The increase of public awareness on ocular conditions leads to an early diagnosis and treatment, as well as an increased demand for more effective and minimally invasive solutions for the treatment of both the anterior and posterior segments of the eye. Despite being the most common route of ophthalmic drug administration, eye drops are associated with compliance issues, drug wastage by lacrimation, and low bioavailability due to the ocular barriers. In order to overcome these problems, the design of drug-eluting ophthalmic lenses constitutes a non-invasive and patient-friendly approach for the sustained drug delivery to the eye. Several examples of therapeutic contact lenses and intraocular lenses have been developed, by means of different strategies of drug loading, leading to promising results.
- therapeutic contact lenses
- therapeutic intraocular lenses
- eye diseases
- glaucoma
- cataract
- corneal diseases
- posterior segment of the eye diseases
1. Drug Loading Methods
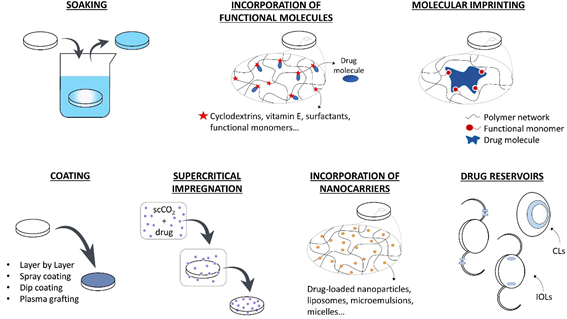
The use of drug-loaded CLs can substitute the topical application of eye drops increasing the drug residence time on the cornea, the drug bioavailability, and the patient compliance. Therapeutic IOLs, which are directly implanted in the anterior chamber, are expected to perform an efficient prophylaxis for post-surgical inflammation and infection or temporarily substitute intraocular injections in case of chronic diseases. In the following section, the various strategies that have been developed for loading drugs in therapeutic lenses are summarized. In Figure 1, a schematic description of these methods is presented.
1.1. Soaking
The simplest method for obtaining therapeutic lenses is soaking into a drug solution [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][24,30–45]. The amount of drug loaded and released depends on the material and the structure of the lens (e.g., porosity, swelling capacity), on the drug characteristics (e.g., molecular structure, molecular weight, charge), and on eventual interactions that may be established between the drugs and the lens material [18][22]. Besides, the soaking parameters also affects drug loading, i.e., the concentration of the solution [19][46], loading time [12][40], and environmental factors, such as temperature and pH [20][47]. The main issue of the soaking method still is the limited control over the drug release profile, which is usually characterized by a high initial release rate and a short delivery time after lens placement onto the eye [20][21][47,48]. Furthermore, economic and environmental concerns rise due to the waste of the drug present in the soaking solution [19][46]. Most drug-loaded lenses that are obtained by soaking are able to retain their therapeutic effect for a few hours or for some days [21][48], although a few examples of sustained release over weeks do exist [1][24]. This fast release kinetics could be suitable in the case of disposable CLs with a daily use, but it is not compatible with the development of IOLs with a long-term therapeutic purpose [22][49].
Figure 1. Strategies f[23]or the development of therapeutic ophthalmic lenses: soaking into a drug solution, incorporation of functional molecules with a high affinity to the drug, molecular imprinting, drug-eluting or drug-barrier coating, supercritical impregnation, incorporation of nanocarriers and incorporation of drug reservoirs.
1.2. Incorporation of Functional Molecules
The incorporation of functional molecules into the lens polymer (e.g., cyclodextrins, vitamin E, surfactants, functional monomers) proved to be a potential strategy for enhancing drug loading during the soaking step and tuning the release kinetics. Cyclodextrins present a hydrophobic cavity that is suitable for accommodating hydrophobic drugs. They have been successfully co-polymerized with the lens backbone material to control drug delivery over time [24][25,50], or mixed to the drug solution prior to lens soaking in order to enhance the apparent aqueous solubility of hydrophobic drugs [25][51]. Vitamin E is considered to be a promising molecule for providing a hydrophobic diffusion barrier for the release of hydrophilic drugs [26][27][28][29][30][52–56]. When hydrophobic drugs are involved, drug molecules diffuse through the highly viscous vitamin E agglomerates, resulting in a slower release kinetic [23][57]. Because of the hydrophobic nature of vitamin E, it can be easily incorporated into the lenses by soaking in a vitamin E-ethanol solution. After ethanol evaporation, vitamin agglomerates remain trapped into the polymer network. However, a loss in oxygen permeability, protein adsorption, and changes in the mechanical properties can be associated with the use of this functional molecule in therapeutic lenses [18][22]. Aggregates that are composed of long-chain surfactants and oppositely charged ionic drugs can be added to the lens pre-polymer mixture to extend drug release over time by entrapment of the drug molecules [31][58]. The suitability of the lens polymer network to load and release drugs in a sustained fashion can also be improved by co-polymerization with functional monomers presenting a stronger affinity with the target drugs [32][33][34][59–61] or by the modification of the lens charge [35][36][62,63].
1.3. Coating
Several methods have been suggested to produce coatings on drug-eluting biomedical devices (e.g., layer-by-layer deposition, spray coating, dip coating, plasma-assisted grafting) with the purpose of implementing drug-eluting reservoirs [37][38][39][40][41][42][43][44][45][46][64–73]. Besides, these coatings may be used in order to modify the hydrophilicity of the device surface [47][74] and, also, as diffusion barriers to drug release [48][49][50][75–77]. The main concern with the design of coatings to be applied onto CLs or IOLs is the preservation of the optical properties of the original lenses. In the case of coatings with biodegradable polymers, the degradation products must be biocompatible with the surrounding tissues. Furthermore, the coating adhesion to the lens material should be sufficient for avoiding the presence of floating debris on the cornea or in the anterior chamber, which results from the coating detachment from CLs or IOLs, respectively.
1.4. Molecular Imprinting
Molecular imprinting can be obtained by two different methods, which involve either covalent or noncovalent bonds (e.g., ionic, hydrophobic, hydrogen bonds) between the template drug and functional monomers. The latter method is the most widely used in drug delivery, as it is associated to an easier and faster drug dissociation kinetics. Stable noncovalent drug-monomer complexes are established by solubilizing both the template drug and the functional monomers in the prepolymer mixture [51][78]. With the subsequent polymerization step, the functional monomers are covalently bonded to the polymer backbone, but not the drug, due to the presence of aromatic rings or other highly stable molecular structures that are less prone to react. The drug is removed during the washing cycles to which the newly polymerized biomaterials are subjected to eliminate potentially toxic unreacted monomers [52][79]. After drug removal, tailored memory sites remain imprinted in the polymer and, by mimicking the drug’s natural receptors, they create an oriented functional material with high affinity to the template drug [53][80]. The drug is then re-loaded in the polymer by soaking and interacting with the memory sites. This process can lead to a higher drug loading and a slower release profile [54][81], and it was successfully applied to therapeutic CLs and IOLs [55][56][57][58][59][60][61][82–88]. The use of miscible combinations of drugs and monomers is generally preferable over non-miscible ones in order to avoid the use of solvents, since their presence could prevent the orientation of monomers around the drug molecules and result in a less effective imprinting [62][89]. The drug affinity and the release kinetics are determined by the type of functional monomers and their concentration in the matrix: the ratio between functional monomers and drug molecules must be optimized for each combination of drug and monomers [62][63][64][22,89–91]. This ratio has to be sufficiently high to create enough interactions and retard drug release; however, an excessive monomer concentration could interfere with drug diffusion during the loading phase and could also impede an organized orientation of molecules, thus hindering any effect on the template drug.
1.5. Supercritical Impregnation
Supercritical fluids are characterized by a high density, low viscosity, high diffusivity, and low interfacial tension [65][92]. These features are favorable for the diffusion of such fluids in the polymer matrices of CLs or IOLs [65][66][67][68][69][70][26,60,92–97]. Impregnation with a supercritical fluid consists in the dissolution of the drug in the solvent (generally supercritical CO2), followed by the interaction with the target material [18][22]. Supercritical CO2 is a better solvent for drugs than water, and also a better plasticizer for polymer networks: therefore, drug loading is enhanced when compared to traditional techniques like soaking [19][46]. This method is considered a ‘green’ alternative, as it forces the impregnation of the drug in the lens without using organic solvents. Moreover, CO2 is spontaneously released from the lens after impregnation, avoiding the purification steps that are usually associated to the use of solvents [65][92]. An optimized loading and release can be obtained by tuning the processing parameters, like pressure, temperature, presence of a co-solvent, duration of impregnation, and depressurization rate [19][22,46]. The choice of suitable parameters is fundamental: a too rapid depressurization, for example, can damage the lens material (foaming phenomenon [71][26]) and, therefore, compromise the optical functionality of the device. However, a too slow depressurization limits the efficacy of impregnation, as it reduces drug deposition into the polymer network [72][98]. The complex set-up is a limitation in the use of supercritical impregnation, as compared to other drug-loading techniques, and it can hinder its incorporation into the manufacturing process [62][89]. When applied to IOLs, supercritical impregnation allowed obtaining a sustained drug release over several weeks with various types of drugs (e.g., dexamethasone sodium and ciprofloxacin [68][26,92,95], methotrexate [70][97], cefuroxime sodium, and timolol maleate [72][98]). However, when the technology was applied to the thinner CLs, an initial burst release was commonly detected, followed by a sustained release for a few hours [66][67][73][93,94,99]. In order to overcome this issue, the combination of molecular imprinting and impregnation demonstrated to be an interesting alternative for the achievement of a gradual release, even in the first hours of CL wearing [74][100].
1.6. Incorporation of Nanocarriers
Colloidal nanocarriers entrapping active substances (e.g., polymeric biodegradable or non-degradable nanoparticles [75][76][77][78][79][80][81][82][74,101–108], liposomes, microemulsions, micelles) can be easily incorporated into the polymeric matrix of the lens [18][22]. Most of the nanoparticles for drug-delivery (homogeneous nanospheres or heterogeneous nanocapsules) are biodegradable polymers, which can release the drugs by degradation. Other mechanisms, such as light-induced release, can be implemented on non-degradable carriers [83][109]. Changes in pH and temperature, drug diffusion through the particle core/shell and matrix swelling also influence the drug delivery profile [84][110]. Liposomes have a structure that is similar to the biological membranes, in which lipophilic drugs can be loaded into the phospholipid bilayer and hydrophilic drugs into the aqueous core [85][111]. Polymeric micelles are spontaneously formed structures with promising applications in the delivery of hydrophobic drugs [86][87][112,113]. Microemulsions increase the drug-loading capacity during soaking and they present good thermodynamic stability, while preserving the ease of fabrication of the therapeutic lenses [88][89][90][114–116]. Despite the advantages of these innovative systems, significant problems need to be overcome during their design as drug-carriers for ophthalmic applications. In fact, changes in the mechanical properties and water content can be associated to their use in hydrogels [18][22]. Moreover, their natural tendency to aggregation must be hampered in order avoid a decrease in transparency [91][10].
1.7. Drug Reservoirs
Drug-eluting ocular implants have long been used as drug delivery platforms. However, their eventual migration after injection in the eye is one possible complication. The physical link of these implants to IOLs may overcome the problem and guarantee their correct positioning in the anterior chamber of the eye. Despite being more commonly applied on IOLs [92][93][94][23,27,117–119], examples of incorporated reservoirs were also implemented on CLs [95][96][97][98][99][100][101][120–126]. Drug reservoirs are usually constituted of biodegradable polymers: the material must be biocompatible, and its degradation products should not cause chronic inflammation or toxicity in the ocular tissues [20][47]. Reservoirs guarantee a prolonged (usually months) and controlled release over time. Moreover, the optical properties of the lens are not compromised, as they can be attached to the haptics of IOLs or incorporated into the peripheral part of CLs [102][12]. On the other hand, the design and fabrication of reservoirs can be complex and the physical link with the lens might be difficult to manage [19][46]. In the case of IOLs, the presence of these reservoirs can also raise problems while loading the lens in the injector system prior to surgery or in the ejection process [20][47].
2. Lenses for Ocular Diseases
2.1. Glaucoma
Glaucoma is the leading cause of irreversible blindness worldwide, and it is characterized by a progressive visual field loss due to the degeneration of retinal ganglion cells and optic nerve changes [103][5,127]. Although the relationship between diabetes and the development of glaucoma is still controversial and further investigation is needed, recent studies [104][105][128,129] have shown a significantly higher intraocular pressure (IOP) in diabetic patients when compared to non-diabetics, possibly due to an impaired autonomic function [105][129] and the progression of microvascular injury that is associated with chronic hyperglycemia [106][130]. An elevated IOP is considered to be a major risk factor for glaucoma, as it is associated to retinal ischemia and mechanical stress, as well as to an impaired ocular blood flow to retinal neurons [104][128]. The current treatment of glaucoma involves the lowering of IOP by pharmacological administration, laser, or surgical procedures [107][131]. The balance between the aqueous humor production and outflow from the anterior chamber of the eye regulates the IOP [104][128]. Therefore, current drug treatments are targeted at the reduction of aqueous humor production and/or the increase of the outflow facility. This can be achieved by use of beta-adrenergic antagonists (e.g., timolol, betaxolol, puerarin), sympathomimetic agents (e.g., epinephrine), parasympathomimetic miotic agents (e.g., pilocarpine), carbonic anhydrase inhibitors (e.g., dorzolamide), or prostaglandin analogues (e.g., latanoprost, bimatoprost) [108][132].
Many examples of therapeutic CLs for the treatment of glaucoma are described in the literature. Several strategies were followed in order to improve drug loading: optimized soaking, the incorporation of functional molecules, coating, molecular imprinting, supercritical impregnation, incorporation of nanocarriers, and reservoir attachment. A prolonged release over days was obtained even by soaking in a drug solution, which constitutes the easiest drug loading method. By the incorporation of drug eluting reservoirs, drug release was extended over weeks. Despite the wide research on the topic, only a few examples of in vivo tests on rabbits, dogs, and monkeys were reported, with a lack of preclinical and clinical tests on glaucoma patients, which are indispensable for the future commercial application of the designed devices.
2.2. Cataract
A cataract is defined as the opacification of the natural crystalline lens with a consequent decrease in the quality of vision [109][140]. It is the leading cause of vision loss in elderly patients, causing visual impairment in about 30% of the population over 65 years old, but its incidence is also increasing in the younger population due to the exposition of UV radiation, smoke, use of steroids, increased incidence of diabetes and malnutrition [110][46,141]. Approximately 20% of cataract surgeries in the western population are performed on diabetic patients [111][142]. Diabetic crystalline lenses are characterized by an increased level of free radicals and impaired antioxidant capacity, which leads to an increased susceptibility to oxidative stress. Cataract development occurs at an earlier age in presence of diabetes, but an appropriate metabolic control can contribute to the lens recovery in young patients [112][143]. The administration of aldose-reductase inhibitors (ARIs) and antioxidants was also demonstrated to be beneficial in the delay of diabetic cataract in the early stage of the pathology and it could be useful for its prevention in patients at risk. The use of CLs that are loaded with aldose-reductase inhibitors or antioxidants for the prevention of cataract in diabetic patients, especially in the young population, demonstrated, in in vitro studies, to be a promising approach.
Cataract surgery currently remains the standard treatment of severe cataractous eyes. The procedure consists in the removal of the damaged lens and a subsequent implant of an IOL. Despite the advances in the technique and the evolution in the different types of IOLs made this procedure one of the most cost-effective in the current healthcare [19][46], some post-operative complications can still occur and cause patient discomfort and visual impairment, as well as a prolonged recovery time [113][145]. An inflammatory response due to the physical trauma that is associated to surgery is considered to be physiological and it is generally treated with topical non-steroidal anti-inflammatory drugs (NSAIDs) or corticosteroids. If untreated, persistent inflammation can lead to pseudophakic cystoid macular edema (PCME), uveitis, iritis, glaucoma, and an increase of IOP [113][114][145,146]. Diabetic patients are at increased risk of developing these diseases. A recent clinical trial by McCafferty et al. [114][146] on 662 patients evidenced that PCME was the most common complication after cataract surgery. Because of the higher sensitivity of the vascular bed in diabetic patients, approximately 4–12% of patients affected by diabetes mellitus [115][142,147] and up to 56% of patients with diabetic retinopathy [116][148] are expected to develop PCME after IOL implant. Ocular infection, often linked to endophthalmitis, is another possible adverse condition, and it is mostly caused by bacterial migration from the lid and conjunctiva to the intraocular space. In this case, prevention and treatment involve the use of topical corticosteroids and antibiotics [113][145]. The migration and proliferation of epithelial cells to the posterior surface of the IOL can also lead to visual impairment after surgery; the phenomenon is known as posterior capsule opacification (PCO), and it is commonly treated by a laser procedure [117][149]. Postoperative endophthalmitis and early PCO are both reported to develop at a higher incidence rate in diabetic patients [118][119][150,151].
The current prophylaxis after cataract surgery consists in the administration of antibiotics and anti-inflammatory drugs (NSAIDs and/or corticosteroids) in the form of eye drops for two to four weeks after IOL implantation. The Food and Drug Administration approved a new intracameral drug-release device (DEXYCU, Icon Bioscience Inc., Newark, CA) in 2018 for the treatment of postoperative inflammation associated to cataract surgery [120][152]. The device is constituted by an injectable dexamethasone suspension, which is able to guarantee a 21-days long therapeutic level of drug release. The suspension is injected at the end of cataract surgery, forming a surface tension-based sphere positioned aside the IOL and behind the iris. The use of CLs as drug depots for antibiotics and anti-inflammatories has been proposed as a less-invasive alternative [85][21,111]. However, in the case of cataract surgery, postoperative prophylaxis could be more efficiently achieved by use of drug-loaded IOLs, in order to overcome the issues that are related to patient compliance and poor drug permeability through the cornea, to which both eye drops administration and drug-eluting CLs are subjected [20][47]. The possibility of a dual loading of both antibiotics and anti-inflammatory drugs on IOLs constitutes an interesting solution for addressing both post-surgical infection and inflammation. Drug release for 15 days or longer can be obtained with various drugs, thus confirming the suitability of double loaded IOLs for the substitution of the current eye drops prophylaxis, usually administered for two weeks after surgery. Examples of drug eluting IOLs for the prevention of PCO were also reported and, interestingly, long-term effects were observed in rabbits, even in the case of short-term drug release. As previously stated for drug-eluting CLs, extended in vivo tests and subsequent pre-clinical tests are also missing for drug-eluting IOLs, despite the promising results that were obtained in rabbit models.
2.3. Corneal Diseases
Keratoconjunctivitis sicca, or dry eye syndrome, is a common condition of the anterior segment of the eye, which can be associated to discomfort, burning sensation, external body sensation, ocular pain, light sensitivity, and intermittent blurred vision [121][159]. The presence of a normal tear film is fundamental for corneal health and immune protection [122][160] and the prevention of corneal injuries. Ocular lubrication can be compromised by reduced tear production and/or instability of the tear film, leading to a rapid evaporation from the eye surface. Various causes have been identified, including environmental factors (low ambient humidity, excessive wind or dust, temperature extremes, air conditioning), ageing, allergies, a prolonged computer use, metabolic conditions and nutritional deficiencies, contact lens use, and the prolonged administration of systemic or topical drugs (e.g., antiglaucoma medications or preservative-containing eye drops) [123][161]. Patients that are affected by neuropathic disorders, Sjögren’s syndrome, lupus, blepharitis, or congenital abnormalities of the lid are more prone to developing dry eye symptoms [124][162].
Other common corneal diseases are keratitis, a corneal inflammation that is usually treated by topical or systemic administration of antibiotics, biocides, antifungals, or antivirals, depending on the type of infection [125][163], and exposure keratopathy, often caused by an inadequate eyelid closure and treated by the use of lubricating drops or by surgical procedure [126][164]. Bullous keratopathy and Fuch’s dystrophy, on the contrary, are caused by an impairment of the corneal endothelium which loses the ability to drain fluid out of the cornea. Hypertonic topical solutions are usually prescribed to reduce the resulting corneal edema [127][165], and the concomitant wearing of bandage contact lenses is suggested to increase the residence time of eyedrops on the corneal surface [128][129][166,167]. Inherited corneal dystrophies, such as keratoconus, lattice dystrophy, and map-dot-fingerprint dystrophy, can lead to a major visual impairment at their advanced stage [130][168].
Although often overlooked, complications in the anterior segment of the eye are also common in diabetic patients [131][169]. It is estimated that diabetic keratopathy affects approximately 47–64% of diabetic patients and, if not treated, it can lead to major visual impairment [132][170]. Keratopathy is associated with an increased risk of corneal epithelial defects, recurrent erosions, delayed epithelial wound healing, tear film alteration, edema, and neurotropic corneal ulcers [131][132][160,169,170]. In severe cases, hyperglycemia and microvascular damage cause corneal neurotrophic lesions and a progressive decrease in corneal nerve fiber density, which lead to a loss of sensitivity, an impairment of the epithelial healing process, and a lack of feedback control over tear secretion [133][169,171]. An abnormal regulation of the healing mechanism can cause corneal opacity and blindness [134][172]. Despite the suggested causality between peripheral neuropathy and diabetic keratopathy, direct alterations in the corneal epithelium were also observed in less severe conditions, without signs of neuropathy [122][160]. In the early stage of the pathology, diabetic patients frequently experience dry eye symptoms[133] [171]. Hyperglycemia, in fact, can cause a microvascular damage to the lacrimal gland, also contributing to the low tear secretion [131][169]. Alterations in the tear film were also associated to the presence of inflammation, oxidative stress, and the accumulation of advanced glycation end products (AGEs) in the lacrimal gland [122][160]. Tear film changes, such as a reduced lipid layer quality and film stability, were also registered in diabetics [135][173].
The first-line treatment of the cornea is based on the use of artificial tears to maintain a lubricated ocular surface, but a few examples of CLs releasing moisturizing agents are already present on the market. The current research is focused on the optimization of the release profiles of those agents, alone or in association with drugs for pathological conditions. In this latter case, the administration of eye drops is the standard of care, but many therapeutic CLs eluting corticosteroids and NSAIDs were reported in literature. More recently, specific drugs addressed to the treatment of keratopathy were suggested (i.e., naltrexone, growth factors, aldose reductase inhibitors, and antioxidants), and a few examples reported their sustained release by drug-eluting CLs. Preliminary clinical tests were conducted with therapeutic CLs releasing epidermal growth factors with encouraging results. However, extended experimentation is required due to the limited number of patients involved. Interestingly, the use of therapeutic CLs eluting aldose reductase inhibitors or antioxidants could have a double effect on both corneal issues and cataract prevention in the diabetic eye. In fact, the possibility of simultaneous treatment of different diseases could be an appealing objective in the design of therapeutic lenses that are addressed to diabetic patients due to the relationship between several ocular pathologies and chronic hyperglycemia.
2.4. Posterior Segment Diseases
Macular degeneration, diabetic retinopathy and diabetic macular edema are the most common vision-impairing diseases of the back of the eye [136][207]. AMD is the leading cause of elderly blindness in the United States, affecting 9.2% of individuals over 50 years old [137][138][208,209]. The pathology can be manifested under two forms, namely dry and wet AMD. Although dry AMD is more frequent, with an incidence above 85% in AMD patients, wet AMD is responsible for 90% of severe vision loss cases [138][209]. In the case of dry AMD, a thinning of the retinal pigment epithelium is observed, leading to blurred central vision [138][209]. Wet AMD is associated to retinal hemorrhage and fibrovascular tissue formation, due to an abnormal neovascularization, with the accumulation of subretinal or intraretinal fluid [139][210]. Cardiovascular diseases, which are linked to a higher hydrostatic pressure in the eye vessels, constitute a risk factor for the development of wet AMD [137][208], as well as smoking, obesity, and hereditary factors. Most of the pathologies of the back of the eye in diabetic patients, such as DR and DME, are caused by the long term and chronic progression of microvascular damage in the retina associated with persistent hyperglycemia [140][211]. In its non-proliferative stage, DR is associated to an abnormal vessel permeability or the presence of microaneurysms in the capillaries. Consequently, the leaking of fluid and its accumulation in the surrounding tissue can lead to the progression of macular edema [141][6] and, if the subsequent swelling or thickening of the retina occurs in the fovea, DME can significantly affect vision [140][211]. DME can be diagnosed at any stage of DR, and its incidence increases with the progression of DR and with the duration of diabetes. Poorly controlled blood pressure, smoking, a high cholesterol level, and a reduced kidney function are also considered to be risk factors for the development of the pathology [140][211]. At its proliferative stage, DR can be directly related to vision impairment. In fact, neovascularization is promoted on the retinal surface due to the occlusion of capillaries, but the fragility of the newly formed capillaries leads to frequent hemorrhages. The accumulation of blood in the vitreous affects vision, while the formation of fibrotic tissue can lead to traction retinal detachment and permanent blindness [140][6,211]. Approximately one-third of diabetic patients are expected to develop DR, and one-tenth of these are associated to sight-threatening states [142][212]. Even if strict blood glucose and blood pressure control have a protective effect on the development and progression of retinopathy, they are not associated to the complete elimination of the threat.
The treatment of disorders of the posterior segment of the eye is a challenging topic in ocular drug delivery. The standard of care consists in laser treatments or invasive intravitreal injections. The possibility of using therapeutic lenses for the treatment of the posterior segment of the eye is almost unexplored. Despite this, a few in vivo studies detected drug amounts in the retina after drug delivery through therapeutic CLs. Based on the reported cases, further research on the ocular drug delivery pathway to the posterior segment and the therapeutic efficacy of ophthalmic lenses to target tissues in the back of the eye is suggested. In fact, future in vivo studies could evidence the suitability of previously developed devices for drug delivery to the retina. The incorporation of innovative drug delivery methods, such as drug-eluting nanoparticles, into ophthalmic lenses could also constitute an interesting future approach for a minimally invasive sustained drug delivery to the back of the eye.
3. Concluding Remarks and Future Perspectives
Surprisingly, the impressive efforts that were made by researchers around the world on the optimization of drug-eluting ophthalmic lenses did not yet result in the commercialization of these devices. As recently described by Lanier et al. [143][215], several reasons may be pointed out for the apparent lack of interest of the pharmaceutical industry to invest in those systems. One common limitation of all innovative methods referred above is the need for the optimization of each specific system drug/lens. In fact, it is not possible to extrapolate the results that were obtained with a so-called model drug, because the drug release behavior and eventual alterations of the physical properties of the lenses after loading depend on the specific interactions between the drug and the polymeric matrix. For example, when using the molecular imprinting technique, the optimization of each combination polymer/monomer/crosslinker/template must be done. The control of the drug release by coating the lens strongly depends on the characteristics of the drug molecule: a very efficient coating for one drug may be inefficient for other similar drugs. Besides choosing the ideal combinations of components, it is also necessary to determine the adequate amounts of drug loaded: it has to be sufficient to ensure clinically relevant therapeutic release, but it cannot affect key aspects of the lens, namely transparency, Young modulus, ionic and oxygen permeability, wettability, and water content. In the case of the addition of other agents capable of sustaining the drug release, such as the functional monomers in the imprinting technique, vitamin E or surfactants, the preservation of the lens properties has to be ensured. The optimization of the combination of materials and loading conditions may be still more demanding when multiple drugs are needed for the treatment. Some of the methods of preparation of drug-loaded lenses involve complex manufacturing, namely the incorporation of nanoparticles or drug reservoirs, the LbL coating, supercritical impregnation, which may be a drawback for scaling up production. Important issues, like the lack of drug stability during processing, the prevention of burst release, protein adherence, sterilization, and storage conditions, have been addressed, but need more intense investigation. In general, several innovative drug-eluting lenses have been submitted to in vivo studies, which demonstrated promising results; however, further studies involving the assessment of long-term safety are missing as well as extended clinical tests. Fortunately, there are solutions for many of the technical problems described above. The minimization of burst release and protein adherence may be achieved with adequate coatings and/or optimized formulations. The storage conditions may involve immersion of the lenses in drug solutions, due to the equilibrium loading method, or keeping the lenses in dry state. Sterilization methods, which have no detrimental effects, have been proposed. Thus, the last step before commercialization needs a positive evaluation of the costs and benefits. The benefits seem to be huge, when considering that drug-eluting CLs may decrease the risks that are associated with their usual wear (keratitis, corneal erosion, dry eye syndrome, conjunctivitis) and avoid the frequent administration of eye drops for the treatment of ocular diseases, while drug-loaded IOLs may substitute the invasive intracameral and intravitreal injections. Thus, the commercialization of drug-loaded ophthalmic lenses is probable in the near future, and ongoing research on this subject continues to be relevant.