Most cancer treatment modalities efficient in other malignancies display limited efficacy in pancreatic cancer, and novel therapeutic strategies in a multidisciplinary approach are highly warranted. Targeting the gut microbiota represents a big challenge for precision medicine in the near future. A growing body of evidence suggested the prognostic value of pancreatic tumor microbiome for cancer treatment efficacy. Pilot results showed that bacteria found in pancreatic ductal adenocarcinoma (PDAC) samples could modulate sensitivity to therapeutic agents via immune activation in the pancreatic tumor microenvironment. Thus, the microbiota-derived approach might represent an emerging trend for improving the immunotherapy and chemotherapy response in this devastating disease.
- pancreatic ductal adenocarcinoma
- microbiome
- cancer treatment efficacy
- tumor microenvironment
- immune activation
- probiotics
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC), accounting for about 90% of all pancreatic cancer cases, is expected to become the second leading cause of cancer deaths before 2030 [1]. Since dense desmoplastic stroma represents one of the main factors responsible for the failure of currently applied PDAC treatment, focusing on the tumor microenvironment components might represent an option to overcome the chemoresistance and immune tolerance. Recently, the pancreatic microbiota has been recognized as an integral part of the PDAC microenvironment and the microbial remodeling of the tumor microenvironment towards immune tolerance might be associated with the inefficiency of antitumor immunotherapy. Thus, a microbiota-derived approach should also be taken into account and numerous clinical trials evaluating the effect of the microbiome in pancreatic cancer are currently ongoing (Table 1).
Table 1. Pancreatic cancer, solid tumors, and the microbiome. The table summarizes the list of ongoing and completed clinical trials dealing with the impact of microbiome on the risk, prognosis, and treatment efficacy in pancreatic cancer and solid tumors (according to http://clinicaltrials.gov/).
| Study | Study Design | Disease | Purpose | Patients (n) | Intervention | Study Status |
|---|---|---|---|---|---|---|
| NCT03302637 | A prospective, observational, case-control study | Pancreatic cancer | To determine the relationship of oral and pancreatic microbiome, and their impact on pancreatic cancer risk. | 732 | 16S rRNA gene sequencing assay, extraction of genomic DNA from oral samples | Completed-results not posted |
| NCT04274972 | A prospective, observational, cohort study | Pancreatic cancer | Qualitative and quantitative analysis of the pancreatic microbiome in patients with PDAC submitted to pancreaticoduodenectomy, sampling the lesion intraoperatively. | Estimated enrollment 20 | The oral and rectal microbiome samples will be collected preoperatively. The PDAC tissue from the surgical specimen, the intestinal mucosal tissue from the enteric side of the pancreatic anastomosis, and the bile sample will be collected intraoperatively. On the 30th postoperative day, the oral and rectal samples will be repeated. | Recruiting |
| NCT04189393 | A prospective, observational, cohort study | Gastrointestinal cancer | To assess changes in microbiome composition during surgical treatment quantified as alpha diversity by 16S rRNA sequencing. | Estimated enrollment 60 | Four types of samples will be collected for microbiome analysis: saliva, feces, intraoperative mucosal swabs, and drain fluid | Active, not recruiting |
| NCT04193904 | An interventional open-label phase I study | Pancreatic cancer | To assess the safety of MRx0518 in combination with hypofractionated preoperative radiation through the collection of adverse events. | Estimated enrollment 15 | Drug: MRx0518 Radiation: hypofractionated preoperative radiation |
Recruiting |
| NCT04579978 | A prospective, observational, cohort study | Advanced Solid tumors | To investigate relative abundance and composition of immunotherapy response-associated bacterial species in patients with advanced/unresectable or metastatic solid tumors. | Estimated enrollment 60 | Fecal microbial composition analyzed by 16S rRNA and metagenomic sequencing. | Recruiting |
| NCT04243720 | A prospective, observational, cohort study | Solid tumors | To assess several outcomes; fecal microbiome changes associated with primary or acquired resistance to immunotherapy given alone or in combination in patients with advanced solid tumors. | Estimated enrollment 100 | Stool sample will be collected for DNA extraction. | Recruiting |
| NCT01706393 | An interventional, randomized study |
Solid tumors | To evaluate the effect of probiotics to change the intestinal microbiome in patients undergoing concurrent pelvic/abdominal RT. | Estimated enrollment 26 | Dietary supplement: probiotics (six probiotic cultures); 2 capsules bid orally for 6 weeks, 1 capsule (500 mg). The subjects will start eating probiotics 1 week prior of radiation therapy. | Unknown |
| NCT04600154 | An interventional, randomized study |
Pancreatic cancer | To evaluate the effects of MS-20 on gut microbiota and risk/severity of cachexia in patients receiving chemotherapy for pancreatic cancer. | Estimated enrollment 40 | MS-20 or placebo will be orally administered twice per day in treatment period. | Active, not recruiting |
| NCT03840460 | A prospective observational cohort study | Pancreatic cancer |
|
Estimated enrollment 200 | Blood, urine, stool, saliva, bile, and tissue samples from patients undergoing a tissue biopsy or surgery for suspected or known pancreatic cancer will be collected. Molecular analyses including miRNA analysis, DNA and RNA sequencing, nanostring, RT-PCR, and immunohistochemistry will be carried out. |
Recruiting |
| NCT03891979 | A pilot study | Pancreatic cancer | To determine the change in immune activation in pancreatic tumor tissue following treatment with antibiotics and pembrolizumab. | 0 | Drug: pembrolizumab Drug: ciprofloxacin 500 mg PO BID days 1–29 Drug: metronidazole 500 mg PO TID days 1–29 |
Withdrawn (Suspended due to Primary Investigator’s decision) |
| NCT04203459 | A prospective observational cohort study | Pancreatic cancer | To study the mechanism of enhancing the antitumor effects of human chimeric antigen receptor T cells on pancreatic cancer by gut microbiota regulation. | Estimated enrollment 80 | The collected blood and tissue will undergo molecular analyses, including but not limited to, miRNA analysis, DNA and RNA sequencing, nanostring, real-time PCR, and immunohistochemistry. | Recruiting |
| NCT01562626 | An interventional phase I/II study | Solid tumors | To evaluate the safety and tolerability of APS001F treatment plus 5-FC and maltose. | Estimated enrollment 75 | Drug: APS001F APS001F infusion on days 1, 2, and 3 of each 28-day cycle Drug: 5-FC oral doses on days 11–15 and 18–22, each 28-day cycle Drug: 10% maltose 10% maltose infusion will be administered on days 1–5, 8–12, and 15–19, each 28-day cycle |
Recruiting |
| NCT03637803 | An interventional phase I/II study | Solid tumors | To assess the safety and tolerability of MRx0518 in combination with pembrolizumab through the collection of adverse events. | Estimated enrollment 132 | Drug: MRx0518 Drug: pembrolizumab 25 mg/1 mL intravenous solution |
Recruiting |
Abbreviations: PDAC, pancreatic ductal adenocarcinoma; rRNA, ribosomal ribonucleic acid; RT, radiation therapy; 5-FC, 5-fluorocytosine; RT-PCR, real-time polymerase chain reaction; miRNA, microRNA; DNA, deoxyribonucleic acid.
Oral gavage with fluorescently-labeled Enterococcus faecalis or Escherichia coli confirmed that gut bacteria were able to access the pancreas and might affect the pancreatic microenvironment. Amplicon 16S rRNA gene sequencing showed distinct stage-specific gut and pancreatic microbiome in tumor samples, inducing intratumoral immune suppression and PDAC progression. Furthermore, fecal transfer from PDAC-bearing mice contributed to disease progression. According to the results, deficiency in pattern recognition receptor (PRR) signaling slowed PDAC progression [2][3]. Activation of PRRs and TLR ligation by microbial lipopolysaccharides and flagellins in peritumoral milieu might promote tumor microenvironment reprogramming, and accelerate the PDAC tumorigenesis [4]. Moreover, modification of microbiome by oral antibiotic administration induced tumor microenvironment remodeling leading to a reduction in MDSC and an increase in M1 macrophage differentiation and intratumoral CD4+ and CD8+ T cell activation. Together, bacterial ablation enhanced antitumor immunity and increased susceptibility to αPD-1 immunotherapy by upregulating PD-1 expression on effector T cells in a PDAC orthotopic mouse model. Hence, a combination of specific microbiota ablation with checkpoint-directed immunotherapy might represent a potential treatment strategy for PDAC patients [4].
Gemcitabine is used as a key chemotherapeutic agent in the treatment of PDAC patients, therefore a deeper understanding of the mechanism of resistance would be of particular interest. Expression of the bacterial cytidine deaminase (CDDL) by intratumor Gammaproteobacteria was shown to be responsible for gemcitabine resistance in a mouse model of colorectal cancer due to the ability to metabolize the chemotherapeutic drug gemcitabine (2′,2′-difluorodeoxycytidine) into its inactive form (2′,2′-difluorodeoxyuridine). Moreover, cotreatment with ciprofloxacin has been shown to abrogate gemcitabine resistance. Interestingly, 16S rRNA sequencing of 65 human PDAC tumors identified Gammaproteobacteria (mostly members of Enterobacteriaceae and Pseudomonadaceae), as the most common bacterial taxa representing 51.7% of all reads. Geller et al. suggested the potential role of intrapancreatic microbiota in modulation of tumor resistance to gemcitabine, since cultivation of bacteria from 15 fresh PDAC tumors with colorectal carcinoma cell cultures mediated a complete resistance to a chemotherapeutic agent [5]. Discoveries from PDAC-bearing mice on gemcitabine-treated and nontreated controls revealed the substantial modifications in the gut bacterial composition. The shift towards an inflammation-related bacterial profile with increased Proteobacteria and Verrucomicrobia phylum is assumed to aggravate the pancreatic inflammatory state. Furthermore, bacterial translocation through the bloodstream or direct reflux through the pancreatic ducts might promote immune remodeling of the peritumoral microenvironment [6].
Re-establishing an effective intestinal ecosystem with a favorable enteric microbiota might increase the efficacy of cancer treatment (Figure 2). Despite the emerging role of the microbiome in PDAC, there is a limited number of controlled trials with a consistent design regarding the potential role of the gut and/or tumor microbiome modulation towards tumor progression or improving the sensitivity to therapeutics.
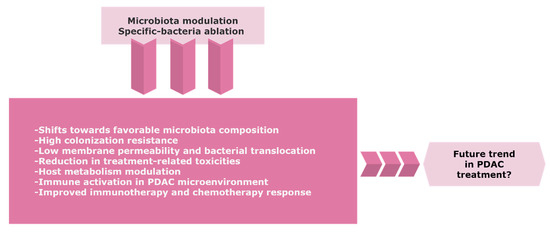
Figure 2. The possible trend of the gut and/or tumor microbiome modulation in PDAC. Precise targeting of microbiota composition might represent a novel approach to improve the therapeutic efficacy and clinical outcome for PDAC patients. Further research and randomized control trials with careful benefit-risk assessment are warranted due to the considerable risks of infection in immunosuppressive cancer patients. Abbreviations: PDAC, pancreatic ductal adenocarcinoma.
Oral antibiotics lead to an antitumor immune activation and restrained tumor burden in mice models bearing PDAC [7]. Coadministration of the PDAC drug gemcitabine with ciprofloxacin significantly reduced the level of detectable bacteria via in vivo imaging and improved the response to the chemotherapeutic agent in colon mouse models [5]. In addition, bacterial ablation via oral antibiotics was found to be protective in pancreatic tumorigenesis and to augment the sensitivity to immunotherapy [4]. Mohindroo et al. retrospectively analyzed the clinical data of 148 metastatic PDAC patients (135 patients exposed to antibiotics) showing prolonged OS and PFS (progression-free survival) after macrolide consumption longer than 3 days [8]. However, a retrospective single-center cohort study on resectable PDAC patients found that tetracycline treatment was associated with clinically significant decreased PFS and statistically significant worse OS [9]. Recently, the reanalysis of the comparator arm of the MPACT clinical trial (comprising 430 metastatic PDAC patients on antibiotic therapy) demonstrated increased gemcitabine-associated toxicity during and after antibiotic exposure [10].
2. Discussion
Numerous studies highlight the positive effects of probiotics and prebiotics on gastrointestinal cancers through the activation of the host’s immune system, maintenance of intestinal barrier integrity, reduction in microbial activity by decreased intestinal pH, as well as inhibition of bacteria involved in the conversion of procarcinogens to carcinogens [11]. Probiotics are described as “mono- or mixed cultures of live microorganisms able to beneficially affect the host by improving the properties of the indigenous flora” [12]. Bacterial translocation is thought to be a possible route of communication between the gut and pancreatic microbiota. Hence, the effects of probiotic modulation in patients with pancreatitis have been evaluated as a risk factor for PDAC development. The first randomized, controlled, and double-blind study in a small cohort of 45 patients with severe acute pancreatitis (SAP) reported a significant reduction in pancreatic sepsis and the number of surgical interventions [13]. However, these results were not able to be reproduced in a second trial [14]. Importantly, the multicenter, randomized, and double-blind versus placebo PROPATRIA study, comprising 296 patients, reported that probiotic prophylaxis did not reduce the risk of infectious complications and was associated with an increased risk of mortality in patients with predicted SAP [15]. Moreover, the result of meta-analysis of six clinical trials found no significant effects of probiotics on the clinical outcomes of patients with SAP [16].
Fecal microbiota transplantation (FMT) contains a greater quantity of microbiota than commonly used probiotic supplements and may represent a promising trend in overcoming the immunosuppression and resistance to therapy in cancer patients likely to have relatively short survival [17]. Animal studies suggest the protective effect of gut and tumor bacteria in PDAC patients who had survived more than 5 years without evidence of disease (long-term survivors). Mice that received FMT from patients with advanced disease harbored much larger tumors compared to the animals receiving FMT from long-term survivors of PDAC or healthy controls [18]. To evaluate the results from preclinical findings, the first clinical trial on resectable PDAC patients receiving FMT from healthy donors delivered through both colonoscopy and oral pills is in preparation.
Due to the contribution of microbiota to an enormous variety of metabolic and immunological pathways, the particular composition of patient´s microbiome should be taken into account to achieve the most efficient therapy response. According to recent studies, a combination of chemotherapy and immunotherapy with proper microbiota modulation might improve the efficacy of cancer treatment and outcome for PDAC patients.
Acknowledgments: The research was funded by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and Slovak Academy of Sciences VEGA, project number 2/0052/18.
The research was funded by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and Slovak Academy of Sciences VEGA, project number 2/0052/18.
This entry is adapted from
The article is from
References
- 1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM.; Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913-21, 10.1158/0008-5472.
- Zambirinis, C.P.; Levie, E.; Nguy, S.; Avanzi, A.; Barilla, R.; Xu, Y.; Seifert, L.; Daley, D.; Greco, S.H.; Deutsch, M.; et al.et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J. Exp. Med. 2015, 212, 2077–2094, 10.1084/jem.20142162.
- Seifert, L.; Werba, G.; Tiwari, S.; Giao Ly, N.N.; Alothman, S.; Alqunaibit, D.; Avanzi, A.; Barilla, R.; Daley, D.; Greco, S.H.; et al.et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 2016, 532, 245–249, 10.1038/nature17403.
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al.et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416, 10.1158/2159-8290.
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al.et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160, 10.1126/science.aah5043.
- Panebianco, C.; Adamberg, K.; Jaagura, M.; Copetti, M.; Fontana, A.; Adamberg, S.; Kolk, K.; Vilu, R.; Andriulli, A.; Pazienza, V.; et al. Influence of gemcitabine chemotherapy on the microbiota of pancreatic cancer xenografted mice. Cancer Chemother. Pharmacol. 2018, 81, 773–782, 10.1007/s00280-018-3549-0.
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al.et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018, 155, 33–37.e36, 10.1053/j.gastro.2018.04.001.
- Mohindroo, Ch.; Rogers, J.E.; Hasanov M.; Mizrahi J.; Overman M.J.; Varadhachary G.R.; Wolff R.A.; Javle M.M.; Fogelman D.R.; Pant S., et al.; et al. A retrospective analysis of antibiotics usage and effect on overall survival and progressive free survival in patients with metastatic PC. J Clin Oncol 2019, 37, 15_suppl, e15781-e15781.
- Hasanov, Z.; Ruckdeschel, T.; König, C.; Mogler, C.; Kapel, S.S.; Korn, C.; Spegg, C.; Eichwald, V.; Wieland, M.; Appak, S.; et al.et al. Endosialin Promotes Atherosclerosis Through Phenotypic Remodeling of Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 495–505.
- Corty, R.W.; Langworthy, B.W.; Fine, J.P.; Buse, J.B.; Sanoff, H.K.; Lund, J.L.; Antibacterial Use Is Associated with an Increased Risk of Hematologic and Gastrointestinal Adverse Events in Patients Treated with Gemcitabine for Stage IV Pancreatic Cancer. Oncologist 2020, 25, 579–584, 10.1634/theoncologist.2019-0570.
- Ciernikova, S.; Mego, M.; Hainova, K.; Adamcikova, Z.; Stevurkova, V.; Zajac, V.; Modification of microflora imbalance: Future directions for prevention and treatment of colorectal cancer?. Neoplasma 2015, 62, 345–352, 10.4149/neo_2015_042.
- Bengmark, S.; Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut 1998, 42, 2-7, 10.1136/gut.42.1.2.
- Oláh, A.; Belágyi, T.; Issekutz, A.; Gamal, M.E.; Bengmark, S.; Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br. J. Surg. 2002, 89, 1103–1107, 10.1046/j.1365-2168.2002.02189.x.
- Oláh, A.; Belágyi, T.; Pótó, L.; Romics, L., Jr.; Bengmark, S.; Synbiotic control of inflammation and infection in severe acute pancreatitis: A prospective, randomized, double blind study. Hepatogastroenterology 2007, 54, 590–594.
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; et al.et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659, 10.1016/S0140-6736(08)60207-X.
- Gou, S.; Yang, Z.; Liu, T.; Wu, H.; Wang, C.; Use of probiotics in the treatment of severe acute pancreatitis: A systematic review and meta-analysis of randomized controlled trials. Crit. Care 2014, 18, R57, 10.1186/cc13809.
- Pitt, J.M.; Vétizou, M.; Gomperts Boneca, I.; Lepage, P.; Chamaillard, M.; Zitvogel, L.; Enhancing the clinical coverage and anticancer efficacy of immune checkpoint blockade through manipulation of the gut microbiota. Oncoimmunology 2017, 6, e1132137, 10.1080/2162402X.2015.1132137.
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al.et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e712, 10.1016/j.cell.2019.07.008.
