The Ca2+-activated K+ (KCa) channels can be grouped into three categories: large (BK, KCa 1.1), intermediate (SK4/IK/KCa3.1), and small (SK1, SK2, SK3/KCa2.1, KCa2.2, KCa2.3) conductance ion channels. They possess a unique feature to connect intracellular Ca2+ signals to cell excitability. KCa channels are widely expressed in the neurons of the central nervous system (CNS), where they are involved in the control of excitability, synaptic signal transduction, and firing pattern.
- Ca2+ Ions,KCa2+ Channels,SK4 channel
1. Introduction
Ca2+ ions entering the cell are a crucial source for the activation of a diversity of Ca2+-sensing proteins. The latter include Ca2+-activated potassium (KCa) ion channels, which have been reported to be controlled via the Ca2+ entry across diverse Ca2+ channels.
In non-excitable cells, KCa channels organize K+ homeostasis and cell volume. Additionally, they trigger hormone secretion and the release of neurotransmitters. The plethora of functions of KCa channels reflects their imperative in living organisms. Defective working mechanisms or overexpression of KCa channels have been associated with neuronal disease [275,295][1][2] and many cancer phenotypes [72,296,297,298,299][3][4][5][6][7].
BK channels define the membrane potential and possess a very high single-channel conductance of ~100–300 pS. They are activated by both, voltage and enhanced cytosolic Ca2+ levels. The single-channel conductance of SK4 channels is in the range of 20–85 pS, while that of SK1–3 channels exhibit 4–14 pS. Small and intermediate KCa channels activate at low intracellular Ca2+ concentration (300 nM) in a voltage-independent manner. Despite BK and SK channels show a rather low homology, both are regulated by Ca2+. Nevertheless, their gating mechanism is completely distinct. While BK channels are directly gated by Ca2+ ions, the activation of SK channels is triggered upon Ca2+ binding to calmodulin (CaM), which is constitutively bound to SK channels [300,301,302,303][8][9][10][11]. This entry highlights the activation mechanisms of SK channels.
2.1. SK Channels
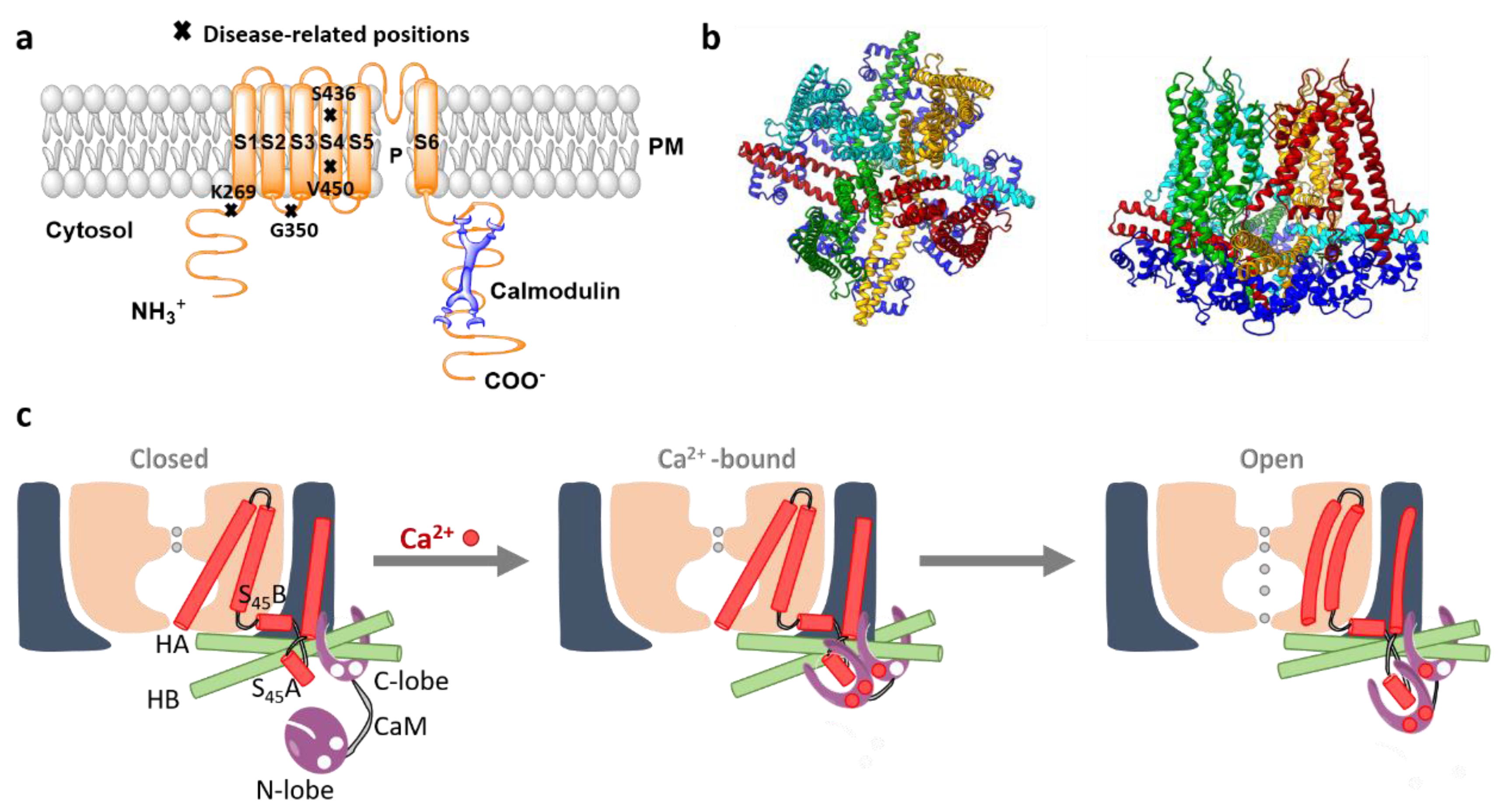
SK channel complexes possess a tetrameric stoichiometry with each of the four subunits composed of six transmembrane (S1–S6) domains. Thus, they resemble the overall structure of voltage-gated (Kv) K+ channels, whereas the voltage sensor S4 is absent. Both, N- and C-termini of SK channels are situated in the cytosol. The pore region of SK channels is formed by a re-entrant loop between the S5 and S6 domains [300][8] (Figure 1a).
Figure 1. The structure and activation mechanism of SK channels. (a) The proposed structure of the SK channel with constitutively bound CaM. P represents the pore region of the channel. Moreover, residues that have been associated with the rare developmental disorder, the Zimmermann–Laband Syndrome, are highlighted. (b) The top and side view of SK4 tetramer (PDB ID 6CNM; 4 subunits colored distinct in red, yellow, light blue, green) bound to 4 CaM (dark blue). (c) The scheme depicts the stepwise gating mechanism of the SK channel. Under the Ca2+ free conditions (left panel), the SK channel remains closed. The C-lobe of CaM is constitutively associated with the channel, whereas the CaM N-lobe possesses diverse conformations due to a high level of flexibility and almost no present interaction to the channel. The binding pocket of the CaM N-lobe at this stage stays closed. In the presence of Ca2+, the ions bind to the CaM N-lobe, which structurally rearranges into a more open conformation (middle panel). The latter rearrangement allows the interaction of CaM N-lobe with the S45A helix. In the following, the N-lobe pulls the S45A toward the cytosol, displacing S45B away from the pore axis (right panel). Subsequently, the S6 helical bundle expands and potentially opens the pore. Modified from [304] [12].
Recently, two cryo-EM structures of the SK4-CaM complex in the closed and open conformation with a resolution of 3.4 and 3.5 Å [304][12], respectively, have been reported. These structures confirm that four SK4 subunits assemble in a four-fold-symmetric tetramer which is ~95-Å long and 120-Å wide [304][12]. The pore region formed by the re-entrant loop between S5 and S6 domains is surrounded by S1 and S4 helices. The topology of SK channels resembles that of BK channels, however, there are two essential differences in the length of S1 and S2 helices and the structure of the S4-S5 linker. In the SK channel, the S1 and S2 expand to the cytosol and are much longer (60 Å) than those of the BK channels. The S4-S5 linker in the SK channel comprises two α-helices, S45A and S45B, whereas it forms a short turn in the BK channel. The structure of the S4-S5 linker is assumed to be responsible to transfer Ca2+ sensitivity to the SK channel gate mediated by CaM (Figure 1).
At the C-terminal end of S6, two helices HA and HB positioned in parallel to the membrane plane were resolved. The peripheral ends of HA and HB of each SK4 subunit represent the binding site for CaM C-lobes. Four CaMs bind to one SK channel tetramer. HB is still followed by another helix, HC, which forms a coiled-coil region positioned in the center of the complex. This region is essential for channel assembly and trafficking [304][12] (Figure 1).
While the structure-function relationship of all SK channels is comparable, they exhibit distinct expression patterns in specific cell types. SK1–3 channels are found in different kinds of cells including neurons, smooth muscle, and sensory cells [305][13]. The SK4 channel is mainly expressed in the epithelial cells [296][4].
2.2. Activation Mechanism of a Human SK-Calmodulin Channel Complex
SK channel gating is accomplished by submicromolar changes in cytosolic Ca2+ levels (KD = 0.5 µM) [306][14]. Ca2+-dependent regulation of KCa channels is established via constitutively bound calmodulin (CaM) [302][10] to the calmodulin-binding domain (CaMBD) at the C-terminus. The first indications of KCa2+ activation via CaM have been obtained upon CaM binding to partially purified KCa channels from the kidney. Furthermore, SK2 channel deletion mutants have unraveled the proximal C-terminus as the CaM-binding domain, CaMBD. GST fusion protein experiments have revealed that CaM was efficiently bound to the CaMBD, both, in the absence as well as the presence of Ca2+ [307,308][15][16].
CaM binding to the C-terminus of SK channels has been further verified via the structural resolution of a complex of a C-terminal fragment of SK2 channels together with Ca2+/CaM. This structure shows an elongated dimer of two C-termini containing a CaM attached at each end [308][15]. Each CaM twists around three alpha-helices, whereas two are from one CaM-binding domain and one is from the other CaMBD subunit. These findings have suggested that a CaMBD dimerization process induced via the Ca2+/CaM complex establishes SK channel gating [308,309][15][16].
Structural resolutions of the SK4 channel in complex with CaM have suggested that the C-terminal CaM lobe (C-lobe) is constitutively bound to SK4, whereas the N-terminal lobe (N-lobe) controls SK4 gating in a Ca2+-dependent manner [304][12] (Figure 1). Indeed, in the Ca2+-free environment, the cryo EM structure reveals a tight association of the CaM C-lobe to the HA and HB helices, while the CaM N-lobe displays high mobility. The latter likely allows fast detection of and response to the local Ca2+ signals [310][17]. In the presence of Ca2+, the cryo EM structure reveals that the CaM N-lobe is attached to SK4, thus, forming a novel interaction network. Specifically, CaM binds to the S1 and S2 helices and directly contacts the HA and HC helices of a neighboring subunit. Overall, each CaM molecule has been reported to interact during SK channel activation with three subunits of the SK channel tetramer. The binding pocket for CaM is formed by S45A, with S45B forming a bridge to S6 which enables the indirect contact of CaM to the pore. In support, several residues of S45A helix face the CaM N-lobe pocket directly. This region of the helix is highly conserved among the SK channel family potentially reflecting its importance for the channel function. Moreover, several amino acids, that form interactions within one subunit and between two subunits (N201 with R287 and K197 with E295, respectively) have been reported to hold the structural elements together. The gate formed by the residues V282 at the S6 helices represents the narrowest part of the channel pore with a radius <1 Å in the closed state. The SK4 V282G mutant has been reported to form a leaky channel that allows activation of currents also in the absence of Ca2+. Moreover, two disease-related gain-of-function mutants SK4 V282Q and SK4 V282M associated with a type of hemolytic anemia are currently known [304][12].
Overall, structural and functional analysis has demonstrated that the C-lobe is responsible for Ca2+-independent tight association to the SK channel subunit, whereas Ca2+-induced gating is established via EF hands in the N-lobe. Ca2+-bound CaM triggers structural alterations within the SK channel that leads to pore opening [304,306][12][14]. The current idea of the SK channel activation mechanism suggests that upon the increase of [Ca2+]i, CaM N-lobe binds to Ca2+ ions, which induces a conformational change. Hence, the affinity of the N-lobe for binding to S45A helix is enhanced. Subsequently, CaM couples to the S45A and moves it toward the cytosol, whereas S45B helix is displaced from the pore axis (Figure 1c). This movement leads to structural changes of the S6 helices allowing pore opening. Ca2+-independent CaM binding has been shown to control the SK channel trafficking to the membrane [311][18].
2. Ca2+-Activated K+ Channels in Diseases
2.1. SK Channel in Neurons and Neuronal Disease
SK channels play an essential role in neurons for the intrinsic excitability and synaptic function. Dysregulation of SK channels has been connected to neuropsychiatric/neurodegenerative disorders such as epilepsy, Parkinson’s disease, schizophrenia, or bipolar disorder [275,295][1][2]. In human patients diagnosed with schizophrenia, a spontaneous N-terminal deletion mutation of the SK channel gene has been detected [331,332][19][20]. Similarly, significantly suppressed expression and function of SK channels are responsible for the development of epilepsy [333][21]. Down-regulation of SK channels has been determined after induced status epilepticus (30 min continuous seizure or repeated seizures). The role of SK channels in Parkinson’s disease has remained elusive because of the contradictory evidence. Although some reports have demonstrated that enhanced SK channel activity could mitigate symptoms of Parkinson’s disease [270,334,335,336,337][22][23][24][25][26]. The reason for the different results is probably that Parkinson’s disease consists of different stages. Besides the role of the SK channels in neurons and epithelial cells, several publications have already highlighted their role in breast, colon, or prostate cancer cells.
Several SK3 gain-of-function mutations (K269G, G350N, S436C, V450L; positions indicated in Figure 1 A) have been associated with a rare developmental disorder, the Zimmermann–Laband Syndrome. As patients show, among diverse phenotypes, in addition epilepsy, this syndrome has been proposed to belong to neurological channelopathies [338][27].
2.2. SK Channels in Cancer
With respect to the potential role of the KCa channels in cancer only a few studies are currently available. Interestingly, SK channels have been reported to be expressed only in four cancer types. Gene expression of at least one of the KCa members has been identified in medulloblastoma (SK3) [339][28], glioma (SK2) [303][11], melanoma (SK2 and SK3) [297][5], or breast cancer (SK2 and SK3) [340][29]. Interestingly, despite the detection of gene expression in medulloblastoma and brain tumor cells, no SK-typical current activation or other biological effects have been detectable. Thus, diverse reports have assumed that the presence of the gene does not necessarily lead to the subsequent expression of the functional protein [297,341,342,343][5][30][31][32]. Contrarily, in breast cancer and melanoma cells, both SK2 and SK3 channel activity have been proven. Additionally, in colon cancer cells SK3 expression and SK3-mediated currents have been detectable. In particular, SK3 channel activity drives breast and colon cancer cell migration, which is abolished by the SK channel inhibitor apamin. In melanoma cells, SK3 channel expression controls cell motility. The proliferation enhancing the role of SK2 in melanoma cells appears only under hypoxia [269,343][32][33].
Among other members of the Ca2+-activated K+ channel family, both, IK and BK channels, have been found to play a significant role in cancer. The association of BK and IK channels to specific cancer hallmarks and tumor progression has already been reported for many diverse tumor cell lines such as breast, prostate, colon, glioblastoma, melanoma, cervical carcinoma, and others. This knowledge has already been discussed in many excellent reviews [344,345,346,347,348,349,350,351,352,353][34][35][36][37][38][39][40][41][42][43]. Specifically, BK channels have been found to determine glioma, breast, and prostate cancer cell growth [276,299,354,355,356,357,358][7][44][45][46][47][48][49]. IK channels are involved in the control of the typical cancer hallmarks of glioma, colon, prostate, breast cancer, and cervical carcinoma [43,64,273,296,303,359,360][4][50][51][52][53]. The most frequently occurring type of cancer-cell-hijacked biological function appears to be the upregulation of the protein expression which induces cell proliferation, migration, and finally triggers bone metastasis [344,345,346,347][45][46][47][48].
References
- Kshatri, A.S.; Gonzalez-Hernandez, A.; Giraldez, T. Physiological roles and therapeutic potential of Ca2+ activated potassium channels in the nervous system. Front. Mol. Neurosci. 2018, 11, 258.
- Trombetta-Lima, M.; Krabbendam, I.E.; Dolga, A.M. Calcium-activated potassium channels: Implications for aging and age-related neurodegeneration. Int. J. Biochem. Cell Biol. 2020, 123, 105748.
- Duffy, S.M.; Ashmole, I.; Smallwood, D.T.; Leyland, M.L.; Bradding, P. Orai/CRACM1 and KCa3.1 ion channels interact in the human lung mast cell plasma membrane. Cell Commun. Signal. 2015, 13, 32.
- Lallet-Daher, H.; Roudbaraki, M.; Bavencoffe, A.; Mariot, P.; Gackiere, F.; Bidaux, G.; Urbain, R.; Gosset, P.; Delcourt, P.; Fleurisse, L.; et al. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene 2009, 28, 1792–1806.
- Chantome, A.; Girault, A.; Potier-Cartereau, M.; Collin, C.; Vaudin, P.; Pagès, J.-C.; Vandier, C.; Joulin, V. KCa2.3 channel-dependent hyperpolarization increases melanoma cell motility. Exp. Cell Res. 2009, 315, 3620–3630.
- Potier, M.-C.; Tran, T.A.; Chantome, A.; Girault, A.; Joulin, V.; Bougnoux, P.; Vandier, C.; Pierre, F. Altered SK3/KCa2.3-mediated migration in adenomatous polyposis coli (Apc) mutated mouse colon epithelial cells. Biochem. Biophys. Res. Commun. 2010, 397, 42–47.
- Gackière, F.; Warnier, M.; Katsogiannou, M.; Derouiche, S.; Delcourt, P.; Dewailly, E.; Slomianny, C.; Humez, S.; Prevarskaya, N.; Roudbaraki, M.; et al. Functional coupling between large-conductance potassium channels and Cav3.2 voltage-dependent calcium channels participates in prostate cancer cell growth. Biol. Open 2013, 2, 941–951.
- Berkefeld, H.; Fakler, B.; Schulte, U. Ca2+-Activated K+ channels: From protein complexes to function. Physiol. Rev. 2010, 90, 1437–1459.
- Berkefeld, H.; Sailer, C.A.; Bildl, W.; Rohde, V.; Thumfart, J.-O.; Eble, S.; Klugbauer, N.; Reisinger, E.; Bischofberger, J.; Oliver, D.; et al. BKCa-Cav channel complexes mediate rapid and localized Ca2+-Activated K+ signaling. Science 2006, 314, 615–620.
- Lee, W.S.; Ngo-Anh, T.J.; Bruening-Wright, A.; Maylie, J.; Adelman, J.P. Small conductance Ca2+-activated K+ channels and calmodulin—Cell surface expression and gating. J. Biol. Chem. 2003, 278, 25940–25946.
- Weaver, A.K.; Bomben, V.C.; Sontheimer, H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia 2006, 54, 223–233.
- Wulff, H.; MacKinnon, R. Activation mechanism of a human SK-calmodulin channel complex elucidated by cryo-EM structures. Science 2018, 360, 508–513.
- Guéguinou, M.; Chantôme, A.; Fromont, G.; Bougnoux, P.; Vandier, C.; Potier-Cartereau, M. KCa and Ca2+ channels: The complex thought. Biochim. Biophys. Acta 2014, 1843, 2322–2333.
- Maylie, J.; Bond, C.T.; Herson, P.S.; Lee, C.W.-S.; Adelman, J.P. Small conductance Ca2+-activated K+Channels and calmodulin. J. Physiol. 2004, 554, 255–261.
- Xia, X.-M.; Fakler, B.; Rivard, A.L.; A Wayman, G.; Johnsonpais, T.L.; E Keen, J.; Ishii, T.; Hirschberg, B.; Bond, C.T.; Lutsenko, S.V.; et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nat. Cell Biol. 1998, 395, 503–507.
- A Schumacher, M.; Rivard, A.F.; Bächinger, H.P.; Adelman, J.P. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nat. Cell Biol. 2001, 410, 1120–1124.
- Tidow, H.; Nissen, P. Structural diversity of calmodulin binding to its target sites. FEBS J. 2013, 280, 5551–5565.
- Adelman, J.P. SK channels and calmodulin. Channels 2015, 10, 1–6.
- Miller, M.J.; Rauer, H.; Tomita, H.; Gargus, J.J.; Gutman, G.A.; Cahalan, M.D.; Chandy, K.G. Nuclear localization and dominant-negative suppression by a mutant SKCa3 N-terminal channel fragment identified in a patient with schizophrenia. J. Biol. Chem. 2001, 276, 27753–27756.
- Bowen, T.; Williams, N.; Norton, N.; Spurlock, G.; Wittekindt, O.H.; Dj, M.-R.; Brzustowicz, L.; Hoogendoorn, B.; Zammit, S.; Jones, G.; et al. Mutation screening of the KCNN3 gene reveals a rare frameshift mutation. Mol. Psychiatry 2001, 6, 259–260.
- Oliveira, M.S.; Skinner, F.; Arshadmansab, M.F.; Garcia, I.; Mello, C.F.; Knaus, H.-G.; Ermolinsky, B.S.; Otalora, L.F.P.; Garrido-Sanabria, E.R. Altered expression and function of small-conductance (SK) Ca2+-activated K+ channels in pilocarpine-treated epileptic rats. Brain Res. 2010, 1348, 187–199.
- Ji, H.; Shepard, P.D. SK Ca2+-activated K+ channel ligands alter the firing pattern of dopamine-containing neurons in vivo. Neuroscience 2006, 140, 623–633.
- Dolga, A.M.; De Andrade, A.; Meissner, L.; Knaus, H.-G.; Hollerhage, M.; Christophersen, P.; Zischka, H.; Plesnila, N.; Hoglinger, G.U.; Culmsee, C. Subcellular expression and neuroprotective effects of SK channels in human dopaminergic neurons. Cell Death Dis. 2014, 5, e999.
- Alvarez-Fischer, D.; Noelker, C.; Vulinović, F.; Grünewald, A.; Chevarin, C.; Klein, C.; Oertel, W.H.; Hirsch, E.C.; Michel, P.P.; Hartmann, A. Bee venom and its component apamin as neuroprotective agents in a parkinson disease mouse model. PLoS ONE 2013, 8, e61700.
- Kim, J.I.; Yang, E.J.; Lee, M.S.; Kim, Y.S.; Huh, Y.; Cho, I.H.; Kang, S.; Koh, H.K. Bee venom reduces neuroinflammation in the MPTP-induced model of Parkinson’s disease. Int. J. Neurosci. 2011, 121, 209–217.
- Doo, A.-R.; Kim, S.-T.; Moon, W.; Yin, C.S.; Chae, Y.; Park, H.-K.; Lee, H.; Park, H.-J. Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Neurol. Res. 2010, 32, 88–91.
- Bauer, C.K.; Schneeberger, P.E.; Kortüm, F.; Altmüller, J.; Santos-Simarro, F.; Baker, L.; Keller-Ramey, J.; White, S.M.; Campeau, P.M.; Gripp, K.W.; et al. Gain-of-function mutations in KCNN3 Encoding the small-conductance Ca2+-Activated K+ Channel SK3 cause Zimmermann-Laband syndrome. Am. J. Hum. Genet. 2019, 104, 1139–1157.
- Carignani, C.; Roncarati, R.; Rimini, R.; Terstappen, G.C. Pharmacological and molecular characterisation of SK3 channels in the TE671 human medulloblastoma cell line. Brain Res. 2002, 939, 11–18.
- Potier, M.; Joulin, V.; Roger, S.; Besson, P.; Jourdan, M.-L.; LeGuennec, J.-Y.; Bougnoux, P.; Vandier, C. Identification of SK3 channel as a new mediator of breast cancer cell migration. Mol. Cancer Ther. 2006, 5, 2946–2953.
- Tajima, N.; Schönherr, K.; Niedling, S.; Kaatz, M.; Kanno, H.; Schönherr, R.; Heinemann, S.H. Ca2+-activated K+ channels in human melanoma cells are up-regulated by hypoxia involving hypoxia-inducible factor-1α and the von Hippel-Lindau protein. J. Physiol. 2006, 571, 349–359.
- Meyer, R.; Schönherr, R.; Gavrilova-Ruch, O.; Wohlrab, W.; Heinemann, S.H. Identification of ether à go-go and calcium-activated potassium channels in human melanoma cells. J. Membr. Biol. 1999, 171, 107–115.
- Girault, A.; Haelters, J.-P.; Potier-Cartereau, M.; Chantôme, A.; Jaffres, P.-A.; Bougnoux, P.; Joulin, V.; Vandier, C. Targeting SKCa channels in cancer: Potential new therapeutic approaches. Curr. Med. Chem. 2012, 19, 697–713.
- Chen, M.X.; Gorman, S.A.; Benson, B.; Singh, K.; Hieble, J.P.; Michel, M.C.; Tate, S.N.; Trezise, D.J. Small and intermediate conductance Ca 2+ -activated K + channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedeberg’s Arch. Pharmacol. 2004, 369, 602–615.
- Arcangeli, A.; Crociani, O.; Lastraioli, E.; Masi, A.; Pillozzi, S.; Becchetti, A. Targeting ion channels in cancer: A novel frontier in antineoplastic therapy. Curr. Med. Chem. 2009, 16, 66–93.
- Cuddapah, V.A.; Sontheimer, H. Ion channels and tranporters in cancer. Ion channels and the control of cancer cell migration. Am. J. Physiol. Physiol. 2011, 301, C541–C549.
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of ion channels and transporters in cell migration. Physiol. Rev. 2012, 92, 1865–1913.
- Pardo, L.A.; Stühmer, W. The roles of K+ channels in cancer. Nat. Rev. Cancer 2014, 14, 39–48.
- Villalonga, N.; Ferreres, J.C.; Argilés, J.M.; Condom, E.; Felipe, A. Potassium channels are a new target field in anticancer drug design. Recent Pat. Anticancer Drug Discov. 2007, 2, 212–223.
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in tumor metastasis: New roles for known actors. Nat. Rev. Cancer 2011, 11, 609–618.
- Monteith, G.; Davis, F.M.; Roberts-Thomson, S.J. Calcium channels and pumps in cancer: Changes and consequences. J. Biol. Chem. 2012, 287, 31666–31673.
- Chen, Y.-F.; Chen, Y.-T.; Chiu, W.; Shen, M.-R. Remodeling of calcium signaling in tumor progression. J. Biomed. Sci. 2013, 20, 23.
- Contreras, G.F.; Castillo, K.; Enrique, N.; Carrasquel-Ursulaez, W.; Castillo, J.P.; Milesi, V.; Neely, A.; Alvarez, O.; Ferreira, G.; Gonzalez, C.; et al. A BK (Slo1) channel journey from molecule to physiology. Channels 2013, 7, 442–458.
- D’Amico, M.; Gasparoli, L.; Arcangeli, A. Potassium channels: Novel emerging biomarkers and targets for therapy in cancer. Recent Pat. Anticancer Drug Discov. 2013, 8, 53–65.
- Liu, X.; Chang, Y.; Reinhart, P.H.; Sontheimer, H. Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J. Neurosci. 2002, 22, 1840–1849.
- Weaver, A.K.; Liu, X.; Sontheimer, H. Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J. Neurosci. Res. 2004, 78, 224–234.
- Sontheimer, H. Ion channels and amino acid transporters support the growth and invasion of primary brain tumors. Mol. Neurobiol. 2004, 29, 61–72.
- Mu, D.; Chen, L.; Zhang, X.; See, L.-H.; Koch, C.M.; Yen, C.; Tong, J.J.; Spiegel, L.; Nguyen, K.C.; Servoss, A.; et al. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell 2003, 3, 297–302.
- Oeggerli, M.; Tian, Y.; Ruiz, C.; Wijker, B.; Sauter, G.; Obermann, E.; Güth, U.; Zlobec, I.; Sausbier, M.; Kunzelmann, K.; et al. Role of KCNMA1 in breast cancer. PLoS ONE 2012, 7, e41664.
- Bloch, M.; Ousingsawat, J.; Simon, R.; Schraml, P.; Gasser, T.C.; Mihatsch, M.J.; Kunzelmann, K.; Bubendorf, L. KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene 2006, 26, 2525–2534.
- Steudel, F.A.; Mohr, C.J.; Stegen, B.; Nguyen, H.Y.; Barnert, A.; Steinle, M.; Beer-Hammer, S.; Koch, P.; Lo, W.Y.; Schroth, W.; et al. SK4 channels modulate Ca2+ signalling and cell cycle progression in murine breast cancer. Mol. Oncol. 2017, 11, 1172–1188.
- Gueguinou, M.; Gambade, A.; Felix, R.; Chantome, A.; Fourbon, Y.; Bougnoux, P.; Weber, G.; Potier-Cartereau, M.; Vandier, C. Lipid rafts, KCa/ClCa/Ca2+ channel complexes and EGFR signaling: Novel targets to reduce tumor development by lipids? Biochim. Biophys. Acta. 2015, 1848 (10 Pt B), 2603–2620.
- Mohr, C.J.; Steudel, F.A.; Gross, D.; Ruth, P.; Lo, W.-Y.; Hoppe, R.; Schroth, W.; Brauch, H.; Huber, S.M.; Lukowski, R. Cancer-associated intermediate conductance Ca2+-Activated K+ channel KCa3. Cancers 2019, 11, 109.
- Thurber, A.E.; Nelson, M.; Frost, C.L.; Levin, M.; Brackenbury, W.J.; Kaplan, D.L. IK channel activation increases tumor growth and induces differential behavioral responses in two breast epithelial cell lines. Oncotarget 2017, 8, 42382–42397.