Plant bioactive compounds (PBC) are widespread in the plant kingdom, including in forage species, but their impact on silage fermentation and ruminant use of PBC-containing silage has been under-researched.
- silage quality,animal health,product quality,tannins,fermentation
1. Introduction
Several approaches have been proposed in an effort to improve the sustainability of animal production systems by adapting agroecological principles[1] [1]: reducing inputs needed for production, reducing pollution, integrating the management of animal health, exploiting system diversity and preserving biological diversity[2] [2]. Applying these general principles and approaches to resource use for ruminant nutrition creates a number of challenges. There is a need to increase nutrient use efficiency by the animals while decreasing nitrogen (N) excretion and methane (CH4) emissions at the animal level[3] [3], to reduce livestock exposure to pathogens, toxins and oxidative stress[4] [4], and to improve animal product quality, for instance through better meat fatty acid (FA) profiles or oxidative stability[5] [5].
All these challenges come into play for silages, which are an important source of forage outside the growing season in many countries. The demand for high-quality silage to provide forage for ruminants is also made more acute by heightening food–feed competition over limited arable land resources, especially for monogastric livestock. The issue of energy and protein losses (with concomitant pollutant emissions) is even more crucial, as they begin with enzymatic and microbial processes in the silos, long before actual intake of the silage. These losses come in the form of fermentation gases and juices that contain various amounts of energy and soluble N depending on harvest-plant and fermentative process factors[6] [6]. Moreover, silage quality strongly affects silage use by the animals, especially as previously partly-degraded protein will be more degraded in the rumen and thus more N will get excreted as urea[7][8] [7,8]. There are also potential health issues specific to silage due to the presence of pathogens or mycotoxins produced during the silage-making process[9][10] [9,10] that can have impacts on ruminant health and performances[11][12] [11,12] or lead to residues in animal products[13] [13].
Plant bioactive compounds (PBC) have long been recognized as a fertile source of new drugs in the pharmaceutical industry. Mounting public concern over the use of chemicals and pharmaceuticals in ruminant production systems has driven interest in PBC as alternative rumen modifiers and performance enhancers[14] [14]. The biological activity of PBC depends mainly on their chemical nature and concentration in animal diets, with contrasted effects on animal responses. The main desired effects are those promoting animal health (antimicrobial, anthelmintic, anti-bloating, antioxidant, immune stimulator) and performance (production, N use efficiency) and product quality[15] [15], although some PBC (including the beneficial ones used at an undesirable concentration) can be anti-nutritional or even toxic to animals. There has been extensive investigation into the use of PBC in ruminant nutrition, but the value of PBC in silage remains under-researched.
Here we review the state of the art on PBC use in silage as part of practices to promote more sustainable ruminant production systems. Our scope includes opportunities opened up by PBC to improve: (i) silage lactic fermentation and plant protein protection to increase nutrient use efficiency and decrease pollutant losses in the form of greenhouse gases and ammonia (NH3), (ii) animal health (e.g., digestive diseases), and (iii) product quality, for example through FA profiles in meat and milk or increased oxidative stability. We also report results from the EU-funded Marie Curie project LegumePlus (FP7-PEOPLE-2011-ITN-289377, 2012–2015) in which these different aspects were covered together. One objective of the LegumePlus project was to check whether different pillars of sustainability (productivity, environment and product quality) can be co-achieved using two PBC-containing forage legumes, i.e., sainfoin (containing condensed tannins) and red clover (containing polyphenol oxidase; PPO) included in grass-based silage. Their potential benefits and associative effects on silage quality, voluntary intake, digestion, animal performances and meat quality were measured in sheep[16] [16]. The results of this work are reported throughout the manuscript and summarized in Figure 1. The paper concludes by identifying gaps in the research needed to integrate PBC-enhanced silage into farming practices.
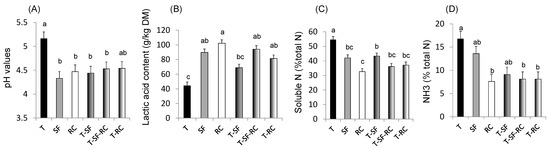
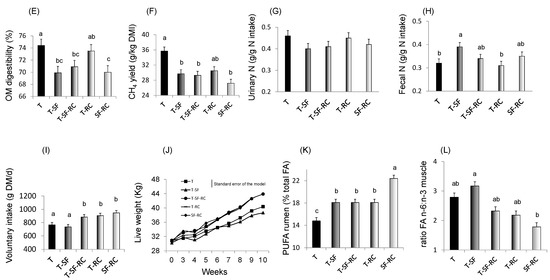
Figure 1. (A) pH values, (B) lactic acid concentration, and proportions of (C) soluble nitrogen (N) and of (D) ammonia (NH3) in total N in microscale silos of pure timothy (T, black bars), pure sainfoin (SF, grey bars), pure red clover (RC, white bars), binary mixture T–SF (50:50%), ternary mixture T–SF–RC (50:25:25%), and binary mixture T–RC (50:50%) (n = 3 for each type of silage)[33] [33]. (E) Organic matter (OM) digestibility, (F) methane (CH4) yield, (G) urinary and (H) faecal N in sheep fed silages of T, T–SF, T–SF–RC, T–RC and SF–RC (binary mixtures: 50:50%, ternary mixture: 50:25:25%) (n = 10 sheep allocated in a replicated Latin square design)[36] [36]. (I) Voluntary intake, (J) kinetics of live weigh, (K) polyunsaturated fatty acids (PUFA) in rumen content and (L) ratio of n-6:n-3 fatty acids (FA) in Longissimus muscle of growing lambs (n = 8 lambs per group) fed the same silages as in (E–H)[37][38] [37,38]. a,b,c For each sub-figure, values with different letters significantly differ (p < 0.05).
2. Classes of Plant Bioactive Compounds and Silages
The plant kingdom features a hugely diverse array of bioactive compounds, many of which are found in forage whole plants, feeds or plant extracts that can be incorporated into silage. PBC can be found in grassland plants, crops, agroindustry byproducts, and plant extracts or essential oils in which PBC have all been characterized to varying degrees.
Despite growing interest in the use of PBC in animal nutrition, their use in silages remains limited. Table 1 recaps the results of a literature search on specific classes of plant compounds. Plant molecules were searched based on chemical structure (lipid, aromatic, alkaloid, polyphenol, tannin, saponin, terpene) in the Web of Science, and each class was refined using the terms “bioactive or antimicrobial or anthelmintic or antibiotic”, “silage”, or both. The results highlight the contrast between the large number of articles reporting bioactivity for these compounds and the little literature focused on their effects in silage. Reasons that may explain this substantive lack of research are the low value of silage, the need for farmers to make silage as easily as possible, and other factors known to improve silage quality (ensiling techniques, inoculants). However, although difficult to assess, the economic impact of poor-quality silage, animal disease and reduced performances can be significant. Some bioactive compounds naturally present in a number of forage species or byproducts are potentially cheap yet could bring complementary benefits to existing solutions. The small amount of literature available on PBC bioactivity in silage tends to focus on polyphenols, and especially tannins.
Table 1. Number of references associated to plant bioactive compounds and silage.
| Compound Class | Number of References 1 | |||
|---|---|---|---|---|
| Total | Bioactive | Silage | Bioactive × Silage | |
| Lipid | 650,783 | 22,544 | 676 | 10 |
| Aromatic | 306,292 | 1616 | 24 | 2 |
| Alkaloid | 88,000 | 6603 | 52 | 3 |
| Polyphenol | 63,497 | 8476 | 128 | 11 |
| Tannin | 24,994 | 3248 | 332 | 18 |
| Saponin | 24,299 | 3269 | 39 | 2 |
| Terpene | 15,830 | 1412 | 21 | 2 |
1 Searched on 21/04/2020 using Web of Science. Each compound class was first searched as an independent topic then refined using: “Bioactive or antimicrobial or anthelmintic or antibiotic’’, “silage”, and both.
3. Improving Silage Quality
Silage quality is highly dependent on the capacity to maintain the integrity of plants at harvest. Plant degradation and decrease in nutritive value start with the post-harvest continuation of plant respiration leading to hydrolysis of carbohydrates into CO2 and consumption of sugars, which are essential substrates for lactic fermentation[17] [17]. Consequently, the delay between harvest and ensiling should be reduced as much as possible. During silage fermentation, the proteins, which typically represent 70–80% of the total N of the plant, are partly degraded by plant proteases to soluble N and especially amino acids and NH3. Proteolysis raises the soluble N content to 40–60% of total N and continues until the pH drops below 4.0[17] [17]. The usual way to reach anaerobiosis and the desired pH as rapidly as possible is through an optimal management of silage: correct dry matter concentration at harvest, rapid silo filling, perfect mass sealing, high mass compaction[6] [6]. Unappropriated management procedures and unloading technique, or the use of machinery not suitable to ensure sufficient compaction can lead to air penetration and consequent aerobic deterioration of the ensiled mass. The use of inoculants based on homo- and/or hetero-fermentative lactic acid bacteria can offer a significant help in some situations[18] [18]. Chemical additives can provide a more consistent effect than inoculants because they are less dependent on biological processes, but their higher cost or toxicity may be a limit to adoption by farmers. For instance, benzoate or sorbate have been shown to be safe and of high effectiveness against molds and yeasts, while formaldehyde could be toxic due to irritation of the skin, eye and respiratory organs[19] [19] (for a detailed review on the silage additives, see[18] [18]).
PBCs offer a complementary option to these solutions as they can be used at negligible cost when incorporated in the form of bioactive forages or agroindustry byproducts. Research efforts have focused more on the ability of PBC to reduce protein degradation in the silo, and less on their ability to improve lactic fermentation. A recent meta-analysis unequivocally showed that tannins effectively and efficiently improve silage quality, especially by limiting the extensive proteolysis that may occur during ensiling[20] [20]. Tannin-rich forage legumes are also packed with protein, which make them particularly good candidates to include in silage. Ensiling temperate tannin-rich legumes such as sainfoin or sulla can protect plant proteins during fermentation, reduce NH3 production, and improve nutritive value compared to tannin-free forages[21][22][23] [21,22,23]. Tannins have also been shown to limit proteolysis in high-protein silage such as alfalfa, moringa, indigofera and other legume silages[20][24][25] [20,24,25]. In a study comparing the effects of tannin levels in sorghum silages supplemented or not with tannin-inactivating polyethylene glycol (PEG), silages without PEG had higher protein levels and lower NH3 concentrations than silages with PEG[26] [26]. Tannins can also be successfully supplemented in silage via byproducts (e.g., green tea waste,[27] [27] and plant extracts containing hydrolysable tannins from chestnut and oak[28][29] [28,29] or condensed tannins from quebracho[30] [30].
PPO is another bioactive compound found in forages, especially red clover. PPO is an enzyme that can catalyze the oxidation of phenols to quinones, which are highly reactive protein-binding molecules, and thus provide effective protection against proteolysis and lipolysis[31] [31]. PPO is particularly relevant for silages because PPO action requires (i) cellular damages in order to obtain contact between PPO located in chloroplasts and phenols located in vacuoles, and these damages are obtained during harvest, wilting and silage-making processes, and (ii) a time window of aerobiosis for oxidation, which occurs before the stabilization of the silo but not at grazing where the period of aerobiosis between chewing the forage and entry into the rumen is not enough long[32] [32].
Mixing a grass (timothy) with sainfoin and/or red clover in micro-scale silos has been demonstrated to improve fermentation and protein protection against degradation through lower pH values, decreased soluble N and NH3 contents, and higher concentrations of lactic acid compared to pure ensiled grass (Figure 1A–D), thus confirming the benefits of condensed tannins and PPO for silage quality[33] [33].
Other PBC including erythritol byproduct solution and lignosulphonates from wood molasses were studied as silage additives to reduce in-silo proteolysis but were shown to be less efficient compared to tannin solutions[29] [29]. In contrast, spraying essential oils (eugenol, thymol, cinnamaldehyde and carvacrol) before ensiling ryegrass was shown to reduce protein degradation, but usually had to be sprayed at a concentration of 2 g/kg fresh forage to observe inhibition of amino acid deamination[34] [34]. Finally, without really being considered as PBC, sugars added at ensiling or present in high-sugar grasses are well known to facilitate lactic fermentation, especially when the sugar content of forage is a limiting factor[35] [35]. It should be noted that sugars can also be a substrate source for undesirable microorganisms if other inhibitory factors are missing or the silo management is inadequate. In particular, the saccharolytic Clostridia are able to ferment sugars to butyric acid, and excessive concentration of sugars can decrease aerobic stability, as residual sugars are rapidly used by spoilage yeasts and molds[18] [18].
Reference
(we'll rearrange the references after you submitted it)
- Altieri, M.A. Agroecological Principles and Strategies for Sustainable Agriculture. In Agroecological Innovations: Increasing Food Production with Participatory Development; Uphoff, N.T., Ed.; Earthscan Publication Ltd.: London, UK, 2002; pp. 40–46.
- Dumont, B.; Fortun-Lamothe, L.; Jouven, M.; Thomas, M.; Tichit, M. Prospects from Agroecology and Industrial Ecology for Animal Production in the 21st Century. Animal 2013, 7, 1028–1043.
- Gislon, G.; Ferrero, F.; Bava, L.; Borreani, G.; Dal Prà, A.; Pacchioli, M.T.; Sandruccia, A.; Zucalia, M.; Tabacco, E. Forage Systems and Sustainability of Milk Production: Feed Efficiency, Environmental Impacts and Soil Carbon Stocks. J. Clean. Prod. 2020, 260, 121012.
- Lykkesfeldt, J.; Svendsen, O. Oxidants and Antioxidants in Disease: Oxidative Stress in Farm Animals. Vet. J. 2007, 173, 502–511.
- Ponnampalam, E.N.; Bekhit, A.E.D.; Bruce, H.; Scollan, N.D.; Muchenje, V.; Silva, P.; Jacobs, J.L. Production Strategies and Processing Systems of Meat: Current Status and Future Outlook for Innovation—A Global Perspective. In Sustainable Meat Production and Processing; Galanakis, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 17–44.
- Muck, R.E. Factors Influencing Silage Quality and Their Implications for Management. J. Dairy Sci. 1998, 71, 2992–3002.
- Peyraud, J.L.; Vérité, R.; Delaby, L. Nitrogen Excretion by Dairy Cows: Influence of the diet and of the Level of Production. Fourrages 1995, 142, 131–144.
- Charmley, E. Towards Improved Silage Quality—A Review. Can. J. Anim. Sci. 2001, 81, 157–168.
- Queiroz, O.C.M.; Ogunade, I.M.; Weinberg, Z.; Adesogan, A.T. Silage Review: Foodborne Pathogens in Silage and Their Mitigation by Silage Additives. J. Dairy Sci. 2018, 101, 4132–4142.
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vias, D.; Adesogan, A.T. Silage Review: Mycotoxins in Silage: Occurrence, Effects, Prevention, and Mitigation. J. Dairy Sci. 2018, 101, 4034–4059.
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on Mycotoxin Issues in Ruminants: Occurrence in Forages, Effects of Mycotoxin Ingestion on Health Status and Animal Performance and Practical Strategies to Counteract Their Negative Effects. Toxins 2015, 7, 3057–3111.
- Driehuis, F.; Wilkinson, J.M.; Jiang, Y.; Ogunade, I.; Adesogan, A.T. Silage Review: Animal and Human Health Risks from Silage. J. Dairy Sci. 2018, 101, 4093–4110.
- Flores-Flores, M.E.; Lizarraga, E.; de Cerain, A.L.; González-Peñas, E. Presence of Mycotoxins in Animal Milk: A Review. Food Control 2015, 53, 163–176.
- Greathead, H. Plants and Plant Extracts for Improving Animal Productivity. Proc. Nutr. Soc. 2003, 62, 279–290.
- Rochfort, S.; Parker, A.J.; Dunshea, F.R. Plant Bioactives for Ruminant Health and Productivity. Phytochemistry 2008, 69, 299–322.
- Copani, G. Benefit of Including Bioactive Legumes (Sainfoin, Red Clover) in Grass-Based Silages on Ruminant Production and Pollutant Emissions. Ph.D. Thesis, Université Clermont Auvergne, Clermont-Ferrand, France, 10 September 2015. Available online: https://hal.archives-ouvertes.fr/tel-01276667/ (accessed on 23 December 2020).
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Aberystwyth, UK, 1991.
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung Jr, L. Silage Review: Recent Advances and Future Uses of Silage Additives. J. Dairy Sci. 2018, 101, 3980–4000.
- Golden, R. Identifying an Indoor Air Exposure Limit for Formaldehyde Considering Both Irritation and Cancer Hazards. Crit. Rev. Toxicol. 2011, 41, 672–721.
- Jayanegara, A.; Sujarnoko, T.U.; Ridla, M.; Kondo, M.; Kreuzer, M. Silage Quality as Influenced by Concentration and Type of tannins Present in the Material Ensiled: A Meta-Analysis. J. Anim. Physiol. Anim. Nutr. 2019, 103, 456–465.
- Albrecht, K.A.; Muck, R.E. Proteolysis in Ensiled Forage Legumes That Vary in Tannin Concentration. Crop Sci. 1991, 31, 464–469.
- Niezen, J.H.; Waghorn, G.C.; Lyons, T.B.; Corson, D.C. The Potential Benefits of Ensiling the Forage Legume Sulla Compared with Pasture. Proc. New Zealand Grassl. Assoc. 1998, 60, 105–109.
- Lorenz, M.M.; Eriksson, T.; Uden, P. Effect of Wilting, Silage Additive, PEG Treatment and Tannin Content on the Distribution of N between Different Fractions after Ensiling of Three Different Sainfoin (Onobrychis viciifolia) Varieties. Grass Forage Sci. 2010, 65, 175–184.
- Aboagye, I.A.; Oba, M.; Koenig, K.M.; Zhao, G.Y.; Beauchemin, K.A. Use of Gallic Acid and Hydrolyzable Tannins to Reduce Methane Emission and Nitrogen Excretion in Beef Cattle Fed a Diet Containing Alfalfa Silage. J. Anim. Sci. 2019, 97, 2230–2244.
- He, L.; Lv, H.; Chen, N.; Wang, C.; Zhou, W.; Chen, X.; Zhang, Q. Improving Fermentation, Protein Preservation and Antioxidant Activity of Moringa oleifera Leaves Silage with Gallic Acid and Tannin Acid. Bioresour. Technol. 2020, 297, 122390.
- De Oliveira, S.G.; Berchielli, T.T.; Reis, R.; Vechetini, M.E.; dos Santos Pedreira, M. Fermentative Characteristics and Aerobic Stability of Sorghum Silages Containing Different Tannin Levels. Anim. Feed Sci. Technol. 2009, 154, 1–8.
- Kondo, M.; Naoki, N.; Kazumi, K.; Yokota, H. Enhanced Lactic Acid Fermentation of Silage by the Addition of Green Tea Waste. J. Agric. Food Sci. 2004, 84, 728–734.
- Tabacco, E.; Borreani, G.; Crovetto, G.M.; Galassi, G.; Colombo, D.; Cavallarin, L. Effect of Chestnut Tannin on Fermentation Quality, Proteolysis, and Protein Rumen Degradability of Alfalfa Silage. J. Dairy Sci. 2006, 89, 4736–4746.
- Herremans, S.; Decruyenaere, V.; Beckers, Y.; Froidmont, E. Silage Additives to Reduce Protein Degradation during Ensiling and Evaluation of In Vitro Ruminal Nitrogen Degradability. Grass Forage Sci. 2019, 74, 86–96.
- Adesogan, A.T.; Salawu, M.B. The Effect of Different Additives on the Fermentation Quality, Aerobic Stability and In Vitro Digestibility of Pea/Wheat Bi-crop Silages Containing Contrasting Pea to Wheat Ratios. Grass Forage Sci. 2002, 57, 25–32.
- Lee, M.R.F.; Scott, M.B.; Tweed, J.K.S.; Minchin, F.R.; Davies, D.R. Effects of Polyphenol Oxidase on Lipolysis and Proteolysis of Red Clover Silage with and without a Silage Inoculant (Lactobacillus plantarum L54). Anim. Feed Sci. Technol. 2008, 144, 125–136.
- Lee, M.R.F.; Tweed, J.K.; Minchin, F.R.; Winters, A.L. Red Clover Polyphenol Oxidase: Activation, Activity and Efficacy under Grazing. Anim. Feed Sci. Technol. 2009, 149, 250–264.
- Copani, G.; Ginane, C.; Le Morvan, A.; Niderkorn, V. Bioactive Forage Legumes as a Strategy to Improve Silage Quality and Minimise Nitrogenous Losses. Anim. Prod. Sci. 2014, 54, 1826–1829.
- Foskolos, A.; Cavini, S.; Ferret, A.; Calsamiglia, S. Effects of Essential Oil Compounds Addition on Ryegrass Silage Protein Degradation. Can. J. Anim. Sci. 2016, 96, 100–103.
- Ferris, C.P.; Mayne, C.S. The Effects of Incorporating Sugar-Beet Pulp with Herbage at Ensiling on Silage Fermentation, Effluent Output and In-Silo Losses. Grass Forage Sci. 1994, 49, 216–228.
