Tremor is a prevalent symptom associated with multiple conditions, including essential tremor (ET), Parkinson’'s disease (PD), multiple sclerosis (MS), stroke and trauma. The surgical management of tremor evolved from stereotactic lesions to deep-brain stimulation (DBS), which allowed safe and reversible interference with specific neural networks.
- DBS,tremor,essential tremor,Parkinson’s disease,multiple sclerosis,stroke,trauma,Holmes tremor
1. Introduction
Tremor is a prevalent symptom associated with multiple conditions, including neurodegenerative diseases (e.g., essential tremor (ET), Parkinson disease (PD), multiple system atrophy and spinocerebellar ataxias), inflammatory diseases (e.g., multiple sclerosis (MS)), drug toxicity (e.g., lithium, sympathomimetics and chemotherapeutic agents), stroke, trauma and many others [1]. While mild tremor can often be controlled medically, many patients have refractory and intractable syndromes that significantly affect their quality of life [2].
The surgical treatment of tremor evolved from a series of serendipitous discoveries that were initially reported in 1953, when Irving Cooper accidentally generated an anterior choroidal artery stroke during a pedunculotomy procedure for PD [3]. It was realized that the globus pallidus internus (GPi) lesion led to improvement in bradykinesia and rigidity without creating motor deficits. A subsequent series of chemopallidotomies for PD further highlighted that patients with tremor improvement typically had thalamic involvement (Figure 1) [4]. Thalamotomy was then studied and demonstrated effective for the relief of tremor, achieving control rates between 69% and 91% [5,6,7][5][6][7], at the cost of adverse side effects that could reach an incidence of 67% when bilateral lesions were performed [7,8][7][8]. Deep-brain stimulation (DBS) was introduced as a way to reversibly inhibit the ventrolateral thalamus in patients who had previously undergone a contralateral thalamotomy, with the rationale that it would allow a safer treatment of the second side [9]. After the demonstration that DBS could achieve tremor reduction rates similar to a thalamotomy and with a better side-effect profile, even in unilateral cases [10[10][11],11], the technique rapidly replaced lesioning procedures as the preferred surgical option for tremor.
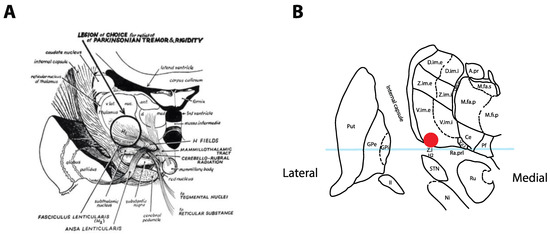
Figure 1. Historical tremor target. (A) Coronal representation by Cooper in 1960 of the ideal lesioning target for the control of tremor. Note how the lesion encompasses the ventrolateral thalamus in addition to the zona incerta (Zi) and posterior subthalamic area (PSA). Reproduced from [4] with permission. (B) Optimal location for thalamic stimulation based on current data [12] plotted on a coronal representation of the thalamus, 5 mm posterior to the midcommissural point [13]. The light-blue overlay represents the midcommissural plane.
2. Tremor Targets
Tremors of various etiology (e.g., ET, PD, MS) have clearly distinct pathophysiological origins [14]. However, the circuits involved appear to converge at the ventrolateral thalamic area. Multiple structures have been targeted, including the ventral intermediate nucleus of the thalamus (Vim), the nucleus ventralis oralis of the thalamus (Vo) and the posterior subthalamic area (PSA).
The Vim nucleus is the classic tremor target for both DBS and thalamotomy procedures (Figure 2). It is a 3 mm-thick layer of cell bodies receiving fibers from the contralateral deep cerebellar nuclei (dentate, interposed and fastigial). Both structures are connected by the dentato-rubro-thalamic tract (DRTT, alternatively named fasciculus cerebellothalamicus or the cerebellothalamic tract), which travels through the superior cerebellar peduncle, mostly decussates in the brachium conjunctivum, passing through and anterior to the red nucleus before ascending into the Vim thalamus and, to a lesser extent, the Vop [15]. The Vim then projects to the ipsilateral motor (M1) and association cortices (the premotor cortex, supplementary motor area and pre-supplementary motor area). The Vim is bordered by the ventralis caudalis (Vc) nucleus posteriorly (receiving sensory information through the medial lemniscus) and the pallidal receiving area (Vop) nucleus anteriorly (receiving pallidal fibers through the thalamic fasciculus (H1 field of Forel)). Immediately ventral to the Vim and Vop lies a white matter region named prelemniscal radiations (Raprl). In a recent tractography study, the Raprl contained (1) the DRTT fibers arriving from the cerebellum en route to the Vim, (2) tractography streamlines connecting the brainstem to the orbitofrontal and prefrontal cortices, (3) streamlines connecting the pallidum to the pedunculopontine nucleus and (4) streamlines directly connected to the motor and premotor cortices through the internal capsule [16]. The Raprl is itself bordered by the medial lemniscus latero-posteriorly on its way to Vc, by the zona incerta (Zi) latero-anteriorly and by the red nucleus medially. The Zi is a shell of cell bodies embryologically derived from the thalamus and extending from the reticulate nucleus of the thalamus [17]. Together, the caudal portion of the Zi, pallidothalamic tracts (thalamic fasciculus (H1) and lenticular fasciculus (H2)) and the Raprl form the posterior subthalamic area (PSA), and the various terms are often used interchangeably in the literature [18]. The different targets are illustrated in Figure 2 and listed in Table 1 with average coordinates and targeting methods.
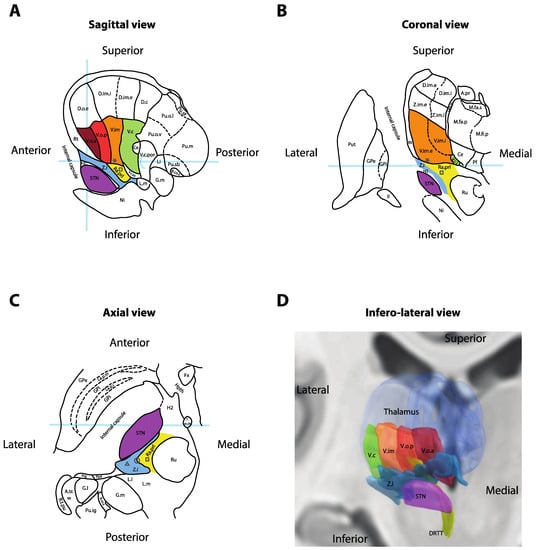
Figure 2. Anatomy of the ventral thalamic area. (A) Sagittal cross-section of the thalamus, 14.5 mm lateral to the midline. Light-blue overlays represent the intercommissural plane (horizontally), the midcommissural plane (vertically) and the posterior commissure (half-circle). (B) Coronal cross-section 5 mm posterior to the mid-commissural point. Light-blue overlay represents the intercommissural plane. (C) Axial cross-section 3.5 mm inferior to the intercommissural plane. Light-blue overlay represents the midcommissural plane. Panels A, B and C redrawn and adapted from [13]. (D) Three-dimensional reconstruction of the right thalamus seen from an antero-infero-lateral oblique view. Nuclei rendered in Lead-DBS v2 [19] using the DISTAL atlas (ventralis caudalis (Vc), ventral intermediate nucleus of the thalamus (Vim), pallidal receiving area (Vop), Voa and subthalamic nucleus (STN)) [20], Zona incerta atlas (Zi) [21] and Brainstem connectome atlas (DRTT) [22]. * = Standard Vim target for deep-brain stimulation (DBS); square = standard prelemniscal radiations (Raprl) target for DBS; triangle = standard caudal Zi target for DBS; circle = intermediate PSA target at the interface between Zi and Raprl; II = optic tract; A.pr = nucleus anteroprincipalis thalami; A.tr.w = area triangularis (Wernicke); B.co.s = brachium colliculi superioris; Ce = nucleus centralis; D.c = nucleus dorso-caudalis; D.im.e = nucleus dorso-intermedius externus; D.im.i = nucleus dorso-intermedius internus; D.o.e = nucleus dorso-oralis externus; DRTT = dentato-rubro-thalamic tract; Fx = fornix; G.l. = corpus geniculatum laterale; G.m = corpus geniculatum mediale; GPe = globus pallidus externus; GPi = globus pallidus internus; H2 = fasciculus lenticularis; Hpth = hypothalamus; La.m.ip = lamina medialis interpolaris; La.p.i = lamina pallidi incompleta; La.p.m = lamina pallidi medialis; Li = nucleus limitans thalami; L.l = lemniscus lateralis; L.m = lemniscus medialis; M.fa.p = nucleus medialis fasciculosus posterior; M.fa.s = nucleus medialis fasciculosus superior; M.fi.p = nucleus medialis fibrosus posterior; Ni = substantia nigra; Pf = nucleus parafascicularis thalami; Ppd = nucleus peripeduncularis; Prg = praegeniculatum; Pu.ig = nucleus pulvinaris intergeniculatus; Pu.m = pulvinar mediale; Pu.o.l = nucleus pulvinaris orolateralis; Pu.o.v = nucleus pulvinaris oroventralis; Pu.sb = nucleus pulvinaris suprabrachialis; Pu.sf = nucleus pulvinaris superficialis; Put = putamen; Ra.prl = radiatio praelemniscalis; Rt = reticulatum thalami; R.t.pu; Ru = red nucleus; STN = subthalamic nucleus; T.m.th = tractus mammillo-thalamicus; V.c = nucleus ventrocaudalis; V.c.i = nucleus ventrocaudalis internus; V.c.pc = nucleus ventrocaudalis parvocellularis; V.c.por = nucleus ventrocaudalis portae; V.im = nucleus ventrointermedius; V.im.e = nucleus ventrointermedius externus; V.im.i = nucleus ventrointermedius internus; V.o.a = nucleus ventrooralis anterior; V.o.p = nucleus ventrooralis posterior; Z.i = zona incerta; Z.im.e = nucleus zentrolateralis intermedius externus; Z.im.i = nucleus zentrolateralis intermedius internus. * = Standard Vim target for DBS; square = standard Raprl target for DBS.
Table 1. Coordinates and anatomical targeting of common tremor targets.
|
Target |
Indirect Coordinates |
Anatomical Targeting |
|
Vim |
X = 15 mm lateral to MCP (or 11 mm lateral from wall of third ventricle) Y = 25% AC–PC distance posterior to MCP Z = level of MCP |
N/A |
|
PSA (intermediate target) [23] |
X = 11 ± 1.36 mm lateral to MCP Y = 5.65 ± 1.42 posterior to MCP Z = 1.87 ± 0.62 mm inferior to MCP |
Halfway between the lateral border of the red nucleus and the medial border of the STN, on a line perpendicularly intersecting the STN axis [24]. |
|
Raprl [25] |
X = 11.63 ± 0.66 mm lateral to MCP Y = 6.73 ± 1.62 mm posterior to MCP Z = 4.38 ± 1.02 mm inferior to MCP |
Perirubral white matter |
|
Caudal Zi [25] |
X = 14 ± 1.56 mm lateral to MCP Y = 5.8 ± 1.46 mm posterior to MCP Z = 2.1 ± 1.05 inferior to MCP |
Behind posterior end of STN |
3. Tremor Syndromes
3.1. Essential Tremor
Essential tremor is seen as neurodegenerative disorder of the cerebellum in which an unknown trigger leads to loss of Purkinje cells [82][26]. This reduces the GABAergic input to cerebellar output nuclei, inducing excessive cerebellar output to the thalamus. Combined with a chronic reorganization or the remaining Purkinje cell synapses in the cerebellar cortex, oscillations arise in motor networks that generate an action tremor [83][27]. Most patients with ET initially respond to propranolol or GABAergic agents, such as topiramate, gabapentin, primidone and ethanol [84][28]. As the disease progresses, patients may become refractory to medication, resulting in a reduced quality of life, and may be considered for surgical intervention. DBS in ET is thought to act by inhibiting the pathological oscillations in the cerebello-thalamic pathways. The various targets discussed above are all nodes in this network that have been targeted and shown to reduce action tremor in ET (see above).
When considering all targets together, a meta-analysis was recently performed and identified 37 studies (14 retrospective and 23 prospective) published between 1996 and 2019 and reporting the results of 1202 patients treated with DBS for medically refractory ET [85][29]. The average improvement in tremor score was 60.1% ± 9.7 (SD) at an average follow-up of 16.6 months. Tremor reduction was superior in patients receiving bilateral DBS (61.2% ± 5.2, SD, n = 69) versus unilateral DBS (56.4% ± 9.7, SD, n = 472, p < 0.001). Across studies, DBS for ET improved quality of life by 52.5% ± 16.2 (SD) at an average follow-up of 16.6 months [85][29]. Perioperative and hardware-related complications included lead problems (11.4%), local adverse symptoms (10.9%), infection (1.8%), intracranial hemorrhage (1.1%), seizures (0.5%) and death (0.1%). Stimulation-related side-effects included speech disturbance (11.1%), gait disturbance (10.9%), paresthesias (8.8%), neuropsychiatric issues (5.1%) and other symptoms (8.3%). Across all complications, 76.6% were transient [85][29]. Overall, patient satisfaction with DBS for ET is very high [86][30], and outcomes appear to have been slightly improving over time (average of 0.4% improvement in tremor score per year in studies published between 1996 and 2019, Spearman’'s correlation coefficient = 0.357, p = 0.035), suggesting progress in the surgical techniques and hardware [85][29].
Many challenges remain in this field. Habituation to stimulation, which occurs in 73% of patients within 5 years of implantation [38][31], is a significant concern to which there is yet to be a convincing solution. The complexity and cost of DBS for ET may also be challenged as thalamotomies are being revisited through the introduction of less invasive lesioning modalities such as radiosurgery and focused ultrasound, both of which may trend to being more cost-effective than DBS [87,88][32][33]. However, these types of analysis may be difficult to interpret, as robust long-term data for lesions and recurrence of tremor are not as clear in large cohorts. This also needs to be weighed against new devices with longer battery life and increased programming options. The demonstrated safety of bilateral thalamic DBS currently maintains neuromodulation’'s edge over lesions, although this may change when current ongoing studies of modern bilateral lesions are completed (NCT04501484 and NCT03465761). If these studies are successful, the main question for the field will be the long-term benefit and frequency of complications with each approach.
3.2. Dystonic Tremor
Dystonic tremor (DT) is a tremor syndrome “"combining tremor and dystonia as the leading neurological signs”" [1]. There is considerable overlap with ET and ET plus syndromes, and many patients are often misclassified in these categories, which may represent a continuum [89][34]. The pathophysiology of dystonic tremor is even less understood than ET, but might involve similar cerebellar pathology, in addition to dystonia-specific loss of inhibition in spinal and brainstem circuits [90][35].
In the largest series of Vim DBS for DT, 26 patients followed for more than 6 months showed a 44–56% improvement in tremor score sustained over 5 years [91][36]. Tremor control was not inferior to ET patients similarly treated at the same institution. Functional status was improved by 56% after 6 months and 1 year, but did not reach statistical significance past 2 years, suggesting suboptimal control of the concomitant dystonia symptoms might be limiting activities of daily living over the long term. This might be relieved by GPi DBS, which has known potency against dystonic symptoms, although the only series comparing tremor control from GPi and Vim DBS in 10 DT patients found GPi inferior to Vim [92][37]. As for STN DBS for dystonic tremor, only four cases were reported, but all were successful [60,61][38][39]. Larger trials will be needed to clarify the optimal target. When considering performing DBS for DT, the involvement of a multi-disciplinary team may be important to debate target selection.
3.3. Parkinson’'s Tremor
Resting and action tremors affect 76–100% and 47–90% of PD patients, respectively, and are often the most visible symptom of the disease [93][40]. While DBS in PD is usually performed to alleviate bradykinesia and rigidity, concomitant tremor reduction has consistently been observed. The pathophysiology of PD tremor is poorly understood but might involve loss of segregation of parallel basal ganglia circuits, independent thalamic pacemakers or pathological coupling of cerebellothalamocortical circuits and the basal ganglia [14].
A meta-analysis of 5 randomized controlled trials including 489 patients demonstrated a 23–100% reduction in tremor at various time points, with no overall difference in efficacy between STN and GPi targets [93][40]. In the off-medication state at the follow-up closest to 12 months, tremor reduction was 37–89% for STN and 25–79% for GPi. While many authors have reported that tremor suppression from GPi stimulation might be delayed by days to week, this target was found to have a more stable postoperative efficacy compared to STN, which is more effective at 12 months than at 6 months after implantation. STN was also found to be equally effective as Vim [94][41]. Improvement can be seen in both tremor-predominant and akinetic-rigid variants. Most importantly, the tremor’'s responsiveness to medication is not predictive of successful tremor control with stimulation and can be achieved (>25% reduction) in 94% of cases [56][42]. Arm and chin tremor might be more responsive to DBS than leg tremor.
3.4. MS-Associated Tremor
MS-associated tremor appears to be less responsive to DBS than the previously discussed syndromes, although it can still be improved. In a meta-analysis of 13 studies including 129 DBS patients, stimulation improved the Hedges standardized mean tremor score by 2.15 [95][43]. This effect size is difficult to transpose into clinical scales, as it is a pooled estimate from heterogeneous outcome measures, but the largest study of combined Vim and Vo DBS demonstrated a 29.6% tremor reduction [53][44], while series of PSA DBS showed a 50–60% improvement on average [18]. The number of cases is too small to compare targets to one another, and the low rate of DBS implantation for MS tremor, even in high-volume centers, makes it unlikely that large trials will be conducted in the near future. For now, some authors have suggested using a dual lead/dual target strategy (Figure 3B) to maximize the chance of good outcome [53][44], although the efficacy of this approach remains empirical. Careful discussion of the goals of the treatment with the patient is critical to manage expectations in these complex tremor cases.
3.5. Other Tremors
DBS has been used in a variety of rarer indications, including post-stroke tremors, lesion-related tremors and post-traumatic tremors. Many of these cases also fulfill the criteria for Holmes tremor, which consists of a slow (usually <5 Hz) rest, postural and intention tremor typically appearing up to 2 years following central nervous system injury [1,96][1][45]. The literature on these syndromes is limited to small case reports and case series providing anecdotal evidence of efficacy. In a recent meta-analysis of 35 studies reporting 82 patients, Vim DBS was performed in 63.6% of cases, GPi in 18.2% and other targets (PSA and Vo) in the remaining cases [96][45]. Median tremor improvement was 75%, although a significant publication bias might be present. Post-stroke tremor appeared to have better prognosis than post-traumatic syndromes. Reports have also been published for orthostatic tremor [97][46], tremor with underlying ataxia syndromes [98][47], Wislon’'s disease-associated tremor [99][48], Klinefelter’'s syndrome [100][49] and fragile X-associated tremor ataxia syndrome [101][50]. The reader is referred to these individual publications for further consideration of these rare indications.
Tremor reduction scores derived from the largest series or meta-analyses available. When multiple timepoints are reported, the data closest to 12 months were used. Shaded cells represent combinations for which reliable data or meta-analyses are unavailable. Given the large methodological differences between studies and different patient populations, these numbers cannot demonstrate the superiority or inferiority of one target over another.
References
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G.; et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018, 33, 75–87, doi:10.1002/mds.27121.
- Louis, E.D.; Machado, D.G. Tremor-related quality of life: A comparison of essential tremor vs. Parkinson’s disease patients. Parkinsonism Relat. Disord. 2015, 21, 729–735, doi:10.1016/j.parkreldis.2015.04.019.
- Cooper, I.S. Ligation of the anterior choroidal artery for involuntary movements-parkinsonism. Psychiatr. Q. 1953, 27, 317–319, doi:10.1007/bf01562492.
- COOPER, I.S. Results of 1,000 consecutive basal ganglia operations for parkinsonism. Ann. Intern. Med. 1960, 52, 483–499, doi:10.7326/0003-4819-52-3-483.
- Nagaseki, Y.; Shibazaki, T.; Hirai, T.; Kawashima, Y.; Hirato, M.; Wada, H.; Miyazaki, M.; Ohye, C. Long-term follow-up results of selective VIM-thalamotomy. J. Neurosurg. 1986, 65, 296–302, doi:10.3171/jns.1986.65.3.0296.
- Mohadjer, M.; Goerke, H.; Milios, E.; Etou, A.; Mundinger, F. Long-term results of stereotaxy in the treatment of essential tremor. Stereotact. Funct. Neurosurg. 1990, 54–55, 125–129, doi:10.1159/000100201.
- Zirh, A.; Reich, S.G.; Dougherty, P.M.; Lenz, F.A. Stereotactic thalamotomy in the treatment of essential tremor of the upper extremity: Reassessment including a blinded measure of outcome. J. Neurol. Neurosurg. Psychiatry 1999, 66, 772–775, doi:10.1136/jnnp.66.6.772.
- Alshaikh, J.; Fishman, P.S. Revisiting bilateral thalamotomy for tremor. Clin. Neurol. Neurosurg. 2017, 158, 103–107, doi:10.1016/j.clineuro.2017.04.025.
- Benabid, A.L.; Pollak, P.; Gervason, C.; Hoffmann, D.; Gao, D.M.; Hommel, M.; Perret, J.E.; Rougemont, J. de Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991, 337, 403–406, doi:10.1016/0140-6736(91)91175-t.
- Schuurman, P.R.; Bosch, D.A.; Bossuyt, P.M.; Bonsel, G.J.; Someren, E.J. van; Bie, R.M. de; Merkus, M.P.; Speelman, J.D. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. New Engl. J. Med. 2000, 342, 461–468, doi:10.1056/nejm200002173420703.
- Tasker, R.R. Deep brain stimulation is preferable to thalamotomy for tremor suppression. Surg. Neurol. 1998, 49, 145-153. doi:10.1016/s0090-3019(97)00459-x.
- King, N.K.K.; Krishna, V.; Sammartino, F.; Bari, A.; Reddy, G.D.; Hodaie, M.; Kalia, S.K.; Fasano, A.; Munhoz, R.P.; Lozano, A.M.; et al. Anatomic Targeting of the Optimal Location for Thalamic Deep Brain Stimulation in Patients with Essential Tremor. World Neurosurg 2017, 107, 168–174, doi:10.1016/j.wneu.2017.07.136.
- Schaltebrand, G.; Wahren, W. Atlas for Stereotaxy of the Human Brain; Thieme: Leipzig, Germany, 1977; Volume 1; ISBN 3-13-393702-2.
- Helmich, R.C.; Toni, I.; Deuschl, G.; Bloem, B.R. The pathophysiology of essential tremor and Parkinson’s tremor. Curr. Neurol. Neurosci. Rep. 2013, 13, 378–10, doi:10.1007/s11910-013-0378-8.
- Gallay, M.N.; Jeanmonod, D.; Liu, J.; Morel, A. Human pallidothalamic and cerebellothalamic tracts: Anatomical basis for functional stereotactic neurosurgery. Brain Struct. Funct. 2008, 212, 443–463, doi:10.1007/s00429-007-0170-0.
- García-Gomar, M.G.; Soto-Abraham, J.; Velasco-Campos, F.; Concha, L. Anatomic characterization of prelemniscal radiations by probabilistic tractography: Implications in Parkinson’s disease. Brain Struct. Funct. 2017, 222, 71–81, doi:10.1007/s00429-016-1201-5.
- Yen, C.; Conley, M.; Hendry, S.; Jones, E. The morphology of physiologically identified GABAergic neurons in the somatic sensory part of the thalamic reticular nucleus in the cat. J. Neurosci. 1985, 5, 2254–2268, doi:10.1523/jneurosci.05-08-02254.1985.
- Ramirez-Zamora, A.; Smith, H.; Kumar, V.; Prusik, J.; Phookan, S.; Pilitsis, J.G. Evolving Concepts in Posterior Subthalamic Area Deep Brain Stimulation for Treatment of Tremor: Surgical Neuroanatomy and Practical Considerations. Stereotact. Funct. Neurosurg. 2016, 94, 283–297, doi:10.1159/000449007.
- Horn, A.; Li, N.; Dembek, T.A.; Kappel, A.; Boulay, C.; Ewert, S.; Tietze, A.; Husch, A.; Perera, T.; Neumann, W.-J.; et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. NeuroImage 2019, 184, 293–316, doi:10.1016/j.neuroimage.2018.08.068.
- Ewert, S.; Plettig, P.; Li, N.; Chakravarty, M.M.; Collins, D.L.; Herrington, T.M.; Kühn, A.A.; Horn, A. Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage 2018, 170, 271–282, doi:10.1016/j.neuroimage.2017.05.015.
- Lau, J.C.; Xiao, Y.; Haast, R.A.M.; Gilmore, G.; Uludağ, K.; MacDougall, K.W.; Menon, R.S.; Parrent, A.G.; Peters, T.M.; Khan, A.R. Direct visualization and characterization of the human zona incerta and surrounding structures. Hum. Brain Mapp. 2020, 41, 4500–4517, doi:10.1002/hbm.25137.
- Meola, A.; Yeh, F.-C.; Fellows-Mayle, W.; Weed, J.; Fernandez-Miranda, J.C. Human Connectome-Based Tractographic Atlas of the Brainstem Connections and Surgical Approaches. Neurosurgery 2016, 79, 437–455, doi:10.1227/neu.0000000000001224.
- Barbe, M.T.; Reker, P.; Hamacher, S.; Franklin, J.; Kraus, D.; Dembek, T.A.; Becker, J.; Steffen, J.K.; Allert, N.; Wirths, J.; et al. DBS of the PSA and the VIM in essential tremor: A randomized, double-blind, crossover trial. Neurology 2018, 91, e543–e550, doi:10.1212/wnl.0000000000005956.
- Nowacki, A.; Debove, I.; Rossi, F.; Schlaeppi, J.A.; Petermann, K.; Wiest, R.; Schüpbach, M.; Pollo, C. Targeting the posterior subthalamic area for essential tremor: Proposal for MRI-based anatomical landmarks. J. Neurosurg. 2019, 131, 820–827, doi:10.3171/2018.4.jns18373.
- Velasco, F.; Palfi, S.; ́enez, F.J.; Carrillo-Ruiz, J.D.; Castro, G.; Keravel, Y. Other Targets to Treat. Parkinson’s Disease (Posterior Subthalamic Targets and Motor Cortex); Lozano, A.M., Gildenberg, P.L., Tasker, R.R., Eds.; Springer: New York, NY, USA, 2009; ISBN 978-3-540-70779-0.
- Louis, E.D.; Faust, P.L. Essential tremor pathology: Neurodegeneration and reorganization of neuronal connections. Nat. Rev. Neurol. 2020, 16, 69–83, doi:10.1038/s41582-019-0302-1.
- Pan, M.-K.; Li, Y.-S.; Wong, S.-B.; Ni, C.-L.; Wang, Y.-M.; Liu, W.-C.; Lu, L.-Y.; Lee, J.-C.; Cortes, E.P.; Vonsattel, J.P.G.; et al. Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology. Sci. Transl. Med. 2020, 12, eaay1769, doi:10.1126/scitranslmed.aay1769.
- Zesiewicz, T.A.; Elble, R.J.; Louis, E.D.; Gronseth, G.S.; Ondo, W.G.; Dewey, R.B.; Okun, M.S.; Sullivan, K.L.; Weiner, W.J. Evidence-based guideline update: Treatment of essential tremor: Report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology 2011, 77, 1752–1755, doi:10.1212/wnl.0b013e318236f0fd.
- Giordano, M.; Caccavella, V.M.; Zaed, I.; Manzillo, L.F.; Montano, N.; Olivi, A.; Polli, F.M. Comparison between deep brain stimulation and magnetic resonance-guided focused ultrasound in the treatment of essential tremor: A systematic review and pooled analysis of functional outcomes. J. Neurol. Neurosurg. Psychiatry 2020, doi:10.1136/jnnp-2020-323216.
- Børretzen, M.N.; Bjerknes, S.; Sæhle, T.; Skjelland, M.; Skogseid, I.M.; Toft, M.; Dietrichs, E. Long-term follow-up of thalamic deep brain stimulation for essential tremor—patient satisfaction and mortality. BMC Neurol. 2014, 14, 120, doi:10.1186/1471-2377-14-120.
- Shih, L.C.; LaFaver, K.; Lim, C.; Papavassiliou, E.; Tarsy, D. Loss of benefit in VIM thalamic deep brain stimulation (DBS) for essential tremor (ET): How prevalent is it? Parkinsonism Relat. D 2013, 19, 676–679, doi:10.1016/j.parkreldis.2013.03.006.
- Ravikumar, V.K.; Parker, J.J.; Hornbeck, T.S.; Santini, V.E.; Pauly, K.B.; Wintermark, M.; Ghanouni, P.; Stein, S.C.; Halpern, C.H. Cost-effectiveness of focused ultrasound, radiosurgery, and DBS for essential tremor. Mov. Disord. 2017, 32, 1165–1173, doi:10.1002/mds.26997.
- Dang, T.T.H.; Rowell, D.; Connelly, L.B. Cost-Effectiveness of Deep Brain Stimulation With Movement Disorders: A Systematic Review. Mov. Disord. Clin. Pract. 2019, 6, 348–358, doi:10.1002/mdc3.12780.
- Louis, E.D. Essential tremor: “Plus” or “Minus”. Perhaps now is the time to adopt the term “the essential tremors”. Parkinsonism Relat. Disord. 2018, 56, 111–112, doi:10.1016/j.parkreldis.2018.06.026.
- Panyakaew, P.; Cho, H.J.; Lee, S.W.; Wu, T.; Hallett, M. The Pathophysiology of Dystonic Tremors and Comparison with Essential Tremor. J. Neurosci. 2020, doi:10.1523/jneurosci.1181-20.2020.
- Tsuboi, T.; Jabarkheel, Z.; Zeilman, P.R.; Barabas, M.J.; Foote, K.D.; Okun, M.S.; Shukla, A.W. Longitudinal follow-up with VIM thalamic deep brain stimulation for dystonic or essential tremor. Neurology 2020, 94, e1073–e1084, doi:10.1212/wnl.0000000000008875.
- Hedera, P.; Phibbs, F.T.; Dolhun, R.; Charles, P.D.; Konrad, P.E.; Neimat, J.S.; Davis, T.L. Surgical targets for dystonic tremor: Considerations between the globus pallidus and ventral intermediate thalamic nucleus. Parkinsonism Relat. D 2013, 19, 684–686, doi:10.1016/j.parkreldis.2013.03.010.
- Kleiner-Fisman, G.; Liang, G.S.L.; Moberg, P.J.; Ruocco, A.C.; Hurtig, H.I.; Baltuch, G.H.; Jaggi, J.L.; Stern, M.B. Subthalamic nucleus deep brain stimulation for severe idiopathic dystonia: Impact on severity, neuropsychological status, and quality of life. J. Neurosurg. 2007, 107, 29–36, doi:10.3171/jns-07/07/0029.
- Chou, K.L.; Hurtig, H.I.; Jaggi, J.L.; Baltuch, G.H. Bilateral subthalamic nucleus deep brain stimulation in a patient with cervical dystonia and essential tremor. Mov. Disord. 2005, 20, 377–380, doi:10.1002/mds.20341.
- Wong, J.; Cauraugh, J.H.; Ho, K.W.D.; Broderick, M.; Ramirez-Zamora, A.; Almeida, L.; Shukla, A.W.; Wilson, C.A.; Bie, R.MA. de; Weaver, F.M.; et al. STN vs. GPi deep brain stimulation for tremor suppression in Parkinson disease: A systematic review and meta-analysis. Parkinsonism Relat. D 2018, 58, 56–62, doi:10.1016/j.parkreldis.2018.08.017.
- Parihar, R.; Alterman, R.; Papavassiliou, E.; Tarsy, D.; Shih, L.C. Comparison of VIM and STN DBS for Parkinsonian Resting and Postural/Action Tremor. Tremor. Other Hyperkinetic Mov. 2015, 5, 321, doi:10.7916/d81v5d35.
- Katlowitz, K.; Ko, M.; Mogilner, A.Y.; Pourfar, M. Effect of deep brain simulation on arm, leg, and chin tremor in Parkinson disease. J. Neurosurg. 2019, 131, 1514–1519, doi:10.3171/2018.7.jns18784.
- Brandmeir, N.J.; Murray, A.; Cheyuo, C.; Ferari, C.; Rezai, A.R. Deep Brain Stimulation for Multiple Sclerosis Tremor: A Meta‐Analysis. Neuromodulation Technol. Neural. Interface 2020, 23, 463–468, doi:10.1111/ner.13063.
- Oliveria, S.F.; Rodriguez, R.L.; Bowers, D.; Kantor, D.; Hilliard, J.D.; Monari, E.H.; Scott, B.M.; Okun, M.S.; Foote, K.D. Safety and efficacy of dual-lead thalamic deep brain stimulation for patients with treatment-refractory multiple sclerosis tremor: A single-centre, randomised, single-blind, pilot trial. Lancet. Neurol. 2017, 16, 691–700, doi:10.1016/s1474-4422(17)30166-7.
- Mendonça, M.D.; Meira, B.; Fernandes, M.; Barbosa, R.; Bugalho, P. Deep brain stimulation for lesion-related tremors: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2018, 47, 8–14, doi:10.1016/j.parkreldis.2017.12.014.
- Coleman, R.R.; Starr, P.A.; Katz, M.; Glass, G.A.; Volz, M.; Khandhar, S.M.; Ostrem, J.L. Bilateral Ventral Intermediate Nucleus Thalamic Deep Brain Stimulation in Orthostatic Tremor. Stereot. Funct. Neuros. 2016, 94, 69–74, doi:10.1159/000444127.
- Oyama, G.; Thompson, A.; Foote, K.D.; Limotai, N.; Abd-El-Barr, M.; Maling, N.; Malaty, I.A.; Rodriguez, R.L.; Subramony, S.H.; Ashizawa, T.; et al. Deep Brain Stimulation for Tremor Associated with Underlying Ataxia Syndromes: A Case Series and Discussion of Issues. Tremor. Other Hyperkinetic Mov. 2014, 4, 228, doi:10.7916/d8542kq5.
- Hedera, P. Treatment of Wilson's disease motor complications with deep brain stimulation. Annu. N. Y. Acad. Sci. 2017, 1315, 16–23, doi:10.1111/nyas.12372.
- Koegl-Wallner, M.; Katschnig-Winter, P.; Pendl, T.; Melisch, B.; Trummer, M.; Holl, E.; Werner, U.; Schmidt, R.; Schwingenschuh, P. Tremor associated with Klinefelter syndrome—A case series and review of the literature. Parkinsonism Relat. D 2014, 20, 323–327, doi:10.1016/j.parkreldis.2013.11.010.
- Weiss, D.; Mielke, C.; Wächter, T.; Bender, B.; Liscic, R.M.; Scholten, M.; Naros, G.; Plewnia, C.; Gharabaghi, A.; Krüger, R. Long-term outcome of deep brain stimulation in fragile X-associated tremor/ataxia syndrome. Parkinsonism Relat. Disord. 2015, 21, 310–313, doi:10.1016/j.parkreldis.2014.12.015.
