Consumer demand for plant protein-based products is high and expected to grow considerably in the next decade. Factors contributing to the rise in popularity of plant proteins include: (1) potential health benefits associated with increased intake of plant-based diets; (2) consumer concerns regarding adverse health effects of consuming diets high in animal protein (e.g., increased saturated fat); (3) increased consumer recognition of the need to improve the environmental sustainability of food production; (4) ethical issues regarding the treatment of animals; and (5) general consumer view of protein as a “positive” nutrient (more is better). While there are health and physical function benefits of diets higher in plant-based protein, the nutritional quality of plant proteins may be inferior in some respects relative to animal proteins.
- plant protein
- protein quality
- vegetable protein
- PDCAAS
- DIAAS
- protein requirements
- plant protein,protein quality,vegetable protein,PDCAAS,DIAAS,protein requirements
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
1. Introduction
Protein is a nutrient that has been trending increasingly positive in the minds of consumers, with demand rising for both plant and animal sources of protein [1]. In addition, there is a growing body of clinical evidence, especially in older adults, supporting health benefits associated with protein at or above current dietary protein intake recommendations. Among these health benefits are increases in lean body mass [2][3][4][5][6][2,3,4,5,6], functional benefits such as increased leg power [4] or gait speed [6], and improved bone density [7][8][9][7,8,9]. Thus, on the one hand, there is likely to be a continued push for protein-rich options in the food marketplace. On the other hand, the global production of an increased volume of food protein, especially high-quality animal protein, could present environmental sustainability challenges. The production of 1 kg of high-quality animal protein requires feeding 6 kg plant protein to livestock, which introduces the subsequent strain on land and water resources, as well as potential increases in greenhouse gas emissions, associated with livestock agriculture [1][10][1,10]. Wider and prudent use of plant proteins in the diet can help to supply adequate high-quality protein for the population and may reduce the potential for adverse environmental consequences.
2. Determination of Protein Quality
Two requirements for a protein to be considered high quality, or complete, for humans are having adequate levels of indispensable amino acids (see Table 1) to support human growth and development and being readily digested and absorbed.
Table 1. Indispensable, dispensable, and conditionally indispensable amino acids in the human diet. Adapted from [11].
|
Indispensable |
Protein |
Dispensable |
PDCAAS |
Conditionally Indispensable |
|||||||
1 |
PDCAAS 2 |
PDCAAS 3 |
PDCAAS 4 |
DIAAS 3 |
Limiting Amino Acid(s), When Present |
AA Profile: Materials Analyzed and References |
Fractional Digestibility and References |
Histidine |
Alanine |
Arginine |
|
|
Milk |
1.00 |
1.00 |
1.00 |
1.00 |
1.08 |
None |
Fecal true protein: mean 0.96 [13][20][13,20] Ileal AA: range for individual AA 0.84–0.94 [22] |
Isoleucine |
Aspartic acid |
Cysteine |
|
|
Leucine | |||||||||||
|
Whey |
1.00 |
1.00 |
0.97 |
1.00 |
0.90 |
His |
Fecal true protein: mean 0.96 [20][24][20,24] Ileal AA: range for individual AA 0.89–1.00 [22] |
Asparagine |
Glutamine |
||
|
Lysine |
Glutamic acid |
Glycine |
|||||||||
|
Methionine |
Serine |
Proline |
|||||||||
|
Phenylalanine |
|
Tyrosine |
|||||||||
|
Threonine |
|
|
|||||||||
|
Tryptophan |
|
|
|||||||||
|
Valine |
|
|
Various methods for evaluating protein quality have been developed over the years, but amino acid scoring is currently the recommended method by the Food and Agricultural Organization of the United Nations (FAO) and the U.S. National Academy of Sciences [11][12][11,12]. The Protein Digestibility Corrected Amino Acid Score (PDCAAS) was developed in 1989 by a Joint FAO/WHO Expert Consultation on Protein Quality Evaluation [13] to compare the indispensable amino acid content of a test protein (mg/g protein) to a theoretical reference protein thought to meet indispensable amino acid requirements (mg/g protein) for a given age group, creating a ratio known as the amino acid or chemical score. The indispensable amino acid with the lowest ratio is referred to as the most limiting amino acid. The most limiting amino acid score is corrected for the fecal true digestibility of the protein. To determine fecal true protein digestibility, rats are fed a known amount of nitrogen from the test protein and then fecal nitrogen excretion is measured [14]. This measure represents apparent protein digestibility. The fecal nitrogen excretion from the rats on a protein-free diet is then subtracted from fecal nitrogen excretion on the test protein, which accounts for non-dietary protein nitrogen excretion from bacterial cells and digestive secretions. The result is referred to as true fecal protein digestibility. The calculation equation for the PDCAAS is shown in Figure 1.

Figure 1.
The results can be expressed as either decimals or multiplied by 100 to be expressed as a percent. A PDCAAS of <1.00 indicates that the protein is suboptimal and PDAAS scores >1.00 are truncated to 1.00.
In 2011, the FAO introduced an updated amino acid scoring system, the Digestible Indispensable Amino Acid Score (DIAAS) [16]. The DIAAS is calculated and interpreted similarly to the PDCAAS, but with a few important differences. First, the reference patterns for the indispensable amino acids were revised to reflect advances in the scientific knowledge regarding amino acid requirements. Second, a single estimate of fecal protein digestibility is no longer used. Rather, the concept of the ileal individual amino acid digestibility was incorporated. True fecal digestibility of protein, which is based on nitrogen excretion in the feces, is complicated by the considerable exchange of protein, amino acids, and urea between systemic pools and the lower gastrointestinal tract. In response to this limitation, it was recommended to measure ileal amino acid digestibility, which reflects the concentration of amino acids that reaches the ileum and would hence enter the colon, derived from ileostomy output studies conducted in animals or humans. As such, each indispensable amino acid from a given protein source will have an associated ileal digestibility value and its amino acid score will be corrected for that value. Finally, unlike the PDCAAS, the DIAAS method allows for scores >1.00 to acknowledge that there may be incremental health benefits associated with these higher DIAAS scores.
3. The Quality of Plant Proteins
In general, most animal-based protein sources, such as milk, whey, casein, eggs, and beef, have PDCAAS at or very near 1.00 [13][17][18][13,17,18]. As such, they are generally considered complete protein sources for supporting indispensable amino acid requirements for human growth and development. Plant proteins, however, may have insufficient levels of one or more indispensable amino acids. Legumes are frequently low in the sulfur-containing amino acids methionine and cysteine, while lysine is typically limiting in grains [19]. However, it should be noted that plant proteins differ regarding the amounts of limiting amino acids that are present. Table 2 shows the PDCAAS and DIAAS ratings for milk protein, whey, and several selected plant protein sources. Similar to milk protein and whey, soy protein essentially has a PDCAAS of 1.00, and four more proteins (canola, potato, pea, and quinoa) have a PDCAAS of at least 0.75.
Table 2. Protein quality of whey and selected vegetable protein sources.
Soy |
0.99 |
1.00 |
0.93 |
1.00 |
0.92 |
SAA |
Soy PI, Soy PC [25] Soy PI [20] Soy PI [21] |
Fecal true protein: mean 0.97 [13][20][21][25][13,20,21,25] Ileal AA: range for individual AA 0.95–0.99 [22] |
|
Canola |
0.88 |
1.00 |
0.93 |
1.00 |
NA |
AAA |
Canola PI [26]; Canola PI [27] |
|
|
Potato |
0.87 |
1.00 |
0.87 |
1.00 |
0.85 |
His |
Solanic 100F Potato PI [28] Solanic 206P HMW and LMW [29] Potato protein [30] Potato juice protein concentrate [31] Avg of 6 potato varieties [32] |
Fecal true protein: 0.89 [24] Ileal AA: range for individual AA 0.73–0.90 [22] |
|
Pea |
0.83 |
0.84 |
0.78 |
0.91 |
0.66 |
SAA *, Trp |
Pea PC [33] Pea PC [21] Pea PC [20] |
Fecal true protein: mean 0.97 [20][21][33][20,21,33] Ileal AA: range for individual AA 0.83–0.90 [20] |
|
Quinoa |
0.78 |
0.89 |
0.77 |
0.84 |
NA |
Ile, Leu, Lys *, Thr, Val |
Quinoa, raw [34] Quinoa [35] Quinoa from Salta [36] Uncooked quinoa [37] Field grown quinoa [38] Raw and unwashed quinoa [39] |
|
|
Chickpea |
0.77 |
0.85 |
0.71 |
0.71 |
0.69 |
Leu, Lys, SAA *, Thr, Trp, Val |
Boiled chickpeas [41] |
Fecal true protein: 0.85 [42] Ilea AA: range for individual AA 0.72–0.9 [22] |
|
Lentils |
0.73 |
0.73 |
0.68 |
0.80 |
0.75 |
Leu, SAA *, Thr, Trp, Val |
Lentils, mature seeds, ckd, bld without salt [37] |
Fecal true protein: 0.85 [24] Ileal AA: range for individual AA 0.82–0.98 [22] |
|
Red Kidney beans |
0.68 |
0.68 |
0.63 |
0.74 |
0.61 |
Leu, Lys, SAA *, AAA, Thr, Trp, Val |
Red kidney beans, cnd, drnd solids [37] |
Fecal true protein: 0.81 [24] Ileal AA: range for individual AA 0.72–0.94 [22] |
|
Fava/faba |
0.63 |
0.65 |
0.60 |
0.67 |
NA |
Lys, SAA *, Thr, Trp, Val |
Fava bean PI [43] Cooked fava beans [44] Broadbeans, ckd [37] 8 faba cultivars [45] Faba bean PI [46] |
Fecal true protein: 0.86 [47] |
|
Barley |
0.63 |
0.71 |
0.64 |
0.76 |
0.50 |
Lys * |
Barley, pearled [48] |
Fecal true protein: 0.98 [49] Ileal AA: range for individual AA 0.76–0.83 [22] |
|
Pinto beans |
0.61 |
0.61 |
0.57 |
0.66 |
NA |
His, Ile, Leu, Lys, SAA *, AAA, Thr, Trp, Val |
Pinto beans, cnd, drnd solids [37] |
Fecal true protein: 0.73 [24] |
|
Rice |
0.53 |
0.60 |
0.54 |
0.64 |
0.52 |
Lys *, Thr |
Rice PC [50] Rice endosperm protein [51] Oryzatein 90 and 80 Rice protein [52] Rice PC [21] |
Fecal true protein: mean 0.90 [21][24][51][21,24,51] Ileal AA: mean ranges for individual AA 0.81–0.87 [21][22][21,22] |
|
Oat |
0.51 |
0.59 |
0.52 |
0.62 |
0.44 |
Lys *, Thr |
Oat PC [53]; Rolled oats [21] |
Fecal true protein: 0.91 [13] Ileal AA: range for individual AA 0.70–0.85 [22] |
|
Peanut |
0.46 |
0.52 |
0.47 |
0.55 |
0.47 |
Ile, Leu, Lys *, SAA, Thr, Trp, Val |
Peanut PC and PI [54] Roasted peanuts [21] |
Fecal true protein: 0.93 [24] Ileal AA: mean ranges for individual AA 0.82–0.96 [21][22][21,22] |
|
Wheat |
0.45 |
0.51 |
0.46 |
0.54 |
0.39 |
Ile, Leu *, Lys *, AAA, Thr *, Val |
Whole meal and white flour [55] Wheat bran [21] |
Fecal true protein: mean 0.94 [24] Ileal AA: mean ranges for individual AA 0.81–0.91 [22] (wheat bran, wheat flour, wheat gluten, wheat) |
|
Corn |
0.41 |
0.47 |
0.42 |
0.50 |
0.38 |
Ile, Lys *, SAA, Thr *, Trp*, Val |
Corn meal [56] Corn tortillas [57] |
Fecal true protein: 0.84 [24] Ileal AA: ranges for individual AA 0.75–0.88 [22] |
1 FAO FN Paper 51 1989, ages 2–5 year, AA ref standard (mg/g protein) [13]: His 19, Ile 28, Leu 66, Lys 58, SAA 25, AAA 63, Thr 34, Trp 11, Val 35. 2 IOM 2002/2005, ages 1+ year, AA ref standard (mg/g protein) [11]: His 18, Ile 25, Leu 55, Lys 51, SAA 25, AAA 47, Thr 27, Trp 7, Val 32. 3 FAO FN Paper 92 2011, ages 0.5–3 year, AA ref standard (mg/g protein) [16]: His 20, Ile 32, Leu 66, Lys 57, SAA 27, AAA 52, Thr 31, Trp 8.5, Val 43. 4 FAO FN Paper 92 2011, older child, adolescent, adult, AA ref standard (mg/g protein) [16]: His 16, Ile 30, Leu 61, Lys 48, SAA 23, AAA 41, Thr 25, Trp 6.6, Val 40. PDCAAS, Protein Digestibility Corrected Amino Acid Score; DIAAS, Digestible Indispensable Amino Acid Score; AA, amino acid; His, histidine; Ile, isoleucine; Leu, leucine; Lys, lysine; SAA, sulfur amino acids (methionine and cysteine); AAA, aromatic amino acids (phenylalanine and tyrosine); Thr, threonine; TRP, tryptophan; Val, valine; PI, protein isolate; PC, protein concentrate; bld, boiled; ckd, cooked; cnd, canned; drnd, drained. * Limiting amino acid by all four amino acid reference standards.
While the PDCAAS of most plant proteins may be less than 1.00, the individualized protein scoring system is only one way to evaluate the potential contributions of a protein to the diet. Canada uses a method based on the Protein Efficiency Ratio (PER), which is growth/weight gain assay on rats fed different protein sources. Health Canada provides a list of PER values for different protein foods on their website and suggests that the PER of a protein source can be estimated by multiplying the PDCAAS by 2.5 [43][58]. Several other factors can increase the potential contribution of plant-based proteins to meeting overall dietary protein and indispensable amino acid needs. One aspect to consider is the amount of dietary protein contributed by a specific plant protein source. In the case of plant versus animal proteins, simply consuming more of the plant protein can help to provide higher indispensable amino acid intakes. Given that many whole food sources of plant-protein are less calorie-dense than animal sources of protein, greater overall food intake is needed to meet energy requirements which, in turn, helps meet indispensable amino acid requirements. In addition, it has now become much easier for consumers to boost intake of plant proteins via the availability of multiple plant-based protein isolates and concentrates (soy, pea, canola, potato, fava, etc.) in the food industry. It was once difficult for individuals to take in relatively large amounts of protein from whole plant foods because they typically have a low percentage of protein. However, plant protein isolates and concentrates, which often contain 80% or more protein by weight, make it possible to consume 10–20 g or more of plant-based protein per one serving of a ready-to-drink shake or powder mix.
Dietary protein variety is also key for meeting indispensable amino acid requirements. While the PDCAAS of an individual protein is critical when evaluating the quality of a sole-source protein, it becomes less significant when the diet contains proteins from many sources. For example, lysine is often limiting in grain proteins, but such proteins are good sources of the sulfur-containing amino acids. On the other hand, legumes are often rich sources of lysine but are limiting in sulfur-containing amino acids. Consumption of these two protein sources over the course of the day allows them to “complement” one another, helping to meet requirements for both types of indispensable amino acids. A classic example would be a combination of pea and rice proteins. Protein blends of pea and rice ranging 40–90% pea protein can achieve a PDCAAS of 1.00, using the 2011 FAO amino acid reference pattern for adults [16]. Flexitarian approaches, in which persons consume increased amounts of plant-based proteins but also include some animal proteins, represent another strategy for helping to meet indispensable amino acid requirements. Thus, the quality of protein in the diet may be quite high if the plan is to consume a variety of plant proteins with differing amino acid profiles.
One question that has arisen for vegetarians is whether it is needed to combine complementary protein sources at the same meal. Young and Pellet [19] addressed this issue. They noted that the common limiting amino acid in grains, lysine, has a significant pool in the skeletal muscle. After a protein-rich meal, they estimated that 60% of the adult daily requirement for lysine could be stored in this pool within 3 h. If a person were to consume a lysine-poor meal within 3 h of a lysine-rich meal, there would still be adequate intracellular lysine available to promote protein synthesis. Thus, it is not necessary to consume complementary protein sources at the same meal if the gap between meals is relatively short, around 3 h; the complementary amino acids will be metabolically available for protein synthesis.
An often-neglected aspect of plant proteins is their high content of some important dispensable/conditionally indispensable amino acids. The PDCAAS method of evaluating protein quality focuses only on indispensable amino acids and generally on whole body protein requirements. However, since the development of the PDCAAS concept, the knowledge base around the health- or performance-related effects of individual amino acids, both indispensable and conditionally indispensable has grown dramatically. For example, whey protein has received much attention for muscle building due to its high level of leucine (see Figure 1), which serves as a nutrient signal for initiating the process of muscle protein synthesis [44][54][59,60]. However, it is important not to forget the vital physiologic functions of dispensable/conditionally indispensable amino acids found in large amounts in plant proteins. Soy protein, while not as high as whey in leucine, is nearly three times higher in arginine, 2–3 times higher in glutamine, and has double the glycine content (Figure 2 and Table 3). Other plant proteins can be high in these amino acids as well. Arginine is necessary for the body’s synthesis of nitric oxide (vasodilator) and creatine, for urea cycle function, for regulating hormone secretion, and for immune function [55][58][61,62]. Glutamine is a primary fuel source for rapidly proliferating cells such as those in the immune system and gastrointestinal tract and functions in the synthesis of arginine, ornithine, and several other compounds [55][59][61,63]. Glycine is critical for collagen synthesis, comprising up to 1/3 of the amino acids in collagen and some studies suggest that its biosynthesis in humans may not be adequate to meet requirements [60][61][62][63][64,65,66,67]. Although amino acids such as arginine, glutamine, and glycine might not be classified all the time as indispensable amino acids, they perform many critical functions and plant proteins can be significant sources. Thus, the content of these dispensable/conditionally indispensable amino acids deserves to be taken into consideration when evaluating the value of plant proteins in the diet.
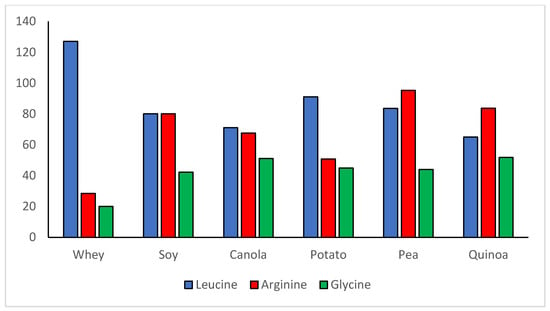
Figure 2. Comparisons of leucine and selected dispensable amino acid concentrations (mg/g protein): whey versus the Top 5 highest quality plant proteins in Table 2.
Table 3. Glutamine concentration of selected plant and dairy proteins. Sources of data: References [64][65][66][67][68,69,70,71] and unpublished data.
|
Protein |
Glutamine Concentration (mg/g Protein, Mean) |
Glutamine Concentration (mg/g Protein, Range) |
|
Wheat protein hydrolysate (n = 15) |
296 |
184–402 |
|
Wheat protein isolate (n = 2) |
208 |
184–232 |
|
Corn protein (n = 1) |
196 |
-- |
|
Rice protein (n = 1) |
130 |
-- |
|
Casein (n = 2) |
102 |
100–104 |
|
Soy protein isolate (n = 2) |
100 |
94–106 |
|
Soy protein concentrate (n = 1) |
94 |
-- |
|
Milk protein concentrate (n = 1) |
94 |
-- |
|
Whey protein concentrate (n = 2) |
57 |
50–63 |
|
Ion exchange whey protein isolate (n = 1) |
34 |
-- |
