The usage of lignocellulosic biomass in energy production for biofuels and other value-added products can extensively decrease the carbon footprint of current and future energy sectors. Therefore, productions and utilization of "Lignocellulolytic Enzymes" are instrumental to achieve this goal.
- Lignocellulolytic enzymes
- cellulase
- hemicellulase
- biomass
- pretreatment
- enzyme production
1. Introduction
Fossil fuels are still consumed at alarming rates, even though lignocellulosic biomass is the most abundant carbon resource, which can be utilized for the production of biofuels [1]. Besides, fossil fuels, which are detrimental to both the environment and human health, are continuously depleting. The industrial infrastructure leans towards the use of fossil fuels after the industrial revolution of the last 250 years. In recent years, it has been speculated that more than 14% of the world’s energy consumption can be met by using lignocellulosic biomass as an alternative to fossil fuel energy [2]. Therefore, it is estimated that lignocellulosic biomass can provide more than 27% of the world’s transportation fuel needs by 2035 [3].
Implementation of lignocellulosic biomass is numerous. Currently, the main usage of lignocellulosic biomass is in the agriculture, forestry, and industrial sectors where it is being used for energy crops, forestry by-products, and wood industry residues [2]. However, lignocellulosic biomass can also be used to produce many other chemical and physical products with the help of a better understanding of its internal structure. Lignocellulosic biomass is usually composed of cellulose (40–60%), hemicellulose (10–40%), and lignin (15–30%) [1]. The cellulose and hemicellulose components can provide sugars for the production of bioethanol, which is one of the most utilized sources of renewable energy. The lignin portion of the lignocellulosic biomass can be used for production of value-added products and heat or steam for electricity production. However, all these applications are not currently being utilized widely because of the challenges in the breaking down of lignocellulosic biomass into its respective usable components such as sugars and lignin.
Among various challenges that are associated with the usage of lignocellulosic biomass, the most prominent one is the use of energy-intensive pretreatment processes to break down the complex biomass into its respective usable components. It is widely accepted that more than 40% of the processing cost of lignocellulosic biomass used as energy products comes from the pretreatment of the biomass [4]. Furthermore, the processing steps can also be energy-intensive, which would ultimately make most of the biomass utilization scenarios inefficient. The most common pretreatment strategies are employed to separate the lignin portion of the biomass so that sugars in the cellulose and hemicellulose components can be released to further process for the relative end-use. Cellulose is mostly present as semi-crystalline microfibers and is the main component of the lignocellulosic biomass. It comprises D-glucose molecules linked by β-1,4-glycosidic bonds with a degree of polymerization ranging from 800 to 10,000 [1], while the hemicellulose mainly contains xylose with arabinose, galactose, and glucose in smaller proportions with a degree of polymerization of 50–600. On the other hand, lignin contains coniferyl, sinapyl, and coumaryl alcohols connected by C-O or C-C bonds. Cellulose fibers are bundled together by hydrogen bonding and van der Waals forces. Lignin and hemicellulose essentially act as the resin between the empty spaces of cellulose. All three components are linked by both physical and chemical interactions (Figure 1).
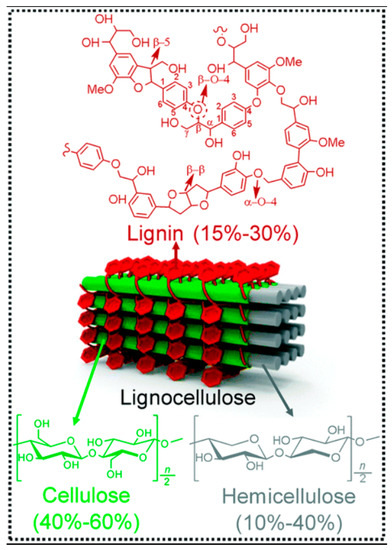
Three main components of lignocellulosic biomass, reproduced with permission from Wu et al., Photocatalytic transformations of lignocellulosic biomass into chemicals, published by Royal Society of Chemistry, 2020. [1].
In addition to the lignocellulosic components described above, there are many other molecular entities that can be present in a lignocellulosic biomass. Some examples of these molecular entities are proteins and their monomers (amino acids), pectin and pectin-like molecules, alkaloids, and inorganic molecules [5]. All these compounds can have different effects on different types of microbial species. For example, a high molar ratio of carbon to nitrogen is needed for the microbial lipid production and accumulation [5]. On the other hand, an abundance of cellulose and hemicellulose fibers can also help in lignocellulolytic enzyme production [6].
Conversion of lignocellulosic biomass into biofuels such as ethanol and biogas can reduce the impact of greenhouse gases emitted from fossil fuels. However, the complex structure including the physical and chemical interactions between the cellulose, hemicellulose, and lignin, along with lignin acting as the physical barrier in the conversion, poses problems in establishing the industrial foundations in the use of lignocellulosic biomass. While the physicochemical methods such as heat, hot water, steam, etc. are effective in removing or excluding the lignin barrier, these energy-intensive methods cannot be controlled to liberate the sugar monomers in the cellulose and hemicellulose components [7]. Furthermore, the physical and chemical methods convert these sugars into chemicals that are then unusable or inhibit the microbial communities converting the biomass into biofuels. Therefore, the biochemical methods, i.e., hydrolytic enzymes, are employed to decrease the energy demands and production of unwanted inhibitory by-products during the conversion process.
Hydrolytic enzymes such as cellulases, hemicellulases, and lignases are produced by a wide variety of organisms including plants and microbial species such as fungi and bacteria. The rate of hydrolytic action along with overall efficiency in the degradation of the lignocellulosic biomass depends on many factors such as the source of the enzyme, substrate, and the number and type of the enzymes involved. For example, a set of different enzymes that must act in synergy is needed for the efficient hydrolysis of any given biomass. The external factors such as the cost of the enzyme production, quality of the enzyme cocktail, and type of pretreatment method required with the enzymatic hydrolysis also play an important role in the determination of the efficiency of the enzymatic conversion of lignocellulosic biomass into biofuels such as bioethanol and biogas.
Therefore, lignocellulosic hydrolytic enzymes are needed for efficient conversion of the lignocellulosic biomass into sugars that can be fermented into valuable products such as biofuels and other bioproducts. Since their discovery in the early 1950s, various enhancement strategies have been employed to increase the production, efficiency, and feasibility of the microbial hydrolytic enzymes [8]. The main barrier in making these enzymes industrially feasible is the cost and type of substrates involved in production of such enzymes. The research on optimization strategies for making these enzymes more competitive has been established for the last four decades.
2. Lignocellulosic Hydrolytic Enzymes
Different pretreatment methods have been proposed in the literature over the past three decades for different types of lignocellulosic biomass [1,9,10][1][9][10]. Among them, biochemical pretreatment, especially enzymatic breakdown, is not only environment-friendly, but also controlled on the molecular level by the type of enzymes used in the degradation, thus regulating the type of product obtained after the biochemical treatment. However, the current status of enzymatic hydrolysis is not well established because of the underlying economic issues in the industrial production of such enzymes. It is extremely important to understand the types and mode of action for such enzymes when they work alone or together on any type of lignocellulosic biomass.
Lignocellulosic hydrolytic enzymes can be roughly categorized based on the type of substrate they act on. Therefore, the most common three categories of these enzymes are cellulases, hemicellulases, and lignases, or lignin modifying enzymes. There can be many subtypes of each category based on the organisms and culture conditions of their production [11]. Overall, it is critical to have an enzyme cocktail for the efficient degradation of lignocellulosic biomass on an industrial scale. However, if the aim is to have just one type of product, such as glucose from cellulose or xylose from the xylan fraction of hemicellulase, the enzyme proportions of the cocktails can be adjusted to obtain that product from the raw biomass. Table 1 gives a concise summary of the broader categories of lignocellulosic hydrolysis enzymes with their relative activity characteristics and areas of industrial applications. The following sections also summarize each of these lignocellulosic hydrolytic enzymes.
Table 1. Enzymes involved in the degradation of lignocellulosic biomass and their industrial applications.
| Enzyme | Type | Enzyme Commission (EC) Number | Activity-Characteristics | Application Areas | References |
|---|---|---|---|---|---|
| Cellulases | Endo-β-glucanase | 3.2.1.4 | Hydrolysis of 1–3 or 1–4 bonds in the beta-D-glucans within the chain | Cereal grains; polishing; feed supplements | [12,13][12][13] |
| β-Glucosidase | 3.2.1.21 | Hydrolysis from non-reducing end | Flavor enhancement; biofuel industry | [12,14][12][14] | |
| Exoglucanases | 3.2.1.91 | reducing or non-reducing end creating cellobiose | Food, pulp and paper industry | [12,15][12][15] | |
| Hemicellulases | Endo-β-1,4-xylanase | 3.2.1.8 | β-1,4 bonds within Xylan chains | Food industry | [16,17][16][17] |
| 1,4-β-Xylosidase | 3.2.1.37 | Hydrolysis from non-reducing end in β-D-Xylan | Food industry | [17,[1718]][18] | |
| Endo-1,4-β-mannosidase | 3.2.1.78 | β-1,4 bonds within mannan chains | Delignification in pulp industry | [17,19][17][19] | |
| 1,2-α-Mannosidase | 3.2.1.113 | Removal of terminal alpha-D-mannose residues | Delignification in pulp industry | [17,20][17][20] | |
| β-Mannosidase | 3.2.1.25 | Hydrolysis from nonreducing end to form d-mannose residues | Delignification in pulp industry | [17,21][17][21] | |
| α-Galactosidase | 3.2.1.22 | Hydrolysis of α-galactoglucomannan | Guar gum digestion | [17,22][17][22] | |
| β-Galactosidase | 3.2.1.23 | Hydrolysis of β-galactoglucomannan | Medicine; guar gum digestion | [17,22][17][22] | |
| α-L-Arabinofuranosidase | 3.2.1.55 | Hydrolysis of arabinoxylan and arabinoglucoronoxylan | Feed industry and baking | [17,22][17][22] | |
| α-Glucuronidase | 3.2.1.139 | Hydrolysis of arabinoglucoronoxylan | Food industry | [22,23][22][23] | |
| Acetyl esterase | 3.1.1.6 | Hydrolysis of the ester bond between arabinose and ferulic acid (Lignin) | Cider clarification | [17,22][17][22] | |
| Acetyl xylan esterase | 3.1.1.72 | Cleaving of Acetyl groups in hemicellulose | Cider clarification | [17,22][17][22] | |
| Lignin modifying enzymes (LMEs) | Lignin peroxidase | 1.11.1.14 | Oxidoreductase | Waste treatment | [24] |
| Manganese peroxidase | 1.11.1.13 | Oxidoreductase | Wastewater treatment in the production of synthetic dyes | [25] | |
| Phenoloxidases | 1.10.3.2 | Multicopper oxidases | Bioremediation | [26] | |
| Hybrid peroxidase | 1.11.1.16 | Oxidoreductase | Industrial waste treatment | [27] |
3. Applications of Lignocellulolytic Enzymes
The concept of using cellulases, hemicellulases, and lignin modifying enzymes to make value-added products from lignocellulosic biomass is relatively new as compared to the applications of such enzymes in other industries. Microbial hydrolytic enzymes have already been explored extensively for their applications in the food industry [39][28]. Table 1 represents some of the most common current applications of these enzymes in different industries. In case of cellulases, the food industry uses cellulases for wine, juice and bakery production, because cellulases provide improved wine filtration, improved maceration in the juice industry and improved texture and quality of bakery products, respectively [40][29]. Cellulases are also used in the paper, textile, and detergent industries [41][30]. For example, in the textile industry, the stone-washing of jeans is treated with pumice stone, which has undesirable effects on the final product, such as the decreased capacity of jean loading. The application of cellulases can produce 50% higher jean load [40][29]. In the juice industry, cellulases are a major component of macerating enzymes that help in the extraction, clarification, and stabilization of fruit juices. Cellulases are also used for the extraction of flavonoids from the seeds and flowers. Cellulases are effective in extraction as they ensure less heat damage and higher yields. Hemicellulases are used in the bread-making industry as these enzymes are effective in the hydrolysis of the non-starch component of the flour. The rheological properties can be improved with the help of xylanases [41][30]. For example, a mixture of hemicellulases increases industrial degradation of various substrates by 12–109% [42][31]. On the other hand, hemicellulases can increase lignin removal from wood pulp by 27.8% [42][31]. Lignin modifying enzymes are usually used in industrial and commercial wastewater treatment (Table 1). Another application of such enzymes, especially phenoloxidases, is in bioremediation [26]. The majority of such enzymes are used in the form of enzyme cocktails with the combination of different enzymes for specific industrial applications. While all these hydrolytic enzymes have a wide range of applications in various industries, the major drawback in the use of such enzymes is their cost and handling conditions. They require specific pH and temperature ranges, which are not suitable for every application or sometimes require the design of additional reaction steps that can increase the cost of the process. However, such limitations can be decreased with the help of extensive research on the underlying enzymatic processes. This can ensure the higher efficiency and lower cost of the enzymatic reaction mixture and method.
References
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223.
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322.
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86.
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass—An overview. Bioresour. Technol. 2016, 199, 76–82.
- Valdés, G.; Mendonça, R.T.; Aggelis, G. Lignocellulosic biomass as a substrate for oleaginous microorganisms: A review. Appl. Sci. 2020, 10, 7698.
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Distillers’ dried grains with solubles (DDGS) and its potential as the fermentation feedstock. Appl. Microbiol. Biotechnol. 2020, 104, 6115–6128.
- Dos Santos, A.C.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Lignin–enzyme interactions in the hydrolysis of lignocellulosic biomass. Trends Biotechnol. 2019, 37, 518–531.
- Mandels, M.; Reese, E.T. Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J. Bacteriol. 1957, 73, 269.
- Kim, Y.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R.; Bals, B.; Balan, V.; Dale, B.E. Enzyme hydrolysis and ethanol fermentation of liquid hot water and AFEX pretreated distillers’ grains at high-solids loadings. Bioresour. Technol. 2008, 99, 5206–5215.
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861.
- Juhasz, T.; Szengyel, Z.; Reczey, K.; Siika-Aho, M.; Viikari, L. Characterization of cellulases and hemicellulases produced by Trichoderma reesei on various carbon sources. Process Biochem. 2005, 40, 3519–3525.
- Knowles, J.; Lehtovaara, P.; Teeri, T. Cellulase families and their genes. Trends Biotechnol. 1987, 5, 255–261.
- Habte-Tsion, H.-M.; Kumar, V. Nonstarch polysaccharide enzymes—General aspects. In Enzymes in Human and Animal Nutrition; Nunes, C., Kumar, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 183–209.
- Ahmed, A.; Batool, K.; Bibi, A. Microbial β-glucosidase: Sources, production and applications. J. Appl. Environ. Microbiol. 2017, 5, 31–46.
- Eriksson, K.-E. Biotechnology in the pulp and paper industry. Wood Sci. Technol. 1990, 24, 79–101.
- Godoy, M.G.; Amorim, G.M.; Barreto, M.S.; Freire, D.M.G. Agricultural residues as animal feed: Protein enrichment and detoxification using solid-state fermentation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–256.
- Kunamneni, A.; Plou, F.J.; Alcalde, M.; Ballesteros, A. Trichoderma enzymes for food industries. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 339–344.
- Álvarez, C.; Reyes-Sosa, F.M.; Díez, B. Enzymatic hydrolysis of biomass from wood. Microb. Biotechnol. 2016, 9, 149–156.
- Songsiriritthigul, C.; Buranabanyat, B.; Haltrich, D.; Yamabhai, M. Efficient recombinant expression and secretion of a thermostable GH26 mannan endo-1, 4-β-mannosidase from Bacillus licheniformis in Escherichia coli. Microb. Cell Fact. 2010, 9, 20.
- Yoshida, T.; Nakajima, T.; Ichishima, E. Overproduction of 1, 2-α-mannosidase, a glycochain processing enzyme, by Aspergillus oryzae. Biosci. Biotechnol. Biochem. 1998, 62, 309–315.
- Ademark, P.; Lundqvist, J.; Hägglund, P.; Tenkanen, M.; Torto, N.; Tjerneld, F.; Stålbrand, H. Hydrolytic properties of a β-mannosidase purified from Aspergillus niger. J. Biotechnol. 1999, 75, 281–289.
- Novy, V.; Nielsen, F.; Seiboth, B.; Nidetzky, B. The influence of feedstock characteristics on enzyme production in Trichoderma reesei: A review on productivity, gene regulation and secretion profiles. Biotechnol. Biofuels 2019, 12, 238.
- Waqasi, M.; Mehmood, Z.; Mahmood, K.; Azam, M.; Khan, G.M.; Ibrahim, M.; Rasool, A.; Ahmed, S. Immobilization of b-glucuronidase on the biomaterials/nano particles and its industrial applications. Indo Am. J. Pharm. Sci. 2018, 5, 2287–2291.
- Wang, H.; Lu, F.; Sun, Y.; Du, L. Heterologous expression of lignin peroxidase of Phanerochaete chrysosporium in Pichia methanolica. Biotechnol. Lett. 2004, 26, 1569–1573.
- Zhang, H.; Zhang, J.; Zhang, X.; Geng, A. Purification and characterization of a novel manganese peroxidase from white-rot fungus Cerrena unicolor BBP6 and its application in dye decolorization and denim bleaching. Process Biochem. 2018, 66, 222–229.
- Mónica, M.-G.A.; Jaime, M.Q. Phenoloxidases of fungi and bioremediation. Fungal Bioremediation Fundam. Appl. 2019, 3, 62–90.
- Siddiqui, K.S.; Ertan, H.; Charlton, T.; Poljak, A.; Khaled, A.K.D.; Yang, X.; Marshall, G.; Cavicchioli, R. Versatile peroxidase degradation of humic substances: Use of isothermal titration calorimetry to assess kinetics, and applications to industrial wastes. J. Biotechnol. 2014, 178, 1–11.
- Gupta, V.G.; Schmoll, M.; Herrera-Estrella, A.; Upadhyay, R.S.; Druzhinina, I.; Tuohy, M. Biotechnology and Biology of Trichoderma; Newnes: Boston, MA, USA, 2014; ISBN 0444595945.
- Singh, A.; Kuhad, R.C.; Ward, O.P. Industrial application of microbial cellulases. In Lignocellulose Biotechnology Future Prospects; I.K. International Publishing House Pvt. Ltd.: New Delhi, India, 2007; pp. 345–358.
- Raveendran, S.; Parameswaran, B.; Beevi Ummalyma, S.; Abraham, A.; Kuruvilla Mathew, A.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of microbial enzymes in food industry. Food Technol. Biotechnol. 2018, 56, 16–30.
- Juturu, V.; Wu, J.C. Insight into microbial hemicellulases other than xylanases: A review. J. Chem. Technol. Biotechnol. 2013, 88, 353–363.
