Connexin 26, one of the smallest connexins, is expressed in diverse epithelial tissue and mutations in this protein are associated with hearing loss, skin and eye conditions of differing severity.
- epithelial tissue
- connexin
- gap junction
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Epithelial tissues line the outer surface of organs and the inner surface of cavities such as the digestive tract and secretory glands, where they project into the lumen connecting with the external environment. Thus, the epithelium separates tissue compartments forming barriers, regulates molecule exchange between those compartments and protects from biological, physical and chemical aggressions [1]. The integrity of the epithelium is maintained by intercellular junctional complexes composed of tight junctions (TJs), adherens junctions (AJs), and desmosomes [2][3][2,3]. These junctions aid in the formation of tight seals or barriers between the external environment. Since the epithelium is avascular, it is believed that the delivery and co-ordination of intercellular signals directly between cell layers is conducted via gap junction intercellular communication channels (GJIC) and paracrine signalling pathways [4][5][4,5].
Connexins (CXs) are the structural building blocks of gap junctions, four-transmembrane domain spanning proteins with intracellular N- and C-tails (Figure 1). In humans, connexins are encoded by a multigene family containing twenty-one members classified by their molecular weight ranging from 23 to 62 kDa in size (CX23-CX62) [6]. Six connexins oligomerise forming hemichannels or connexons linking the cytoplasm with the extracellular space. Two connexons from adjacent cells connect head-to-head to form an axial channel or gap junction, thereby allowing the interchange of ions and water-soluble molecules with a relative molecular mass up to 1.2 kDa. They play a central role in the control of tissue development, homeostasis and a diverse range of cellular functions [7][8][9][10][7–10].
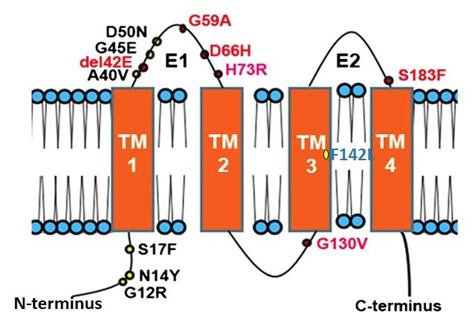
Figure 1. Topology of Connexin 26 and examples of mutations in CX26 associated with epidermal dysplasia and hearing loss. N amino terminus; E1 extracellular loop 1; E2 extracellular loop 2; TM transmembrane domain. Mutations in red indicate ‘Class 2′ black, ‘Class 3′ and blue ‘Class 4′ mutations (see Section 2.2).
The Epithelium and the Tissue Barrier
The structure of epithelial tissue depends on its function. In general, epithelial tissues are classified by their stratification, being simple, transitional or stratified. Simple epithelium composes surface-forming epithelia in contact with the basement membrane (e.g., epithelium of nephric tubules, trachea and secretory glands). The transitional epithelium has some cells in contact with the basement membrane and are surface-forming (e.g., epithelium of urinary bladder). Stratified epithelium is composed of a basal layer, in contact with the basement membrane and the only cells which can divide, and a superficial stratum, where the cells undergo two processes of differentiation: keratinisation non-cornification (e.g., epithelium of oral cavity, oesophagus, vagina) or keratinisation and cornification (e.g., epidermis, nail plate) [1]. The epithelium also plays a central role in body fluid secretions including heat and emotional responses via eccrine, apocrine and sebaceous glandular secretions that are tightly regulated and express a range of connexin proteins [11][12][13][11–13]. Skin appendages including the hair follicles and nails also extend from the stratified epidermis where connexins play a significant role in the hair follicle cycle and reviewed elsewhere [13][14][13,14]. This review will focus primarily on examples of the role of CX26 and CX43 in epithelial tissue including stratified epithelium such as the epidermis and cornea and simple epithelium including the lining of the respiratory and intestinal tracts. Table 1 summarises the expression profile of CX26 in human tissue.
Table 1. Expression of CX26 in human tissue.
|
System or organ |
Tissue or structure |
Cell type |
References |
|
Skin |
Epidermis |
Keratinocyte |
|
|
Spinous layer |
|||
|
Granular layer |
|||
|
Appendages |
Sebaceous gland |
||
|
Eccrine sweat gland and ducts |
|||
|
Hair follicle. |
|||
|
Outer root sheet |
|||
|
Inner root sheet: Henley and Huxlye |
|||
|
Hair shaft: Cortex and Medulla |
|||
|
Matrix |
|||
|
Brain |
Occipital cortex |
Astrocyte (glia) |
[16] |
|
Diencephalon |
Leptomeningeal cells |
||
|
Medulla oblongata |
|||
|
Caudate nucleus |
|||
|
Digestive system |
Stomach |
Epithelial cells |
[17] |
|
Small intestine |
Muscularis externa cells |
||
|
Colon |
|||
|
Endocrine and exocrine glands |
Salivary glands |
Acinar cells |
|
|
Pancreas (serous acini) |
Beta cells |
||
|
Pituitary (adenohypophysis) |
|||
|
Parathyroid |
Principal cells |
||
|
Thyroid (follicles) |
|||
|
Preputial ducts |
|||
|
Lacrimal (serous acini) |
|||
|
Parotid (serous acini) |
|||
|
Liver |
Periportal hepatocytes |
||
|
Kidney |
Proximal tubule |
[21] |
|
|
Reproductive system |
Endometrium (luminal epith.) |
Basal glandular cells |
|
|
Preimplantation embryo |
Blastocysts |
||
|
Placenta |
Syncytiotrophoblasts |
||
|
Myometrium |
Uterine myocytes |
||
|
Endocrine system |
Adrenal cortex |
[24] |
|
|
Ear |
Spiral limbus |
Fibrocytes |
[25] |
|
Spiral ligament |
|||
|
Striavascularis |
|||
|
Cochlea |
Claudius cells |
||
|
Hensen’s cells |
|||
|
Inner sulcus cells |
|||
|
Lung |
Alveolar epithelium |
[26] |
|
System or organ |
Tissue or structure |
Cell type |
References |
|
Skin |
Epidermis |
Keratinocyte |
[13,15] |
|
Spinous layer |
|||
|
Granular layer |
|||
|
Appendages |
Sebaceous gland |
[13,15] |
|
|
Eccrine sweat gland and ducts |
|||
|
Hair follicle. |
|||
|
Outer root sheet |
|||
|
Inner root sheet: Henley and Huxlye |
|||
|
Hair shaft: Cortex and Medulla |
|||
|
Matrix |
|||
|
Brain |
Occipital cortex |
Astrocyte (glia) |
[16] |
|
Diencephalon |
Leptomeningeal cells |
||
|
Medulla oblongata |
|||
|
Caudate nucleus |
|||
|
Digestive system |
Stomach |
Epithelial cells |
[17] |
|
Small intestine |
Muscularis externa cells |
||
|
Colon |
|||
|
Endocrine and exocrine glands |
Salivary glands |
Acinar cells |
[18–20] |
|
Pancreas (serous acini) |
Beta cells |
||
|
Pituitary (adenohypophysis) |
|||
|
Parathyroid |
Principal cells |
||
|
Thyroid (follicles) |
|||
|
Preputial ducts |
|||
|
Lacrimal (serous acini) |
|||
|
Parotid (serous acini) |
|||
|
Liver |
Periportal hepatocytes |
||
|
Kidney |
Proximal tubule |
[21] |
|
|
Reproductive system |
Endometrium (luminal epith.) |
Basal glandular cells |
[22,23] |
|
Preimplantation embryo |
Blastocysts |
||
|
Placenta |
Syncytiotrophoblasts |
||
|
Myometrium |
Uterine myocytes |
||
|
Endocrine system |
Adrenal cortex |
[24] |
|
|
Ear |
Spiral limbus |
Fibrocytes |
[25] |
|
Spiral ligament |
|||
|
Striavascularis |
|||
|
Cochlea |
Claudius cells |
||
|
Hensen’s cells |
|||
|
Inner sulcus cells |
|||
|
Lung |
Alveolar epithelium |
[26] |
2. Connexins and the Skin
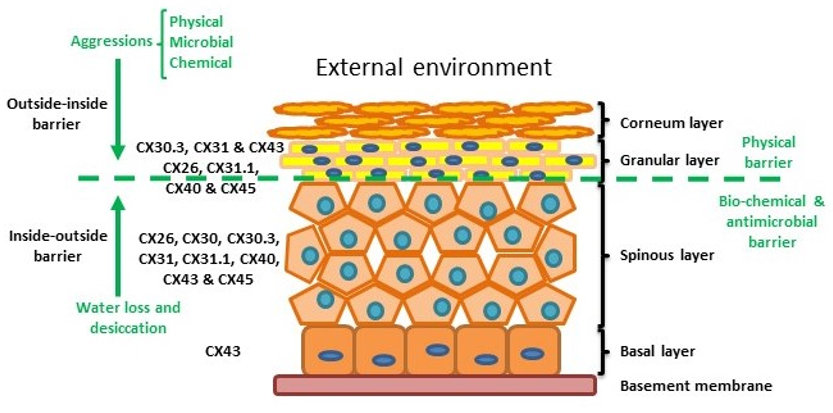
The skin is the human body’s largest organ and is composed of three layers: the hypodermis, dermis and epidermis. The hypodermis and the dermis are connective tissues, while the epidermis is epidermal tissue composed mainly of epithelial cells known as keratinocytes. Keratinocytes are subclassified into four layers: basal (proliferative cells), spinous, granular and corneous (anucleated squamous cells) [27]. The skin provides four different types of barrier: physical, redox, bio-chemical (innate immunity) and the adaptive immune barrier. The physical barrier consists of protein-enriched cells and is mainly located in the stratum corneum and the granular layer, with strong adhesive interactions via tight, adheren and gap junctions. The bio-chemical or antimicrobial barrier consists of lipids, acids, lysozymes and antimicrobial peptides. These two barriers protect from external aggressions (outside-inside barrier), while also avoiding loss of water and solutes (inside-outside barrier) [28][29][28,29] (Figure 2).
Figure 2.
Structure of the epidermis indicating connexin expression profile and barriers.
The epidermis, like all other stratified epithelial tissues, is avascular so gap junctions play an important role in cell-to-cell communication and coordination. Up to 10 different connexins are differentially expressed throughout the epidermis, each presenting a characteristic expression profile dependent on the species, conditions and keratinocyte differentiation status. These Connexin profiles allow the establishment of specific gap junction communication compartments in the different strata. Alteration of connexin activity and compatibility may provoke problems in keratinocyte differentiation [5][30][31][5,30,31]. Several studies have demonstrated that connexins are involved in skin conditions such as chronic non-healing wounds, psoriasis and a variety of genetically related skin syndromes (e.g., [32][33][34][32–34]).
Within normal healthy human skin CX30.3, CX31 and CX43, and at lower levels CX26, C31.1, CX40 and CX45 are expressed in the granular layer. In the spinous layer, CX26, CX30, CX30.3, CX31, CX31.1, CX40, CX43 and CX45 are expressed with CX43 the predominant connexin in the basal layer. [5][30][35][5,30,35] (Figure 2). CX26 is expressed at low levels in proliferating keratinocytes in tissue culture and readily expressed in stratified 3D epidermal cultures by immunocytochemistry [36][37][38][36–38].
2.1. Connexin 26, Trafficking and Assembly
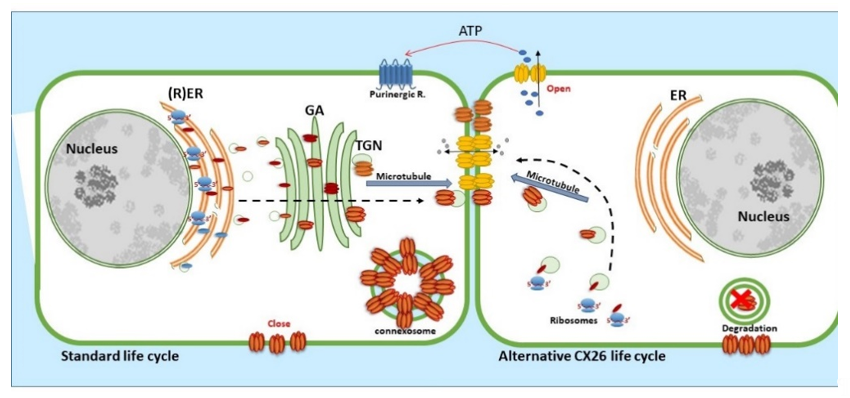
CX26, a 26 kDa protein consisting of 226 amino acids, is encoded by the GJβ2 gene, located on Chromosome 13q12.11 in Homo sapiens [39]. The gene is formed by the non-coding exon 1 (160 bp), an intron (3 kb) and exon 2 containing the complete connexin coding region and the subsequent 3′-UTR [40]. The promoter P1 (−128 bp:+2 bp) upstream of exon 1 has a number of transcription regulatory domains including SP1/SP3 and AP1 [39]. CX26 is also responsive to the NFkB transcription factor, which plays a role in inflammation and immunity, as well as in cell proliferation, differentiation and survival [41][42][41,42]. Human CX26 has a short C-terminal tail, with only 18 amino acids, this characteristic is relevant because it affects its interactome and post-translational modification, where CX26 interacts with a variety of proteins including those associated with the tight junction network [20]. Gap Junction assembly depends on the oligomerisation of connexins to form a closed hexameric connexon or hemichannel in the ER-Golgi environs, which are then escorted and inserted into the plasma membrane via the microtubule network, in association with motor proteins such as consortin [7][43][44][7,43,44]. Evidence suggests that CX26, and closely related CX30, can also follow an alternative Golgi-independent trafficking pathway, possibly providing a means for translation on free ribosomes and an ability to be rapidly translated at site-specific plasma membrane locations when required [45][46][47][48][49][50][45–50]. Entire gap junction plaques are removed from the plasma membrane by the formation of annular gap junctions [4][51][4,51] (Figure 3). In addition, CX26 is unique to the connexin family as it does not contain phosphorylation sites in its C-terminal tail that play a significant role in protein turnover, particularly well characterised for Cx43 [51]. Nevertheless, CX26 presents a variety of putative post-translational modifications, including carbamylation [52], hydroxylation, phosphorylation and methylation, some of which happen at sites of deafness-causing mutations and may be associated with CX26 biogenesis and channel function [53][54][53,54]. Furthermore, hemichannels are normally closed during normal conditions; however, human CX26 hemichannels are an exception as they tend to be open under basal conditions. The 3D molecular structure of CX26 suggests this could be because, in contrast to other species, human CX26 presents an asparagine (uncharged) at amino acid position 159 in place of aspartic acid found in other species [55][56][55,56].
Figure 3. Life cycle of CX26: CX26 can be co-translated and trafficked through the secretory route (standard life cycle). It can also be post-translationally incorporated into ER microsomes and trafficked by Golgi-independent pathway (alternative CX26 life cycle). GJIC mediated communication is indicated between cells and paracrine signalling permitting ATP release indicated via the open hemichannels.
2.2. The Effects of CX26 Mutations
Many diseases have been linked with mutations in connexins, commonly termed connexin channelopathies [57][58][59][60][57–60]. CX43 is the most abundant connexin in the human skin; however, mutations and dysregulation of CX26, which is expressed at very low levels in healthy human epidermis, are related to skin disease characterised by abnormal keratinisation and hyperproliferation of the stratum corneum. Mutations in CX26 are among the most prevalent mutations associated with inherited non syndromic deafness (see Section 4.1) [61][62][63][61–63]. In addition to deafness, dominant mutations are also linked with a range of skin conditions of differing severity, suggesting a complex interrelationship between functional changes in connexin genotype and the phenotypic outcome [59][64][65][66][59,64–66] (Figure 1). Other mutations in the beta connexin subgroup including CX31 and CX30 cause similar epidermal dysplasia [59].
Mutations fall into four main classes. Class 1: trafficked to the plasma membrane with non-functional channels; Class 2: non-functional channels and protein trafficking deficiencies; Class 3: mutations associated with ‘leaky’ hemichannels and inflammatory skin disease; Class 4: trafficking deficiency and cell death, associated with mucositis, inflammatory disease and deafness. Class 1 mutations tend to be linked with non-syndromic deafness and have limited skin pathology and may be related to heterozygous advantage (see Section 4.1). Class 2 mutations align with non-inflammatory skin disorders such as Bart-Pumphrey and Vohwinkel syndrome and deafness. Vohwinkel syndrome (OMIM#124500) is a non-inflammatory disorder caused by CX26 mutations located predominantly on the first portion of EL1 (e.g., D66H). The disease is characterised by keratodermas with constriction bands around the phalanges, which induces autoamputation of the digits [67][68][69][67–69]. Class 3, gain of CX26 function mutations, are associated with inflammatory disorders such as Keratitis ichthyosis deafness (KID), Hystrix-like ichthyosis-deafness (HID). KID syndrome (OMIM#148210) is caused by CX26 mutations on the N-terminal tail, the EL1 (e.g., G12R, N14Y, G45E and D50N) [70][71][72][70–72]. In addition to hearing loss, the disease is characterised by: hyperkeratosis of the palms and soles, erythrokeratoderma on the extremities and face, follicular hyperkeratosis, photophobia and corneal vascularisation that ultimately leads to blindness. Patients experience severe and chronic bacterial and fungal skin infections and are susceptible to the development of squamous cell carcinoma [73][74][73,74]. The molecular mechanisms underlying the condition likely relate to “leaky” hemichannels, with differing sensitivities to pro-inflammatory mediators, and ionic sensitivity including calcium and zinc levels thereby altering channel function [66][75][76][77][78][79][80][81][82][66,75–82]. Several mutations induce lethal phenotypes and are associated with loss of cell viability [83][84][85][83–85]. Recently, we proposed a further Class 4 mutation group associated with hyperkeratosis, mucositis and deafness, where cell model studies revealed cell death and a collapse of the microtubule network, but limited connexin channel function (e.g.,F142L, CX31G45E) [86][87][86,87].
Accumulating evidence suggests that, in addition to changes in CX26 trafficking and channel behavior, a key pathological trigger in the diverse CX26 mutations is alteration in connexin oligomerization compatibility. Normally, oligomerization is connexin subtype-specific with ‘alpha’ and ‘beta’ subgroups being incompatible. As such, CX26 and CX43, critical connexins in epithelial tissue are unable to form heterotypic structures [88][89][88,89]. Recent studies report changes in CX26 mutation oligomerization compatibility, allowing aberrant interactions with CX43 with exacerbated hemichannel activity but non-functional gap junction channels [78][86][90][78,86,90], with each mutation uniquely altering the 3D structure in terms of charge, pore size, hydrophobicity, etc. It is also conceivable that altered CX43:CX26 heteromeric channels influence unique metabolic exchange and influence asymmetric cell division required for the stratification of the epidermis, thereby contributing to the hyperproliferative status of the skin [91]. Further characterisation and understanding of the impact of such aberrant connexin signalling is required to enhance understanding of these complex conditions. Interestingly, a recent report by Laird and colleagues suggest that hearing loss caused by CX26 mutations does not depend on any interaction with CX43 [92].
2.3. The Effects of CX26 Dysregulation
Up-regulation of CX26 is a characteristic of hyperproliferative epidermis in physiological conditions, such as vaginal and buccal epithelium, which shows a high proliferation rate, and pathological conditions, such as psoriatic epidermis, chronic non-healing wounds epidermis and viral warts [93][94][95][96][93–96]. CX26 expression is also induced during wound healing and skin hyperplasia stimulated by tumor promoters [40]. Transgenic mice over-expressing CX26 in the suprabasal layer developed a hyperproliferative phenotype, providing models for several epidermal human CX26 diseases, such as psoriasis [97].
2.3.1. Connexins and Wound Repair
Connexins are closely involved in normal wound repair and show dynamic changes in expression after wounding. After 6 h of injury, CX43 is down-regulated in keratinocytes and fibroblast at the wound edge to allow cell migration followed by recovery of CX43 levels [95]. In contrast, CX26 and CX30 are upregulated in keratinocytes until the wound is closed [96][98][99][96,98,99] in the granular cell layer near the wound margins and in the basal cell layer at some distance from the wound [95]. Thus, connexins play a pivotal role in a variety of aspects of the acute wound healing process and each step is associated with a different connexin environment [51].
At the edge of chronic wounds, epidermal CX43, CX26 and CX30, and dermal CX43 are strikingly up-regulated around wound margins and at some distance from the chronic wound [95]. The up-regulation of CX43 may disrupt fibroblast migration to the wound bed and as a result failure of granulation tissue formation occurs [100][101][102][103][104][100–104]. The up-regulation of CX26 may contribute to the inflammatory and hyperproliferative status of the wound [95][105][95,105]. The overexpression of Cx26 in mouse skin (under the control of the involucrin promoter) kept wounded epidermis in a hyperproliferative state, blocked the transition to remodelling, and led to an infiltration of immune cells. This overexpression also induced ATP release from keratinocytes, which delayed epidermal barrier recovery and promoted an inflammatory response in resident immune cells [97].
2.3.2. Connexins and Psoriasis
Psoriasis is a chronic hyperproliferating skin disorder that manifests sporadic skin lesions characterised by loss of the granular layer and incomplete keratinocyte differentiation associated with a thickened cornified layer. Overexpression of CX26 in psoriatic lesions was originally reported by Labarthe and Lucke in the late 1990s [93][94][93,94]. Subsequent studies in mouse models overexpressing Cx26 in the skin showed mildly acanthotic and hyperkeratotic skin, with a thicker and more compact cornified layer. Areas with frictional trauma, such as axillary areas, presented with scaling and desquamation and hyperkeratotic plaques developed [97]. Other studies revealed that tape stripping of normal human epidermis induced CX26 expression and hyperproliferation [106]. More recently, publication of the psoriatic transcriptome permitted in-depth RNAseq analysis [107] and many of the upregulated genes are related with cell-to-cell adhesion complexes. GJB2, encoding CX26 was the 98th most up-regulated gene detected and its overexpression is used as a marker of genetic predisposition in psoriasis [108][109][108,109]. A variety of other transcriptomic studies confirm this overall increase in CX26 expression in psoriatic tissue yet no changes in CX43 gene expression are reported [110][111][110,111].
References
- Bragulla, H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559, doi:10.1111/j.1469-7580.2009.01066.x.
- Schneeberger, E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228.
- Adil, M.S.; Narayanan, S.P.; Somanath, P.R. Cell-cell junctions: Structure and regulation in physiology and pathology. Tissue Barriers 2020, 1848212, doi:10.1080/21688370.2020.1848212.
- Laird, W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543, doi:10.1042/BJ20051922.
- Chanson, ; Watanabe, M.; O’Shaughnessy, E.M.; Zoso, A.; Martin, P.E. Connexin communication compartments and wound repair in epithelial tissue. Int. J. Mol. Sci. 2018, 19, 1354, doi:10.3390/ijms19051354.
- Segretain, ; Falk, M.M. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim. Et Biophys. Acta 2004, 1662, 3–21, doi:10.1016/j.bbamem.2004.01.007.
- Evans, H.; Martin, P.E. Gap junctions: Structure and function (review). Mol. Membr. Biol. 2002, 19, 121–136, doi:10.1080/09687680210139839.
- Delmar, ; Laird, D.W.; Naus, C.C.; Nielsen, M.S.; Verselis, V.K.; White, T.W. Connexins and disease. Cold Spring Harb. Perspect. Biol. 2017, 10.1101/cshperspect.a029348, doi:10.1101/cshperspect.a029348.
- Evans, H. Cell communication across gap junctions: A historical perspective and current developments. Biochem. Soc. Trans. 2015, 43, 450–459, doi:10.1042/bst20150056.
- Aasen, ; Johnstone, S.; Vidal-Brime, L.; Lynn, K.S.; Koval, M. Connexins: Synthesis, post-translational modifications, and trafficking in health and disease. Int. J. Mol. Sci. 2018, 19, doi:10.3390/ijms19051296.
- Clayton, W.; Göbel, K.; Niessen, C.M.; Paus, R.; van Steensel, M.A.M.; Lim, X. Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis. Br. J. Dermatol. 2019, 181, 677–690, doi:10.1111/bjd.17981.
- Bovell, L. The evolution of eccrine sweat gland research towards developing a model for human sweat gland function. Exp. Derm. 2018, 27, 544–550, doi:10.1111/exd.13556.
- Faniku, ; Wright, C.S.; Martin, P.E. Connexins and pannexins in the integumentary system: The skin and appendages. Cell Mol. Life Sci. 2015, 72, 2937–2947, doi:10.1007/s00018-015-1969-0.
- Arita, ; Akiyama, M.; Tsuji, Y.; McMillan, J.R.; Eady, R.A.; Shimizu, H. Gap junction development in the human fetal hair follicle and bulge region. Br. J. Dermatol. 2004, 150, 429–434, doi:10.1046/j.1365-2133.2004.05775.x.
- Salomon, ; Masgrau, E.; Vischer, S.; Ullrich, S.; Dupont, E.; Sappino, P.; Saurat, J.H.; Meda, P. Topography of mammalian connexins in human skin. J. Investig. Derm. 1994, 103, 240–247, doi:10.1111/1523-1747.ep12393218.
- Li, ; Lu, H.; Cheng, P.L.; Ge, S.; Xu, H.; Shi, S.H.; Dan, Y. Clonally related visual cortical neurons show similar stimulus feature selectivity. Nature 2012, 486, 118–121, doi:10.1038/nature11110.
- Maes, ; Crespo Yanguas, S.; Willebrords, J.; Cogliati, B.; Vinken, M. Connexin and pannexin signaling in gastrointestinal and liver disease. Transl. Res. J. Lab. Clin. Med. 2015, 166, 332–343, doi:10.1016/j.trsl.2015.05.005.
- Nielsen, S.; Axelsen, L.N.; Sorgen, P.L.; Verma, V.; Delmar, M.; Holstein-Rathlou, N.H. Gap junctions. Compr. Physiol. 2012, 2, 1981–2035, doi:10.1002/cphy.c110051.
- Meda, Gap junction proteins are key drivers of endocrine function. Biochim. Et Biophys. Acta. Biomembr. 2018, 1860, 124–140, doi:10.1016/j.bbamem.2017.03.005.
- Batissoco, C.; Salazar-Silva, R.; Oiticica, J.; Bento, R.F.; Mingroni-Netto, R.C.; Haddad, L.A. A cell junctional protein network associated with connexin-26. Int. J. Mol. Sci. 2018, 19, doi:10.3390/ijms19092535.
- Abed, B.; Kavvadas, P.; Chadjichristos, C.E. Functional roles of connexins and pannexins in the kidney. Cell Mol. Life Sci. 2015, 72, 2869–2877, doi:10.1007/s00018-015-1964-5.
- Kibschull, ; Gellhaus, A.; Carette, D.; Segretain, D.; Pointis, G.; Gilleron, J. Physiological roles of connexins and pannexins in reproductive organs. Cell Mol. Life Sci. 2015, 72, 2879–2898, doi:10.1007/s00018-015-1965-4.
- Kibschull, ; Gellhaus, A.; Winterhager, E. Analogous and unique functions of connexins in mouse and human placental development. Placenta 2008, 29, 848–854, doi:10.1016/j.placenta.2008.07.013.
- Hodson, J.; Legros, C.; Desarménien, M.G.; Guérineau, N.C. Roles of connexins and pannexins in (neuro)endocrine physiology. Cell Mol. Life Sci. 2015, 72, 2911–2928, doi:10.1007/s00018-015-1967-2.
- Forge, ; Becker, D.; Casalotti, S.; Edwards, J.; Evans, W.H.; Lench, N.; Souter, M. Gap junctions and connexin expression in the inner ear. Novartis Found. Symp. 1999, 219, 134–150; discussion 151-136, doi:10.1002/9780470515587.ch9.
- Koval, Sharing signals: Connecting lung epithelial cells with gap junction channels. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L875–L893, doi:10.1152/ajplung.00078.2002.
- Baroni, ; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262, doi:10.1016/j.clindermatol.2011.08.007.
- Proksch, ; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Derm. 2008, 17, 1063–1072.
- Gallo, L. The birth of innate immunity. Exp. Derm. 2013, 22, 517, doi:10.1111/exd.12197.
- Di, L.; Rugg, E.L.; Leigh, I.M.; Kelsell, D.P. Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J. Investig. Derm. 2001, 117, 958–964.
- Martin, E.; Easton, J.A.; Hodgins, M.B.; Wright, C.S. Connexins: Sensors of epidermal integrity that are therapeutic targets. FEBS Lett. 2014, 588, 1304–1314, doi:10.1016/j.febslet.2014.02.048.
- Gerido, A.; DeRosa, A.M.; Richard, G.; White, T.W. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am. J. Physiol. Cell Physiol. 2007, 293, C337–C345, doi:10.1152/ajpcell.00626.2006.
- Avshalumova, ; Fabrikant, J.; Koriakos, A. Overview of skin diseases linked to connexin gene mutations. Int. J. Derm. 2014, 53, 192–205, doi:10.1111/ijd.12062.
- Saez, C.; Green, C. Involvement of connexin hemichannels in the inflammatory response of chronic diseases. Int. J. Mol. Sci. 2018, 19, doi:10.3390/ijms19092469.
- Wong, ; Tan, T.; Chan, C.; Laxton, V.; Chan, Y.W.; Liu, T.; Wong, W.T.; Tse, G. The role of connexins in wound healing and repair: Novel therapeutic approaches. Front. Physiol. 2016, 7, 596, doi:10.3389/fphys.2016.00596.
- Kandyba, E.; Hodgins, M.B.; Martin, P.E. A murine living skin equivalent amenable to live-cell imaging: Analysis of the roles of connexins in the epidermis. J. Investig. Derm. 2008, 128, 1039–1049, doi:10.1038/sj.jid.5701125.
- Wright, S.; van Steensel, M.A.; Hodgins, M.B.; Martin, P.E. Connexin mimetic peptides improve cell migration rates of human epidermal keratinocytes and dermal fibroblasts in vitro. Wound Repair Regen 2009, 17, 240–249, doi:10.1111/j.1524-475X.2009.00471.x.
- Pollok, ; Pfeiffer, A.C.; Lobmann, R.; Wright, C.S.; Moll, I.; Martin, P.E.; Brandner, J.M. Connexin 43 mimetic peptide Gap27 reveals potential differences in the role of Cx43 in wound repair between diabetic and non-diabetic cells. J. Cell Mol. Med. 2011, 15, 861–873, doi:10.1111/j.1582-4934.2010.01057.x.
- Oyamada, ; Takebe, K.; Oyamada, Y. Regulation of connexin expression by transcription factors and epigenetic mechanisms. Biochim. Et Biophys. Acta 2013, 1828, 118–133, doi:10.1016/j.bbamem.2011.12.031.
- Iossa, ; Marciano, E.; Franzé, A. GJB2 gene mutations in syndromic skin diseases with sensorineural hearing loss. Curr. Genom. 2011, 12, 475–785, doi:10.2174/138920211797904098.
- Oeckinghaus, ; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034, doi:10.1101/cshperspect.a000034.
- Garcia-Vega, ; O'Shaughnessy, E.M.; Jan, A.; Bartholomew, C.; Martin, P.E. Connexin 26 and 43 play a role in regulating proinflammatory events in the epidermis. J. Cell Physiol. 2019, 10.1002/jcp.28206, doi:10.1002/jcp.28206.
- Epifantseva, ; Shaw, R.M. Intracellular trafficking pathways of Cx43 gap junction channels. Biochim. Et Biophys. Acta Biomembr. 2018, 1860, 40–47, doi:10.1016/j.bbamem.2017.05.018.
- del Castillo, J.; Cohen-Salmon, M.; Charollais, A.; Caille, D.; Lampe, P.D.; Chavrier, P.; Meda, P.; Petit, C. Consortin, a trans-Golgi network cargo receptor for the plasma membrane targeting and recycling of connexins. Hum. Mol. Genet. 2010, 19, 262–275, doi:10.1093/hmg/ddp490.
- Martin, E.; Blundell, G.; Ahmad, S.; Errington, R.J.; Evans, W.H. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J. Cell Sci. 2001, 114, 3845–3855.
- Ahmad, ; Martin, P.E.; Evans, W.H. Assembly of gap junction channels: Mechanism, effects of calmodulin antagonists and identification of connexin oligomerization determinants. Eur. J. Biochem. FEBS 2001, 268, 4544–4552.
- George, H.; Kendall, J.M.; Evans, W.H. Intracellular trafficking pathways in the assembly of connexins into gap junctions. J. Biol. Chem. 1999, 274, 8678–8685.
- Qu, ; Gardner, P.; Schrijver, I. The role of the cytoskeleton in the formation of gap junctions by Connexin 30. Exp. Cell Res. 2009, 315, 1683–1692, doi:10.1016/j.yexcr.2009.03.001.
- Defourny, ; Thelen, N.; Thiry, M. Actin-independent trafficking of cochlear connexin 26 to non-lipid raft gap junction plaques. Hear. Res. 2019, 374, 69–75, doi:10.1016/j.heares.2019.01.020.
- Defourny, ; Thelen, N.; Thiry, M. Cochlear connexin 30 homomeric and heteromeric channels exhibit distinct assembly mechanisms. Mech. Dev. 2019, 155, 8–14, doi:10.1016/j.mod.2018.10.001.
- Solan, L.; Lampe, P.D. Kinase programs spatiotemporally regulate gap junction assembly and disassembly: Effects on wound repair. Semin. Cell Dev. Biol. 2016, 50, 40–48, doi:10.1016/j.semcdb.2015.12.010.
- Meigh, ; Greenhalgh, S.A.; Rodgers, T.L.; Cann, M.J.; Roper, D.I.; Dale, N. CO2 directly modulates connexin 26 by formation of carbamate bridges between subunits. eLife 2013, 2, e01213, doi:10.7554/eLife.01213.
- Locke, ; Bian, S.; Li, H.; Harris, A.L. Post-translational modifications of connexin26 revealed by mass spectrometry. Biochem. J. 2009, 424, 385–398, doi:10.1042/BJ20091140.
- Locke, ; Harris, A.L. Connexin channels and phospholipids: Association and modulation. BMC Biol. 2009, 7, 52, doi:10.1186/1741-7007-7-52.
- González, ; Gómez-Hernández, J.M.; Barrio, L.C. Species specificity of mammalian connexin-26 to form open voltage-gated hemichannels. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 2329–2338, doi:10.1096/fj.06-5828com.
- Oshima, ; Tani, K.; Hiroaki, Y.; Fujiyoshi, Y.; Sosinsky, G.E. Three-dimensional structure of a human connexin26 gap junction channel reveals a plug in the vestibule. Proc. Natl. Acad. Sci. USA 2007, 104, 10034–10039, doi:10.1073/pnas.0703704104.
- Laird, W. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010, 20, 92–101, doi:10.1016/j.tcb.2009.11.001.
- Laird, W.; Naus, C.C.; Lampe, P.D. SnapShot: Connexins and disease. Cell 2017, 170, 1260–1260 e1261, doi:10.1016/j.cell.2017.08.034.
- Martin, E.; van Steensel, M. Connexins and skin disease: Insights into the role of beta connexins in skin homeostasis. Cell Tissue Res. 2015, 360, 645–658, doi:10.1007/s00441-014-2094-3.
- Cocozzelli, G.; White, T.W. Connexin 43 mutations lead to increased hemichannel functionality in skin disease. Int. J. Mol. Sci. 2019, 20, doi:10.3390/ijms20246186.
- Mishra, ; Pandey, H.; Srivastava, P.; Mandal, K.; Phadke, S.R. Connexin 26 (GJB2) mutations associated with Non-Syndromic Hearing Loss (NSHL). Indian J. Pediatrics 2018, 85, 1061–1066, doi:10.1007/s12098-018-2654-8.
- Shen, ; Oza, A.M.; Del Castillo, I.; Duzkale, H.; Matsunaga, T.; Pandya, A.; Kang, H.P.; Mar-Heyming, R.; Guha, S.; Moyer, K.; et al. Consensus interpretation of the p.Met34Thr and p.Val37Ile variants in GJB2 by the ClinGen hearing loss expert panel. Genet. Med. Off. J. Am. Coll. Med. Genet. 2019, 21, 2442–2452, doi:10.1038/s41436-019-0535-9.
- Koohiyan, Genetics of hereditary hearing loss in the Middle East: A systematic review of the carrier frequency of the GJB2 mutation (35delG). Audiol. Neuro-Otol. 2019, 24, 161–165, doi:10.1159/000502201.
- Srinivas, ; Verselis, V.K.; White, T.W. Human diseases associated with connexin mutations. Biochim. Et Biophys. Acta Biomembr. 2018, 1860, 192–201, doi:10.1016/j.bbamem.2017.04.024.
- Lilly, ; Sellitto, C.; Milstone, L.M.; White, T.W. Connexin channels in congenital skin disorders. Semin. Cell Dev. Biol. 2016, 50, 4–12, doi:10.1016/j.semcdb.2015.11.018.
- Levit, A.; Sellitto, C.; Wang, H.Z.; Li, L.; Srinivas, M.; Brink, P.R.; White, T.W. Aberrant connexin26 hemichannels underlying keratitis-ichthyosis-deafness syndrome are potently inhibited by mefloquine. J. Investig. Dermatol. 2015, 135, 1033–1042, doi:10.1038/jid.2014.408.
- Maestrini, ; Korge, B.P.; Ocana-Sierra, J.; Calzolari, E.; Cambiaghi, S.; Scudder, P.M.; Hovnanian, A.; Monaco, A.P.; Munro, C.S. A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel’s syndrome) in three unrelated families. Hum. Mol. Genet. 1999, 8, 1237–1243.
- Bakirtzis, ; Choudhry, R.; Aasen, T.; Shore, L.; Brown, K.; Bryson, S.; Forrow, S.; Tetley, L.; Finbow, M.; Greenhalgh, D.; et al. Targeted epidermal expression of mutant Connexin 26 (D66H) mimics true Vohwinkel syndrome and provides a model for the pathogenesis of dominant connexin disorders. Hum. Mol. Genet. 2003, 12, 1737–1744.
- de Zwart-Storm, A.; van Geel, M.; Veysey, E.; Burge, S.; Cooper, S.; Steijlen, P.M.; Martin, P.E.; van Steensel, M.A. A novel missense mutation in GJB2, p.Tyr65His, causes severe Vohwinkel syndrome. Br. J. Derm. 2011, 164, 197–199, doi:10.1111/j.1365-2133.2010.10058.x.
- van Steensel, A.; van Geel, M.; Nahuys, M.; Smitt, J.H.; Steijlen, P.M. A novel connexin 26 mutation in a patient diagnosed with keratitis-ichthyosis-deafness syndrome. J. Investig. Derm. 2002, 118, 724–727, doi:10.1046/j.1523-1747.2002.01735.x.
- Schutz, ; Auth, T.; Gehrt, A.; Bosen, F.; Korber, I.; Strenzke, N.; Moser, T.; Willecke, K. The connexin26 S17F mouse mutant represents a model for the human hereditary keratitis-ichthyosis-deafness syndrome. Hum. Mol. Genet. 2011, 20, 28–39, doi:10.1093/hmg/ddq429.
- Mayama, ; Fujimura, T.; Asano, M.; Kambayashi, Y.; Numata, Y.; Aiba, S. Squamous cell carcinoma arising from Keratitis-ichthyosis-deafness syndrome. Acta Derm. Venereol. 2013, 93, 583–584, doi:10.2340/00015555-1535.
- Coggshall, ; Farsani, T.; Ruben, B.; McCalmont, T.H.; Berger, T.G.; Fox, L.P.; Shinkai, K. Keratitis, ichthyosis, and deafness syndrome: A review of infectious and neoplastic complications. J. Am. Acad. Dermatol. 2013, 69, 127–134, doi:10.1016/j.jaad.2012.12.965.
- Ma, ; Liang, P.; Chen, J.; Feng, P.; Lai, W. Keratitis-ichthyosis-deafness syndrome accompanied by disseminated cutaneous fungal infection. J. Derm. 2017, 44, 1255–1261, doi:10.1111/1346-8138.13926.
- García, E.; Bosen, F.; Mujica, P.; Pupo, A.; Flores-Muñoz, C.; Jara, O.; González, C.; Willecke, K.; Martínez, A.D. From hyperactive Connexin26 Hemichannels to impairments in epidermal calcium gradient and permeability barrier in the keratitis-ichthyosis-deafness syndrome. J. Investig. Dermatol. 2016, 136, 574–583, doi:10.1016/j.jid.2015.11.017.
- Dalamon, K.; Buonfiglio, P.; Larralde, M.; Craig, P.; Lotersztein, V.; Choate, K.; Pallares, N.; Diamante, V.; Elgoyhen, A.B. Connexin 26 (GJB2) mutation in an Argentinean patient with keratitis-ichthyosis-deafness (KID) syndrome: A case report. BMC Med. Genet. 2016, 17, 37, doi:10.1186/s12881-016-0298-y.
- Homeida, ; Wiley, R.T.; Fatahzadeh, M. Oral squamous cell carcinoma in a patient with keratitis-ichthyosis-deafness syndrome: A rare case. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, e226–e232, doi:10.1016/j.oooo.2015.01.005.
- Garcia, E.; Maripillan, J.; Jara, O.; Ceriani, R.; Palacios-Munoz, A.; Ramachandran, J.; Olivero, P.; Perez-Acle, T.; Gonzalez, C.; Saez, J.C.; et al. Keratitis-ichthyosis-deafness syndrome-associated cx26 mutants produce nonfunctional gap junctions but hyperactive hemichannels when co-expressed with wild type cx43. J. Investig. Derm. 2015, 135, 1338–1347, doi:10.1038/jid.2015.20.
- Sanchez, A.; Verselis, V.K. Aberrant Cx26 hemichannels and keratitis-ichthyosis-deafness syndrome: Insights into syndromic hearing loss. Front. Cell. Neurosci. 2014, 8, 354, doi:10.3389/fncel.2014.00354.
- Koval, Drowning out communication. Focus on “The human Cx26-D50A and Cx26-A88V mutations causing keratitis-ichthyosis-deafness syndrome display increased hemichannel activity”. Am. J. Physiol. Cell Physiol. 2013, 304, C1129–C1130, doi:10.1152/ajpcell.00087.2013.
- Donnelly, S.; English, G.; de Zwart-Storm, E.A.; Lang, S van S;teensel, M.A.; Martin, P.E: Differential susceptibility of Cx26 mutations associated with epidermal dysplasias to peptidoglycan derived from Staphylococcus aureus and Staphylococcus epidermidis. Exp Dermatol 2012, 21, 592–598, doi:1111/j.1600-0625.2012.01521.x.
- Levit, A.; Mese, G.; Basaly, M.G.; White, T.W. Pathological hemichannels associated with human Cx26 mutations causing Keratitis-Ichthyosis-Deafness syndrome. Biochim. Biophys. Acta 2012, 1818, 2014–2019, doi:10.1016/j.bbamem.2011.09.003.
- Lilly, ; Bunick, C.G.; Maley, A.M.; Zhang, S.; Spraker, M.K.; Theos, A.J.; Vivar, K.L.; Seminario-Vidal, L.; Bennett, A.E.; Sidbury, R.; et al. More than keratitis, ichthyosis, and deafness: Multisystem effects of lethal GJB2 mutations. J. Am. Acad. Dermatol. 2019, 80, 617–625, doi:10.1016/j.jaad.2018.09.042.
- Rodriguez-Paris, ; Waldhaus, J.; Gordhandas, J.A.; Pique, L.; Schrijver, I. Comparative functional characterization of novel non-syndromic GJB2 gene variant p.Gly45Arg and lethal syndromic variant p.Gly45Glu. PeerJ 2016, 4, e2494, doi:10.7717/peerj.2494.
- Mese, ; Sellitto, C.; Li, L.; Wang, H.Z.; Valiunas, V.; Richard, G.; Brink, P.R.; White, T.W. The Cx26-G45E mutation displays increased hemichannel activity in a mouse model of the lethal form of keratitis-ichthyosis-deafness syndrome. Mol. Biol. Cell 2011, 22, 4776–4786, doi:10.1091/mbc.E11-09-0778.
- Albuloushi, ; Lovgren, M.L.; Steel, A.; Yeoh, Y.; Waters, A.; Zamiri, M.; Martin, P.E. A heterozygous mutation in GJB2 (Cx26F142L) associated with deafness and recurrent skin rashes results in connexin assembly deficiencies. Exp. Derm. 2020, 10.1111/exd.14187, doi:10.1111/exd.14187.
- Easton, A.; Albuloushi, A.K.; Kamps, M.A.F.; Brouns, G.; Broers, J.L.V.; Coull, B.J.; Oji, V.; van Geel, M.; van Steensel, M.A.M.; Martin, P.E. A rare missense mutation in GJB3 (Cx31G45E) is associated with a unique cellular phenotype resulting in necrotic cell death. Exp. Derm. 2019, 28, 1106–1113, doi:10.1111/exd.13542.
- Koval, ; Molina, S.A.; Burt, J.M. Mix and match: Investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett. 2014, 588, 1193–1204, doi:10.1016/j.febslet.2014.02.025.
- Koval, Pathways and control of connexin oligomerization. Trends Cell Biol. 2006, 16, 159–166, doi:10.1016/j.tcb.2006.01.006.
- Valdez Capuccino, M.; Chatterjee, P.; García, I.E.; Botello-Smith, W.M.; Zhang, H.; Harris, A.L.; Luo, Y.; Contreras, J.E. The connexin26 human mutation N14K disrupts cytosolic intersubunit interactions and promotes channel opening. J. Gen. Physiol. 2019, 151, 328–341, doi:10.1085/jgp.201812219.
- Bazzoun, ; Adissu, H.A.; Wang, L.; Urazaev, A.; Tenvooren, I.; Fostok, S.F.; Chittiboyina, S.; Sturgis, J.; Hodges, K.; Chandramouly, G.; et al. Connexin 43 maintains tissue polarity and regulates mitotic spindle orientation in the breast epithelium. J. Cell Sci. 2019, 132, doi:10.1242/jcs.223313.
- Beach, ; Abitbol, J.M.; Allman, B.L.; Esseltine, J.L.; Shao, Q.; Laird, D.W. GJB2 mutations linked to hearing loss exhibit differential trafficking and functional defects as revealed in cochlear-relevant cells. Front. Cell Dev. Biol. 2020, 8, 215, doi:10.3389/fcell.2020.00215.
- Lucke, ; Choudhry, R.; Thom, R.; Selmer, I.S.; Burden, A.D.; Hodgins, M.B. Upregulation of connexin 26 is a feature of keratinocyte differentiation in hyperproliferative epidermis, vaginal epithelium, and buccal epithelium. J. Investig. Derm. 1999, 112, 354–361, doi:10.1046/j.1523-1747.1999.00512.x.
- Labarthe, P.; Bosco, D.; Saurat, J.H.; Meda, P.; Salomon, D. Upregulation of connexin 26 between keratinocytes of psoriatic lesions. J. Investig. Derm. 1998, 111, 72–76, doi:10.1046/j.1523-1747.1998.00248.x.
- Brandner, M.; Houdek, P.; Husing, B.; Kaiser, C.; Moll, I. Connexins 26, 30, and 43: Differences among spontaneous, chronic, and accelerated human wound healing. J. Investig. Derm. 2004, 122, 1310–1320, doi:10.1111/j.0022-202X.2004.22529.x.
- Phillips, R.J.; Chin, J.S.; Madden, L.; Gilmartin, D.J.; Soon, A.; Thrasivoulou, C.; Jayasinghe, S.J.; Miles, M.; O’Neill, S.; Hu, R.; et al. Targeting Cx26 expression by sustained release of Cx26 antisense from scaffolds reduces inflammation and improves wound healing. Adv. Biosyst. 2018, 2, doi:10.1002/adbi.201800227.
- Djalilian, R.; McGaughey, D.; Patel, S.; Seo, E.Y.; Yang, C.; Cheng, J.; Tomic, M.; Sinha, S.; Ishida-Yamamoto, A.; Segre, J.A. Connexin 26 regulates epidermal barrier and wound remodeling and promotes psoriasiform response. J. Clin. Investig. 2006, 116, 1243–1253, doi:10.1172/JCI27186.
- Sutcliffe, E.; Chin, K.Y.; Thrasivoulou, C.; Serena, T.E.; O'Neil, S.; Hu, R.; White, A.M.; Madden, L.; Richards, T.; Phillips, A.R.; et al. Abnormal connexin expression in human chronic wounds. Br. J. Derm. 2015, 173, 1205–1215, doi:10.1111/bjd.14064.
- Becker, L.; Thrasivoulou, C.; Phillips, A.R. Connexins in wound healing; perspectives in diabetic patients. Biochim. Biophys. Acta 2012, 1818, 2068–2075, doi:10.1016/j.bbamem.2011.11.017.
- Tarzemany, ; Jiang, G.; Jiang, J.X.; Gallant-Behm, C.; Wiebe, C.; Hart, D.A.; Larjava, H.; Hakkinen, L. Connexin 43 regulates the expression of wound healing-related genes in human gingival and skin fibroblasts. Exp. Cell Res. 2018, 367, 150–161, doi:10.1016/j.yexcr.2018.03.031.
- Tarzemany, ; Jiang, G.; Jiang, J.X.; Larjava, H.; Hakkinen, L. Connexin 43 hemichannels regulate the expression of wound healing-associated genes in human gingival fibroblasts. Sci. Rep. 2017, 7, 14157, doi:10.1038/s41598-017-12672-1.
- Tarzemany, ; Jiang, G.; Larjava, H.; Hakkinen, L. Expression and function of connexin 43 in human gingival wound healing and fibroblasts. PLoS ONE 2015, 10, e0115524, doi:10.1371/journal.pone.0115524.
- Faniku, ; O'Shaughnessy, E.; Lorraine, C.; Johnstone, S.R.; Graham, A.; Greenhough, S.; Martin, P.E.M. The connexin mimetic peptide Gap27 and Cx43-knockdown reveal differential roles for Connexin43 in wound closure events in skin model systems. Int. J. Mol. Sci. 2018, 19, doi:10.3390/ijms19020604.
- Mendoza-Naranjo, ; Cormie, P.; Serrano, A.E.; Wang, C.M.; Thrasivoulou, C.; Sutcliffe, J.E.; Gilmartin, D.J.; Tsui, J.; Serena, T.E.; Phillips, A.R.; et al. Overexpression of the gap junction protein Cx43 as found in diabetic foot ulcers can retard fibroblast migration. Cell Biol. Int. 2012, 36, 661–667, doi:10.1042/cbi20110628.
- Kanapathy, ; Simpson, R.; Madden, L.; Thrasivoulou, C.; Mosahebi, A.; Becker, D.L.; Richards, T. Upregulation of epidermal gap junctional proteins in patients with venous disease. Br. J. Surg. 2018, 105, 59–67, doi:10.1002/bjs.10653.
- Wang, ; Ramirez, A.; Budunova, I. Overexpression of connexin26 in the basal keratinocytes reduces sensitivity to tumor promoter TPA. Exp. Derm. 2010, 19, 633–640, doi:10.1111/j.1600-0625.2009.01013.x.
- Li, ; Tsoi, L.C.; Swindell, W.R.; Gudjonsson, J.E.; Tejasvi, T.; Johnston, A.; Ding, J.; Stuart, P.E.; Xing, X.; Kochkodan, J.J.; et al. Transcriptome analysis of psoriasis in a large case-control sample: RNA-seq provides insights into disease mechanisms. J. Investig. Derm. 2014, 134, 1828–1838, doi:10.1038/jid.2014.28.
- Stylianaki, A.; Karpouzis, A.; Tripsianis, G.; Veletza, S. Assessment of gap junction protein beta-2 rs3751385 gene polymorphism in psoriasis vulgaris. J. Clin. Med. Res. 2019, 11, 642–650, doi:10.14740/jocmr3845.
- Sun, D.; Cheng, H.; Wang, Z.X.; Zhang, A.P.; Wang, P.G.; Xu, J.H.; Zhu, Q.X.; Zhou, H.S.; Ellinghaus, E.; Zhang, F.R.; et al. Association analyses identify six new psoriasis susceptibility loci in the Chinese population. Nat. Genet. 2010, 42, 1005–1009.
- Ahn, ; Gupta, R.; Lai, K.; Chopra, N.; Arron, S.T.; Liao, W. Network analysis of psoriasis reveals biological pathways and roles for coding and long non-coding RNAs. BMC Genom. 2016, 17, 841, doi:10.1186/s12864-016-3188-y.
- Ahn, ; Yan, D.; Chang, H.W.; Lee, K.; Bhattarai, S.; Huang, Z.M.; Nakamura, M.; Singh, R.; Afifi, L.; Taravati, K.; et al. RNA-seq and flow-cytometry of conventional, scalp, and palmoplantar psoriasis reveal shared and distinct molecular pathways. Sci. Rep. 2018, 8, 11368, doi:10.1038/s41598-018-29472-w.