A.G.; Lev, O.; Prikhodchenko, P.V.; Churakov, A.V. Hydrogen Peroxide Insular Dodecameric and Pentameric Clusters in Peroxosolvate Structures. Angew. Chem. Int. Ed. 2017, 56, 15241–15245, doi:10.1002/anie.201709699.
- Ravikumar, K.; Sridhar, B.; Manjunatha, S.G.; Thomas, S. Risperidone N-oxide Hydrogen Peroxide Methanol Solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, o2515–o2517, doi:10.1107/S1600536805022002.
- Kay Hon, P.; Mak, T.C.W. Isolation and Crystal Structures of 1,3 Molecular Complexes of TriethylenediamineN,N’-dioxide with Hydrogen Peroxide and Water. J. Crystallogr. Spectrosc. Res. 1987, 17, 419–429, doi:10.1007/BF01180319.
- Churakov, A.V.; Prikhodchenko, P.V.; Medvedev, A.G.; Mikhaylov, A.A. Crystal Structure of (Z)-N-benzylidene-1-phenylmethanamine Oxide Hydrogen Peroxide Monosolvate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 1666–1669, doi:10.1107/S2056989017014499.
- Lynch, W.; Padgett, C.W. 2,2′-Disulfanediylbis(pyridine N-oxide)–hydrogen Peroxide (1/1). IUCrData 2018, 3, x180320, doi:10.1107/S2414314618003206.
- Chandrasekaran, A.; Timosheva, N.V.; Day, R.O.; Holmes, R.R. Pseudoheptacoordination and Pseudohexacoordination in Tris(2-N,N-dimethylbenzylamino)phosphane. Inorg. Chem. 2002, 41, 5235–5240, doi:10.1021/ic020153i.
- Mak, T.C.W.; Lam, Y.-S. Hexamethylenetetramine Oxide–hydrogen Peroxide–water (1:1:1). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 1732–1735, doi:10.1107/S0567740878006536.
- Wang, Y.; Song, S.; Huang, C.; Qi, X.; Wang, K.; Liu, Y.; Zhang, Q. Hunting for Advanced High-energy-density Materials with Well-balanced Energy and Safety through an Energetic Host–guest Inclusion Strategy. J. Mater. Chem. A 2019, 7, 19248–19257, doi:10.1039/C9TA04677A.
- C. J. Fritchie; R. K. McMullan; Neutron diffraction study of the 1:1 urea:hydrogen peroxide complex at 81 K. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 1981, 37, 1086-1091, 10.1107/s0567740881005116.
- Gerhard Laus; Volker Kahlenberg; Klaus Wurst; Thomas Lörting; Herwig Schottenberger; Hydrogen bonding in the perhydrate and hydrates of 1,4-diazabicyclo[2.2.2]octane (DABCO). CrystEngComm 2008, 10, 1638-1644, 10.1039/b807303a.
- Andrei V. Churakov; Petr V. Prikhodchenko; Judith A. K. Howard; Ovadia Lev; Glycine and l-serine crystalline perhydrates. Chemical Communications 2009, -, 4224-4226, 10.1039/b906801e.
- Petr V. Prikhodchenko; Alexander G. Medvedev; Tatiana A. Tripol'skaya; Andrei V. Churakov; Yitzhak Wolanov; Judith A. K. Howard; Ovadia Lev; Crystal structures of natural amino acid perhydrates. CrystEngComm 2011, 13, 2399-2407, 10.1039/c0ce00481b.
- Mger A. Navasardyan; Dmitry A. Grishanov; Tatiana A. Tripol'skaya; L. G. Kuz’Mina; Petr V. Prikhodchenko; Andrei V. Churakov; Churakov Andrei; Crystal structures of non-proteinogenic amino acid peroxosolvates: rare example of H-bonded hydrogen peroxide chains. CrystEngComm 2018, 20, 7413-7416, 10.1039/c8ce01486h.
- Alexander G Medvedev; A. A. Mikhailov; Petr V Prikhodchenko; T. A. Tripol’Skaya; Ovadia Lev; Andrei V. Churakov; Crystal structures of pyridinemonocarboxylic acid peroxosolvates. Russian Chemical Bulletin 2013, 62, 1871-1876, 10.1007/s11172-013-0269-9.
- Andrei V. Churakov; Dmitry A. Grishanov; Alexander G. Medvedev; Alexey A. Mikhaylov; Tatiana A. Tripol'skaya; Mikhail V. Vener; Mger A. Navasardyan; Ovadia Lev; Petr V. Prikhodchenko; Churakov Andrei; et al. Cyclic dipeptide peroxosolvates: first direct evidence for hydrogen bonding between hydrogen peroxide and a peptide backbone. CrystEngComm 2019, 21, 4961-4968, 10.1039/c9ce00892f.
- Yitzhak Wolanov; Petr V. Prikhodchenko; Alexander G. Medvedev; Rami Pedahzur; Ovadia Lev; Zinc Dioxide Nanoparticulates: A Hydrogen Peroxide Source at Moderate pH. Environmental Science & Technology 2013, 47, 8769-8774, 10.1021/es4020629.
- Andrei V. Churakov; Petr V. Prikhodchenko; Ovadia Lev; Alexander G. Medvedev; Tatiana A. Tripol'skaya; Mikhail V. Vener; A model proton-transfer system in the condensed phase: NH4+OOH−, a crystal with short intermolecular H-bonds. The Journal of Chemical Physics 2010, 133, 164506, 10.1063/1.3493688.
- Alexander G. Medvedev; Andrei V. Churakov; Petr V. Prikhodchenko; Ovadia Lev; Mikhail V. Vener; Crystalline Peroxosolvates: Nature of the Coformer, Hydrogen-Bonded Networks and Clusters, Intermolecular Interactions. Molecules 2020, 26, 26, 10.3390/molecules26010026.
